
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Linda Nezbedova | + 2077 word(s) | 2077 | 2021-11-15 04:40:59 | | | |
| 2 | Rita Xu | -63 word(s) | 2014 | 2021-11-30 02:35:43 | | |
Video Upload Options
Population studies have associated a diet high in fruits to lower incidence of cancer. Specifically, research shows that secondary plant metabolites known as phytochemicals, which are commonly found in fruits, have onco-preventive and chemo-protective effects. Apple is a commonly consumed fruit worldwide that is available all year round and is a rich source of phytochemicals. The health benefits of apples are thought to be mainly due to their phytochemical composition. Additionally, apple consumption is associated with lower incidence of some cancers based on animal and cell culture studies.
1. Introduction
Chronic diseases including cancer continues to remain a public health burden globally [1][2][3][4][5]. In 2020, cancer was the second leading chronic illness following cardiovascular disease, with an estimate of 19 million new cases and accounting for 10 million deaths per year, globally [6][7]. Data from GLOBOCAN [7] show that cancers of the breast are the most commonly diagnosed followed by cancers of the lung, colorectal and prostate.
To reduce cancer’s global health burden, it is necessary to promote both cancer treatment as well as cancer prevention.
There is growing evidence that diet, especially phytochemicals found in vegetables and fruits, play a major role in cancer aetiology and prevention. One fruit that is a rich and important source of bioactive phytochemicals in western diet, is apple [8][9][10][11]. Apples are globally consumed due to their year-round availability, their cultivar diversity, low price, and easy storage [8][12].
2. Apple Phytochemical Profile and Bioavailability
To better understand the health benefits of apples, in this section we provide a comprehensive review of the main phytochemical patterns observed in apples and their potential health benefits depending on variety, and part consumed (skin/peel versus the flesh of the apple).
Apples contain a wide variety of phytochemicals, including triterpenoids, organic acids, fatty acids and apple phenolic compounds (Figure 1) [13]. Triterpenoids are components mainly of apple waxes [14]. The main triterpenoids found in apples are oleanolic, betulinic and ursolic acid and their derivates such as maslinic, corosolic, euscaphic, pomaceic and pomolic acids [15].
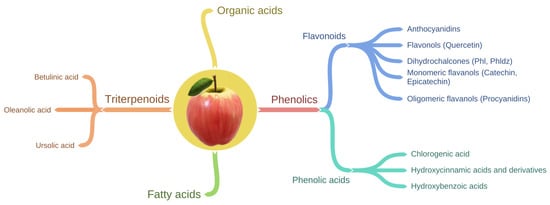
The most well-studied group of apple phytochemicals for their health benefits are phenolic compounds [16]. Studies show that apples are an important source of phenolic compounds in our diet contributing to 22% of phenolic intake [17][18]. Most of the phenolic compounds in the fruit are usually present in the conjugated form such as glycosides or esterified carboxylic acids. However, compared to other fruit, apples contain more of the readily bioavailable free forms of phenolic compounds [19][20][21]. For instance, the ‘Red Delicious’ apple had the highest levels of free forms of phenolic compounds compared to pear, plums, kiwifruit and peach [22].
Phenolic compounds in apple can be sub-divided into two main groups (Table 1, Figure 1) known as flavonoids and phenolic acids. Flavonoids can be further divided into four structural subclasses including anthocyanidins, flavonols, dihydrochalcones and flavan-3-ols (flavanols) which can exist in the monomeric and oligomeric form [23][24] (Table 1). Phenolic acids include chlorogenic acid, hydroxycinnamic acid and hydroxybenzoic acid [23][24]. In general, chlorogenic acid, monomeric and polymeric flavanols are the major phenolic compounds, whereas anthocyanins and dihydrochalcones are minor phenolic compounds of apples [25]. Moreover, anthocyanidins are responsible for the apple redness [26][27]. Therefore, anthocyanidins are abundant in the apple cultivars with red skin (e.g. ‘Red Delicious’) and are not present or present in low concentrations in the green skinned apple cultivars (e.g. ‘Granny Smith’) [27][28].
| Phenolics Group | Phenolic Subgroup | Phenolic Compounds |
|---|---|---|
| Flavonoids | Anthocyanidins | Cyanidin 3-O-arabinoside |
| Cyanidin 3-O-galactoside | ||
| Cyanidin 3-O-xyloside | ||
| Cyanidin 3-O-xylgalactoside | ||
| Flavonols | Quercetin | |
| Quercetin 3-arabinopyranoside | ||
| Quercetin-3-arabinofuranoside | ||
| Quercetin 3-galactoside | ||
| Quercetin 3-glucoside | ||
| Quercetin 3-rhamnoside | ||
| Quercetin 3-rutinoside | ||
| Quercetin 3-xyloside | ||
| Dihydrochalcones | Phloretin | |
| Phloretin-2′-O-xyloglucoside | ||
| Phloridzin | ||
| 3-hydroxyphloridzin | ||
| Flavan-3-ols | Monomeric | |
| (+)-Catechin | ||
| (−)-Epicatechin | ||
| Oligomeric (Procyanidins) | ||
| Procyanidin B1 | ||
| Procyanidin B2 | ||
| Procyanidin B5 | ||
| Procyanidin B7 | ||
| Procyanidin C1 | ||
| Phenolic acids | Chlorogenic acid Hydroxy benzoic acid Hydroxy cinnamic acid |
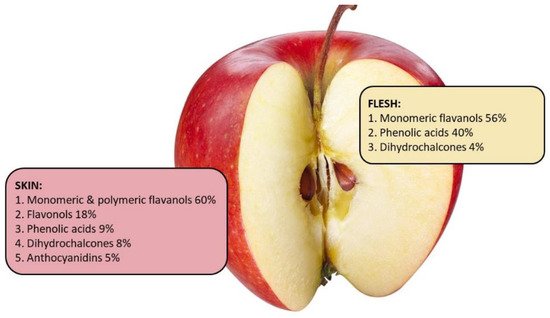
In general, the apple peel contains about 2-4 times higher levels of phenolics, and higher levels of total procyanidins and total flavonoids, compared to flesh [34]. In general, evidence from multiple studies comparing various cultivars of apples, have shown that apple peel contains all groups of phenolic compounds and has greater concentrations of procyanidins, and total flavonoids compared to the flesh [21][35][36][37]. On the other hand, chlorogenic acid can be found in both flesh and peel but tends to be higher in the flesh [21][32]. Taken together these data suggest that apple peel of most apple varieties contain more phenolics than the flesh. More information on the phytochemicals patterns in different cultivars is provided in the review article linked to this entry.
It is important to consider that apple peel contributes only up to 10% of the weight of the whole fruit, therefore the intake of some phenolic compounds from the peel after consumption of a whole apple might not be as significant as the intake from the flesh. Only a few studies have reported on the phenolic compounds content relative to the weight of the peel compared to the whole apple [25][34]. McGhie, et al. [25] demonstrated that peel of ‘Braeburn’, ‘Royal Gala’ and ‘Red Delicious’ contributed 55%, 50% and 52% respectively of the apple’s total phenolics. Data from New Zealand heritage apple cultivar ‘Monty’s Surprise’ and the commercial varieties ‘Braeburn’ and ‘Red Delicious’ (Table 2) showed that the contribution of total phenolics was lower from the peel compared to the flesh. However, anthocyanidins were only present in the peel and flavonols were found only in small quantities in the flesh. Taken together, a combination of unpublished data from New Zealand (Table 2) and other published studies suggests that for most apple varieties the peel is a significant source of phenolic compounds. Therefore, discarding the peel during production of some traditional apple products, such as apple sauce [38] may decrease the health potential of the apples.Table 2. Estimated apple peel contribution to the total phenolics content in whole apple. Phenolic compounds were measured using Liquid chromatography-Mass Spectrometry (LC-MS, Dionex Ultimate RS3000 UHPL and a Bruker micrOTOF-QII) in 2019, Plant and Food research, for 3 apple varieties-Monty’s surprise, Braeburn and Red Delicious. Each compound concentration was quantified by comparison with an authentic standard where possible or as equivalents to standard compounds. Each phenolic compound in the table is presented as a percentage of total concentration measured using LC-MS. Percentage total phenolics was calculated based on the average weight of whole apple (180 g) where apple skin contributed 18 g.
|
|
Monomeric Flavanols |
Procyanidins |
Flavonols |
Dihydrochalcones |
Chlorogenic acid |
Anthocyanins |
Total Phenolics |
|||||||
Cultivar |
Skin (%) |
Flesh (%) |
Skin (%) |
Flesh (%) |
Skin (%) |
Flesh (%) |
Skin (%) |
Flesh (%) |
Skin (%) |
Flesh (%) |
Skin (%) |
Flesh (%) |
Skin (%) |
Flesh (%) |
Monty's Surprise |
33 |
67 |
29 |
71 |
94 |
6 |
42 |
58 |
10 |
90 |
100 |
n.e. |
37 |
63 |
Braeburn |
19 |
81 |
21 |
79 |
99 |
1 |
8 |
92 |
1 |
99 |
100 |
n.e. |
31 |
69 |
Red Delicious |
37 |
63 |
36 |
64 |
94 |
6 |
40 |
60 |
2 |
98 |
100 |
n.e. |
46 |
54 |
* Monty’s Surprise – New Zealand’s heritage apple variety
* n.e. - not evaluated (Anthocyanins were not evaluated in the flesh as they are not present.)
The health benefits of an apple’s bioactive compounds depend on their absorption, metabolism and distribution within the human body [39]. The bioavailability (the fraction of the bioactive that has been absorbed and is available for biology activity) of phenolic compounds is affected by pH, enzymatic activity, their chemical structure, solubility, free and bound form as well as the synergistic effects with the food matrix [40][41][42].
Despite absorption of the phenolic compounds beginning in the small intestine [42], most of the phenolics are released in and absorbed from the large intestine, with aid from gut microbiota [41][43][44]. The gut microbiota is capable of transforming complex phenolic compounds into metabolites that are more easily absorbed [45]. It was demonstrated that once absorbed phenolic compounds can be detected in human plasma and urine after consumption of apple [46], apple juice [47], and apple cider [48]. Bioavailability of the main apple phytochemicals is described in section 5.2 of the main review article linked to this entry.
While apple is a rich source of nutrients and phytochemicals, there is evidence to suggest that the apple food matrix (non-nutrient component) plays an important role in the absorption and bioavailability of apple phytochemicals. Aprikain, et al. [44] demonstrated that ingestion of phenolics rich apple extract and apple pectin together had greater effect on gut microbiota metabolism in the large intestine and lipid metabolism than ingestion of the phenolics-rich apple extract alone, suggesting a beneficial interaction between fibre and phenolics [44].
3. Health Benefits of Apple Phytochemicals: Cancer
Current research attributes the health benefits of apples mainly to the phenolic compounds which exhibit several biological functions beneficial for human health [16]. Apple phenolic compounds are believed to lower incidence of chronic conditions such as cardiovascular disease, cancer, asthma and pulmonary disease, diabetes, and obesity [24][49][8][9][10][11][12][50].
Apple phytochemicals are suggested to have many chemo-preventive and chemo-protective effects (Figure 3) against various types of cancer. Apple phytochemicals were reported to have significant effects on inhibiting multiple ‘hallmarks of cancer’ which are important in growth and progression of cancer [51].
These effects include regulation of proliferation, cell cycle, apoptosis, reactive oxygen species (ROS) and anti-inflammatory activities [45][52][53][54][55]. In this section we discuss health benefits of apple phytochemicals in relation to cancer from epidemiological studies, their ability to alter ROS in cancer cells and impact on cancer biology from in vitro and in vivo studies.
Phenolic compounds from different apple cultivars were positively associated with the higher degree of inhibition of breast cancer cell proliferation [56][57][58] and induction of cell cycle arrest [57][59]. Additionally, apple extracts inhibited growth of prostate [59] and lung [60] cancer cells. Extracts of phenolics from apple pomace of different apple cultivars were reported to inhibit proliferation of oral [61] and colon cancer cells [20]. In addition to in vitro studies, apple polyphenol extracts also inhibited ex vivo proliferation of a hepatoma cell line [62].
Multiple studies have demonstrated that apple phytochemicals can inhibit the activity of p21, growth factors, pyruvate dehydrogenase kinases (PDKs), cyclin-dependent kinases (CDKs) and extracellular protein kinases (ERKs) essential for cell cycle progression [58][63][64][65]. Furthermore, apple phytochemicals can also prevent cell cycle progression by activation of maspin, a tumour suppressor gene [59]. Changes in the key molecules essential for regulating cell cycle by apple phytochemicals leads to cancer cells arrest [57][59]. Apple extracts were reported to inhibit apoptosis in breast cancer cells [56][60]. Apple phenolic compounds were shown to regulate pro-apoptotic genes such as p53, p21, Bax, Bcl-2 [66]. In addition to inhibiting cell proliferation and promoting apoptosis, apple phytochemicals have also been implicated in inhibiting angiogenesis by regulating VEGF [51]; and inhibiting invasion and metastasis [20][62] by regulating matrix metalloproteinases-2,-9 (MMP-2,-9), cadherins and integrins [51] and regulating COX-2 a marker of inflammation [51]. Additionally, the ability of apple phytochemicals to inhibit cell proliferation and in turn reduce incidence of cancer was also observed in rats, where rats fed one human apple equivalent, had reduced appearance of different precancerous markers (ACF, MDF, genes and proteins related to colorectal cancer progression) [67]. Similarly, the incidence of mammary tumours in rats was reduced after two weeks of oral administration of 3.3, 10 and 20 g of apple extract/kg of body weight, which correspond to the human consumption one (200 g), three, and six apples per day [68].
There is evidence that anticancer properties of apples are due to the synergistic effects between apple phytochemicals and between apple phytochemicals and food matrix [69][70][71][72]. Veeriah, et al. [69] demonstrated that the order of inhibition against a colon cancer cells treated with an apple extract (extract from a mixture of different apples) reduced cell proliferation to a greater extend compared to a synthetic apple extract composed of eight apple phenolics or individual apple phenolic compounds [69]. Results from this study indicate the importance of the apple food matrix, which may contain other bioactive compounds present in the apple extract but not in the synthetic mixture.
Taken together, evidence from in vitro, ex vivo and in vivo studies suggest that apple phytochemicals work synergistically, to inhibit multiple ‘hallmarks of cancer’, which in turn can influence cancer incidence and improve outcomes to chemotherapeutic treatments.
References
- Harris, R.E. Epidemiology of chronic disease: global perspectives; Jones & Bartlett Learning: 2019.
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European cardiovascular disease statistics 2017. 2017.
- Bowry, A.D.K.; Lewey, J.; Dugani, S.B.; Choudhry, N.K. The Burden of Cardiovascular Disease in Low- and Middle-Income Countries: Epidemiology and Management. Canadian Journal of Cardiology 2015, 31, 1151-1159, doi:https://doi.org/10.1016/j.cjca.2015.06.028.
- Magliano, D.J.; Islam, R.M.; Barr, E.L.; Gregg, E.W.; Pavkov, M.E.; Harding, J.L.; Tabesh, M.; Koye, D.N.; Shaw, J.E. Trends in incidence of total or type 2 diabetes: systematic review. Bmj 2019, 366.
- Maxwell, O.; Kayode, A.A.; Olusola, Y.O.; Joshua, A.I.; Chinedu, O.V. Lung Cancer: A Chronic Disease Epidemiology; Prevalence Study. Asian Journal of Advanced Research and Reports 2019, 1-7.
- WHO. Global Health Estimated 2020: Deaths bu Cause, bu Age, sex, by country and by region, 2019-2020; World Health Organisation 2020.
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on
- DuPont, M.S.; Bennett, R.N.; Mellon, F.A.; Williamson, G. Polyphenols from Alcoholic Apple Cider Are Absorbed, Metabolized and Excreted by Humans. The Journal of Nutrition 2002, 132, 172-175, doi:10.1093/jn/132.2.172.
- Starowicz, M.; Achrem–Achremowicz, B.; Piskuła, M.K.; Zieliński, H. Phenolic Compounds from Apples: Reviewing their Occurrence, Absorption, Bioavailability, Processing, and Antioxidant Activity–a Review. Polish Journal of Food and Nutrition Sciences 2020, 70, 321-336.
- Zou, T.; Wang, B.; Li, S.; Liu, Y.; You, J. Dietary apple polyphenols promote fat browning in high-fat diet-induced obese mice through activation of adenosine monophosphate-activated protein kinase α. J Sci Food Agric 2020, 100, 2389-2398, doi:10.1002/jsfa.10248.
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598.
- Gayer, B.A.; Avendano, E.E.; Edelson, E.; Nirmala, N.; Johnson, E.J.; Raman, G. Effects of Intake of Apples, Pears, or Their Products on Cardiometabolic Risk Factors and Clinical Outcomes: A Systematic Review and Meta-Analysis. Curr Dev Nutr 2019, 3, nzz109, doi:10.1093/cdn/nzz109.
- Kidoń, M.; Grabowska, J. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT 2021, 136, 110302, doi:https://doi.org/10.1016/j.lwt.2020.110302.
- Dashbaldan, S.; Pączkowski, C.; Szakiel, A. Variations in Triterpenoid Deposition in Cuticular Waxes during Development and Maturation of Selected Fruits of Rosaceae Family. International Journal of Molecular Sciences 2020, 21, 9762.
- Sut, S.; Poloniato, G.; Malagoli, M.; Dall'Acqua, S. Fragmentation of the main triterpene acids of apple by LC-APCI-MSn. Journal of Mass Spectrometry 2018, 53, 882-892, doi:https://doi.org/10.1002/jms.4264.
- Davis, M.A.; Bynum, J.P.W.; Sirovich, B.E. Association Between Apple Consumption and Physician Visits: Appealing the Conventional Wisdom That an Apple a Day Keeps the Doctor Away. JAMA Internal Medicine 2015, 175, 777-783, doi:10.1001/jamainternmed.2014.5466.
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem 2001, 49, 5315-5321, doi:10.1021/jf0009293.
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. The Journal of Nutrition 2000, 130, 2073S-2085S, doi:10.1093/jn/130.8.2073S.
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem 2002, 50, 7449-7454, doi:10.1021/jf0207530.
- McCann, M.J.; Gill, C.I.; G, O.B.; Rao, J.R.; McRoberts, W.C.; Hughes, P.; McEntee, R.; Rowland, I.R. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem Toxicol 2007, 45, 1224-1230, doi:10.1016/j.fct.2007.01.003.
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiology and Biochemistry 2014, 84, 169-188, doi:https://doi.org/10.1016/j.plaphy.2014.09.006.
- Imeh, U.; Khokhar, S. Distribution of Conjugated and Free Phenols in Fruits: Antioxidant Activity and Cultivar Variations. Journal of Agricultural and Food Chemistry 2002, 50, 6301-6306, doi:10.1021/jf020342j.
- Almeida, D.P.F.; Gião, M.S.; Pintado, M.; Gomes, M.H. Bioactive phytochemicals in apple cultivars from the Portuguese protected geographical indication “Maçã de Alcobaça:” Basis for market segmentation. International Journal of Food Properties 2017, 20, 2206-2214, doi:10.1080/10942912.2016.1233431.
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: assessment, analysis and application. Journal of Food Science and Technology 2016, 53, 1727-1738, doi:10.1007/s13197-015-2093-8.
- McGhie, T.K.; Hunt, M.; Barnett, L.E. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apples grown in New Zealand. J Agric Food Chem 2005, 53, 3065-3070, doi:10.1021/jf047832r.
- Honda, C.; Moriya, S. Anthocyanin Biosynthesis in Apple Fruit. The Horticulture Journal 2018, 87, 305-314, doi:10.2503/hortj.OKD-R01.
- De Paepe, D.; Valkenborg, D.; Noten, B.; Servaes, K.; Diels, L.; De Loose, M.; Van Droogenbroeck, B.; Voorspoels, S. Variability of the phenolic profiles in the fruits from old, recent and new apple cultivars cultivated in Belgium. Metabolomics 2015, 11, 739-752, doi:10.1007/s11306-014-0730-2.
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Research International 2014, 59, 41-52, doi:https://doi.org/10.1016/j.foodres.2014.01.046.
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. Journal of Functional Foods 2015, 18, 1086-1094, doi:https://doi.org/10.1016/j.jff.2014.06.013.
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal influence on phenolic constituents and nutritive characteristics of pomace obtained from apples grown in western Himalayas. Journal of Food Science and Technology 2021, 58, 166-174, doi:10.1007/s13197-020-04526-y.
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chemistry 2017, 233, 29-37, doi:https://doi.org/10.1016/j.foodchem.2017.04.067.
- Alberti, A.; Zielinski, A.A.F.; Couto, M.; Judacewski, P.; Mafra, L.I.; Nogueira, A. Distribution of phenolic compounds and antioxidant capacity in apples tissues during ripening. Journal of Food Science and Technology 2017, 54, 1511-1518, doi:10.1007/s13197-017-2582-z.
- van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions. J Agric Food Chem 2001, 49, 3606-3613, doi:10.1021/jf001493u.
- Lata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Scientia Horticulturae 2009, 121, 176-181, doi:https://doi.org/10.1016/j.scienta.2009.01.038.
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). Journal of agricultural and food chemistry 2003, 51, 6347-6353.
- Lata, B. Relationship between apple peel and the whole fruit antioxidant content: year and cultivar variation. J Agric Food Chem 2007, 55, 663-671, doi:10.1021/jf062664j.
- Shehzadi, K.; Rubab, Q.; Asad, L.; Ishfaq, M.; Shafique, B.; Ali Nawaz Ranjha, M.M.; Mahmood, S.; Mueen-Ud-Din, G.; Javaid, T.; Sabtain, B.; et al. A Critical Review on Presence of Polyphenols in Commercial Varieties of Apple Peel, their Extraction and Health Benefits. Open Access Journal of Biogeneric Science and Research 2020, 6, doi:10.46718/JBGSR.2020.06.000141.
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic compounds in some apple (Malus domestica Borkh) cultivars of organic and integrated production. Journal of the Science of Food and Agriculture 2005, 85, 1687-1694, doi:https://doi.org/10.1002/jsfa.2113.
- Francini, A.; Sebastiani, L. Phenolic Compounds in Apple (Malus x domestica Borkh.): Compounds Characterization and Stability during Postharvest and after Processing. Antioxidants (Basel) 2013, 2, 181-193, doi:10.3390/antiox2030181.
- Le Bourvellec, C.; Bouzerzour, K.; Ginies, C.; Regis, S.; Plé, Y.; Renard, C.M.G.C. Phenolic and polysaccharidic composition of applesauce is close to that of apple flesh. Journal of Food Composition and Analysis 2011, 24, 537-547, doi:https://doi.org/10.1016/j.jfca.2010.12.012.
- Selby-Pham, S.N.B.; Miller, R.B.; Howell, K.; Dunshea, F.; Bennett, L.E. Physicochemical properties of dietary phytochemicals can predict their passive absorption in the human small intestine. Scientific Reports 2017, 7, 1931, doi:10.1038/s41598-017-01888-w.
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Research International 2015, 2015, 905215, doi:10.1155/2015/905215.
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends in Food Science & Technology 2017, 69, 243-256, doi:https://doi.org/10.1016/j.tifs.2017.04.012.
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263.
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutrition Journal 2016, 15, 99, doi:10.1186/s12937-016-0217-2.
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Rémésy, C.; Demigné, C. Apple Pectin and a Polyphenol-Rich Apple Concentrate Are More Effective Together Than Separately on Cecal Fermentations and Plasma Lipids in Rats. The Journal of Nutrition 2003, 133, 1860-1865, doi:10.1093/jn/133.6.1860.
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. Journal of Agricultural and Food Chemistry 2009, 57, 6485-6501, doi:10.1021/jf902107d.
- Stracke, B.A.; Rüfer, C.E.; Bub, A.; Seifert, S.; Weibel, F.P.; Kunz, C.; Watzl, B. No effect of the farming system (organic/conventional) on the bioavailability of apple (Malus domestica Bork., cultivar Golden Delicious) polyphenols in healthy men: a comparative study. Eur J Nutr 2010, 49, 301-310, doi:10.1007/s00394-009-0088-9.
- Wruss, J.; Lanzerstorfer, P.; Huemer, S.; Himmelsbach, M.; Mangge, H.; Höglinger, O.; Weghuber, D.; Weghuber, J. Differences in pharmacokinetics of apple polyphenols after standardized oral consumption of unprocessed apple juice. Nutrition Journal 2015, 14, 32, doi:10.1186/s12937-015-0018-z.
- Hyun, T.K.; Jang, K.-I. Apple as a source of dietary phytonutrients: an update on the potential health benefits of apple. EXCLI J 2016, 15, 565-569, doi:10.17179/excli2016-483.
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. International journal of molecular sciences 2019, 20, 3177.
- Gallus, S.; Talamini, R.; Giacosa, A.; Montella, M.; Ramazzotti, V.; Franceschi, S.; Negri, E.; La Vecchia, C. Does an apple a day keep the oncologist away? Annals of Oncology 2005, 16, 1841-1844, doi:https://doi.org/10.1093/annonc/mdi361.
- Basli, A.; Belkacem, N.; Amrani, I. Health benefits of phenolic compounds against cancers. Phenolic compounds–Biological activity. London, UK: IntechOpen 2017, 193-210.
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. BioMed Research International 2018, 2018, 4154185, doi:10.1155/2018/4154185.
- Martínez-Rodríguez, O.P.; Thompson-Bonilla, M.D.R.; Jaramillo-Flores, M.E. Association between obesity and breast cancer: Molecular bases and the effect of flavonoids in signaling pathways. Crit Rev Food Sci Nutr 2020, 60, 3770-3792, doi:10.1080/10408398.2019.1708262.
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. Phytochemical profiles, antioxidant, and antiproliferative activities of four red-fleshed apple varieties in China. J Food Sci 2020, 85, 718-726, doi:10.1111/1750-3841.15056.
- Sun; Liu, R.H. Apple Phytochemical Extracts Inhibit Proliferation of Estrogen-Dependent and Estrogen-Independent Human Breast Cancer Cells through Cell Cycle Modulation. Journal of Agricultural and Food Chemistry 2008, 56, 11661-11667, doi:10.1021/jf8021223.
- Schiavano, G.F.; De Santi, M.; Brandi, G.; Fanelli, M.; Bucchini, A.; Giamperi, L.; Giomaro, G. Inhibition of Breast Cancer Cell Proliferation and In Vitro Tumorigenesis by a New Red Apple Cultivar. PLoS One 2015, 10, e0135840-e0135840, doi:10.1371/journal.pone.0135840.
- Reagan-Shaw, S.; Eggert, D.; Mukhtar, H.; Ahmad, N. Antiproliferative effects of apple peel extract against cancer cells. Nutr Cancer 2010, 62, 517-524, doi:10.1080/01635580903441253.
- Boccellino, M.; Quagliuolo, L.; D'Angelo, S. Annurca Apple Biophenols’ Effects in Combination with Cisplatin on A549 Cells. Current Nutrition & Food Science 2021, 16, doi:10.2174/1573401316999200504093028.
- Rana, S.; Kumar, S.; Rana, A.; Padwad, Y.; Bhushan, S. Biological activity of phenolics enriched extracts from industrial apple pomace. Industrial Crops and Products 2021, 160, 113158, doi:https://doi.org/10.1016/j.indcrop.2020.113158.
- Miura, D.; Miura, Y.; Yagasaki, K. Effect of Apple Polyphenol Extract on Hepatoma Proliferation and Invasion in Culture and on Tumor Growth, Metastasis, and Abnormal Lipoprotein Profiles in Hepatoma-Bearing Rats. Bioscience, Biotechnology, and Biochemistry 2007, 71, 2743-2750, doi:10.1271/bbb.70359.
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, doi:10.3390/molecules26051315.
- Tu, S.-H.; Chen, L.-C.; Ho, Y.-S. An apple a day to prevent cancer formation: Reducing cancer risk with flavonoids. Journal of Food and Drug Analysis 2017, 25, 119-124, doi:https://doi.org/10.1016/j.jfda.2016.10.016.
- Davidson, K.T.; Zhu, Z.; Fang, Y. Phytochemicals in the fight against cancer. Pathology & Oncology Research 2016, 22, 655-660.
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent advances in cancer chemoprevention with phytochemicals. Journal of Food and Drug Analysis 2020, 28, 14-37, doi:https://doi.org/10.1016/j.jfda.2019.11.001.
- Bars-Cortina, D.; Martínez-Bardají, A.; Macià, A.; Motilva, M.J.; Piñol-Felis, C. Consumption evaluation of one apple flesh a day in the initial phases prior to adenoma/adenocarcinoma in an azoxymethane rat colon carcinogenesis model. J Nutr Biochem 2020, 83, 108418, doi:10.1016/j.jnutbio.2020.108418.
- Liu, R.H.; Liu, J.; Chen, B. Apples Prevent Mammary Tumors in Rats. Journal of Agricultural and Food Chemistry 2005, 53, 2341-2343, doi:10.1021/jf058010c.
- Veeriah, S.; Kautenburger, T.; Habermann, N.; Sauer, J.; Dietrich, H.; Will, F.; Pool-Zobel, B.L. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Molecular Carcinogenesis 2006, 45, 164-174, doi:https://doi.org/10.1002/mc.20158.
- Leng, E.; Xiao, Y.; Mo, Z.; Li, Y.; Zhang, Y.; Deng, X.; Zhou, M.; Zhou, C.; He, Z.; He, J.; et al. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complementary and Alternative Medicine 2018, 18, 122, doi:10.1186/s12906-018-2189-6.
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. The Journal of Nutritional Biochemistry 2019, 69, 19-30, doi:https://doi.org/10.1016/j.jnutbio.2019.03.009.
- Yang, J.; Liu, R.H. Synergistic Effect of Apple Extracts and Quercetin 3-β-d-Glucoside Combination on Antiproliferative Activity in MCF-7 Human Breast Cancer Cells in Vitro. Journal of Agricultural and Food Chemistry 2009, 57, 8581-8586, doi:10.1021/jf8039796.
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. The Journal of Nutritional Biochemistry 2019, 69, 19-30, doi:https://doi.org/10.1016/j.jnutbio.2019.03.009.
- Yang, J.; Liu, R.H. Synergistic Effect of Apple Extracts and Quercetin 3-β-d-Glucoside Combination on Antiproliferative Activity in MCF-7 Human Breast Cancer Cells in Vitro. Journal of Agricultural and Food Chemistry 2009, 57, 8581-8586, doi:10.1021/jf8039796.




