
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luca Russo | + 1586 word(s) | 1586 | 2021-11-24 08:05:42 | | | |
| 2 | Camila Xu | + 105 word(s) | 1691 | 2021-11-30 02:01:31 | | |
Video Upload Options
MR imaging provides excellent spatial and contrast resolution to stage locally advanced vulvar cancer (LAVC) for tumor and nodal evaluation in order to facilitate the planning of treatment. Although there are no standard indications for how to estimate the clinical stage of International Federation of Gynecology and Obstetrics at diagnosis, MR imaging can depict the tumor and its extension to the vulvar region and adjacent organs, such as the vagina, urethra, and anus. Optimizing the MR imaging protocol and technique is fundamental for correct staging.
1. Introduction
Vulvar cancer is staged pathologically by an evaluation of tumor size and the depth of invasion determined by (1) physical examination; (2) biopsy/surgical pathology; and (3) lymph node status assessed by physical examination, imaging, or surgical removal via lymphadenectomy or sentinel lymph node biopsy [1][2][3][4]. Some authors report that most vulvar cancers involve the labia majora and minora (70%) with other sites involved in the remaining cases [5][3][6]. Vulvar cancer generally extends slowly and tends to be locally infiltrative prior to invading the local lymph nodes in the groin. Regional extension may involve the vagina, urethra, and anus, but rarely the bladder and bone. In more advanced cases, the pelvic nodes can be involved [5][7]. Metastatic diffusion outside the pelvis is unusual in vulvar cancer aside from malignant melanoma and rare sarcomas [5].
Previous studies show that pelvic MRI and PET/CT help over clinical assessment in the pre-treatment management of patients with vulvar cancer [8][9][10]. MRI is the preferred technique for the assessment of locally advanced vulvar cancer (LAVC) and its extension to the adjacent structures. Kataoka et al. reported an accuracy of 85% for staging vulvar cancer with enhanced MRI [11]. Moreover, some studies have evaluated the role of MRI in detecting lymph node metastasis, reporting large varying sensitivities and specificities ranging from 40% to 89% and 81% to 100%, respectively [3].
LAVC includes unresectable by non-visceral sparing primary surgery large T2 and T3 tumors, as outlined by the National Comprehensive Cancer Network [1]. The expression “locally advanced vulvar carcinoma” has been related to different clinical presentations including bulky primary tumors spreading beyond the vulva or presenting with large positive groin nodes; tumors either near or involving the adjacent organs, such as the vagina, urethra, bladder, anus and/or rectum or pelvic bones; and primary tumors that cannot be locally managed with a radical vulvar resection [12][13].
The treatment of vulvar cancer should be personalized, including tailored primary tumor resection and lymph nodes evaluation and/or primary chemoradiation therapy or exclusive chemo-radiation based on an individual patient’s characteristics [1]. In this setting, patients with vulvar cancer should be referred to a dedicated cancer center as diagnosis and management should be multidisciplinary with a dedicated team, including a gynecologist, radiation oncologist, radiologist, nuclear medicine physician, medical oncologist, pathologist, and plastic surgeon [14][15][16][17].
2. Anatomy and MRI Findings
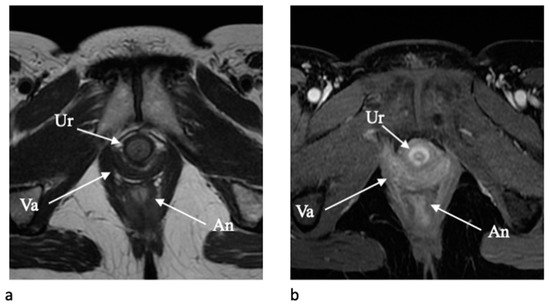
The urethra is clearly depicted on axial T2-weighted and contrast-enhanced T1-weighted images due to a typical target-like appearance with an outer low-signal-intensity layer of external skeletal muscle of the urethral sphincter and a high signal intensity inner layer of smooth muscle and submucosa [18] (Figure 1). The vagina is similarly well depicted on axial T2-WI and axial post-contrast T1-WI, and the shape resemble an “H” form. The vaginal wall includes the mucosal layer (hyperintense on T2-WI with high vascularization) and the submucosal and muscolaris layers (hypointense on T2-WI). The paravaginal fatty tissue presents a high signal intensity on T2-WI [2][18].
The lymphatic drainage of the vulva happens mainly through the superficial inguinal lymph nodes [2][19] located just below the skin and above the fascia lata [20]. From superficial inguinal nodes the metastasis spread to the deep inguinal lymph nodes, located below the fascia lata, along the femoral artery and vein, and subsequently to the external iliac lymph nodes. The cloquet node, the lowest of the external iliac lymph nodes, is at the entrance of the femoral canal and is an important indicator of metastatic spread to the pelvic nodes [5]. The lymphatic drainage of the vulva follows a rich network of lymphatic anastomoses, which continue over the midline. For this reason, the pattern of lymph node metastasis is not restricted to one side and a lesion near the midline can drain to one or both groins [2][19]. Moreover, the clitoris can drain directly to the deep inguinal or external iliac lymph nodes through the hypogastric route [19][21]. The inguinal and femoral lymph nodes are considered locoregional, whereas the involvement of the pelvic lymph nodes is rare and is considered metastatic disease [2][3][19].
In our institution, we use the Daseler classification for inguinal lymph nodes anatomic localization for vulvar cancer. Daseler et al. divided the inguinal region into five zones marked by four quadrants obtained by drawing vertical and horizontal lines over the sapheno-femoral junction, and one zone directly overlying this junction [22]. As a result, we acknowledge one medial superior region, one medial inferior region, one central region, one lateral superior region, and one lateral inferior region (Figure 2).
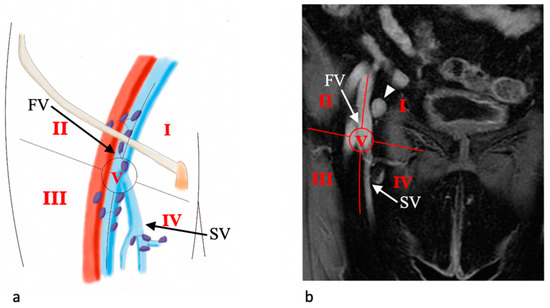
This anatomic classification is important because, looking at the distribution of sentinel lymph nodes (SLNs), it has been shown that lymphatic drainage occurs mainly to the medial regions of the groin as well as the distribution of metastatic SLNs [23]. Moreover, drainage to the lateral inferior region of the groin is only incidental.
3. MRI Protocol
According to ESUR guidelines, the minimal recommended magnet field strength for staging is 1.5 T [24]. Patient preparation and imaging techniques are essential to achieve an optimal examination. Fasting for 4–6 h before the examination, anti-peristaltic agent administration, and bladder voiding are required to limit bowel peristalsis. Vaginal distention with ultrasound gel should be useful for the delineation of small vulvar tumors and/or vaginal infiltration [2][18][24][3]. The patient should be imaged supine, with a phased array pelvic coil or an eight-channel cardiac coil [6]. The standard MRI protocol used in our department for the evaluation of vulvar lesions is summarized in Table 1. It includes axial T1-wieghted images (T1WI) and T2-weighted images (T2WI) with a large field of view (FOV) for an overview of the whole pelvis, allowing detection of lymphadenopathy and pelvic bone metastasis [2][18][3][25]. Sagittal T2WI FSE, oblique axial (perpendicular to the long axis of the urethra), and coronal oblique (parallel to the axis of the urethra) high resolution T2WI provide anatomic detail and tumor delineation [2][18][3][25]. Some authors suggest the use of T2WI with fat suppression (FS) as they provide a better delineation of the tumor, given that the perineal region is rich in fat. Oblique axial diffusion-weighted imaging (DWI) with a high b-value (high b = 1000) (with calculated apparent diffusion coefficient [ADC] map) on the same angle of oblique axial T2WI is also acquired for a better delineation of the primary tumor.
Table 1. MR Imaging protocol for evaluation of patients with vulvar cancer.
| Sequence | Plane | Objective |
|---|---|---|
| T1WI pelvis | Axial | Panoramic view (lymph nodes, uterus, adnexa, bone) |
| T2WI pelvis | Axial | Panoramic view (lymph nodes, uterus, adnexa, bone) |
| T2WI pelvis | Sagittal | Vulva and adjacent structures (urethra, anus, vagina) |
| T2WI (small FOV) | Axial oblique (perpendicular to the long axis of the urethra) | Vulva and adjacent structures (urethra, anus, vagina) |
| T2WI (small FOV) | Coronal oblique (parallel to the long axis of the urethra) | Vulva and adjacent structures (urethra, anus, vagina) |
| DWI (b 0–1000) (small FOV) |
Axial oblique as T2WI (perpendicular to the long axis of the urethra) | Tumor detection and extension |
| T2WI abdomen | Axial | Lymph nodes and hydronephrosis |
| T1WI Multhiphase DCE 3D GRE pelvis | Axial oblique (perpendicular to the long axis of the urethra) |
Tumor detection and extension, lymph nodes |
| T1WI DCE 3D GRE pelvis | Coronal oblique (parallel to the long axis of the urethra) |
Tumor detection and extension, lymph nodes |
Three-dimensional spoiled gradient-pulse fat-saturated T1WI (3D T1WI FS) imaging of the pelvis on the axial or axial oblique plane (perpendicular to the long axis of the urethra) are performed pre- and post-contrast administration, after 18 s of delay and at 15 s intervals for 3 scans to better obtain arterial portal and equilibrium phases. Coronal or oblique coronal plane (parallel to the long axis of the urethra), and eventually sagittal plane, post-contrast enhanced images are subsequently performed. Dynamic contrast-enhanced MRI helps in the evaluation of small tumors and of the involvement of the urethra, anus, and vagina. Multiplanar imaging is essential in evaluating the anatomy and extension of the tumor to the adjacent structures.
The evaluation of the upper abdomen to assess the kidney and lymph nodes should be performed and include axial T2WI (and eventually DWI) from the symphysis to the renal hila.
4. MRI Focused Report
A comprehensive report for staging LAVC requires the subsequent information: tumor dimension (maximum diameter); tumor location (lateral, midline, multifocal); clitoris involvement; tumor adjacent organs/structures extension (urethra and/or vagina with caudo-cranial extension specification: lower 1/3 or upper 2/3; urethral meatus; bladder; fourchette region; and anus/rectum); and lymph-nodes involvement: inguinal and/or pelvic and/or abdominal. Moreover, it is important to report additional findings regarding uterus, adnexa, kidneys, and pelvic bones [24].
References
- National Comprehensive Cancer Network Vulvar Cancer (Squamous Cell Carcinoma) (Version 03.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 27 April 2021).
- Shetty, A.S.; Menias, C.O. MR Imaging of Vulvar and Vaginal Cancer. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 481–502.
- Serrado, M.A.; Horta, M.; Cunha, T.M. State of the Art in Vulvar Cancer Imaging. Radiol. Bras. 2019, 52, 316–324.
- Suneja, G.; Viswanathan, A. Gynecologic Malignancies. Hematol. Oncol. Clin. N. Am. 2020, 34, 71–89.
- Sohaib, S.A.; Moskovic, E.C. Imaging in Vulval Cancer. Best Pract. Res. Clin. Obs. Gynaecol. 2003, 17, 543–556.
- Viswanathan, C.; Kirschner, K.; Truong, M.; Balachandran, A.; Devine, C.; Bhosale, P. Multimodality Imaging of Vulvar Cancer: Staging, Therapeutic Response, and Complications. AJR Am. J. Roentgenol. 2013, 200, 1387–1400.
- Hosseinzadeh, K.; Heller, M.T.; Houshmand, G. Imaging of the Female Perineum in Adults. Radiographics 2012, 32, E129–E168.
- Collarino, A.; Garganese, G.; Valdés Olmos, R.A.; Stefanelli, A.; Perotti, G.; Mirk, P.; Fragomeni, S.M.; Ieria, F.P.; Scambia, G.; Giordano, A.; et al. Evaluation of Dual-Timepoint 18F-FDG PET/CT Imaging for Lymph Node Staging in Vulvar Cancer. J. Nucl. Med. 2017, 58, 1913–1918.
- Collarino, A.; Garganese, G.; Fragomeni, S.M.; Pereira Arias-Bouda, L.M.; Ieria, F.P.; Boellaard, R.; Rufini, V.; de Geus-Oei, L.-F.; Scambia, G.; Valdés Olmos, R.A.; et al. Radiomics in Vulvar Cancer: First Clinical Experience Using 18F-FDG PET/CT Images. J. Nucl. Med. 2018, 60, 199–206.
- Rufini, V.; Garganese, G.; Ieria, F.P.; Pasciuto, T.; Fragomeni, S.M.; Gui, B.; Florit, A.; Inzani, F.; Zannoni, G.F.; Scambia, G.; et al. Diagnostic Performance of Preoperative FDG-PET/CT for Lymph Node Staging in Vulvar Cancer: A Large Single-Centre Study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3303–3314.
- Kataoka, M.Y.; Sala, E.; Baldwin, P.; Reinhold, C.; Farhadi, A.; Hudolin, T.; Hricak, H. The Accuracy of Magnetic Resonance Imaging in Staging of Vulvar Cancer: A Retrospective Multi-Centre Study. Gynecol. Oncol. 2010, 117, 82–87.
- Gadducci, A.; Aletti, G.D. Locally Advanced Squamous Cell Carcinoma of the Vulva: A Challenging Question for Gynecologic Oncologists. Gynecol. Oncol. 2020, 158, 208–217.
- Fanfani, F.; Garganese, G.; Fagotti, A.; Lorusso, D.; Gagliardi, M.L.; Rossi, M.; Salgarello, M.; Scambia, G. Advanced Vulvar Carcinoma: Is It Worth Operating? A Perioperative Management Protocol for Radical and Reconstructive Surgery. Gynecol. Oncol. 2006, 103, 467–472.
- Lancellotta, V.; Macchia, G.; Garganese, G.; Fionda, B.; Fragomeni, S.M.; D’Aviero, A.; Casà, C.; Gui, B.; Gentileschi, S.; Corrado, G.; et al. The Role of Brachytherapy (Interventional Radiotherapy) for Primary and/or Recurrent Vulvar Cancer: A Gemelli Vul.Can Multidisciplinary Team Systematic Review. Clin. Transl. Oncol. 2021, 23, 1611–1619.
- Tagliaferri, L.; Garganese, G.; D’Aviero, A.; Lancellotta, V.; Fragomeni, S.M.; Fionda, B.; Casà, C.; Gui, B.; Perotti, G.; Gentileschi, S.; et al. Multidisciplinary Personalized Approach in the Management of Vulvar Cancer—The Vul.Can Team Experience. Int. J. Gynecol. Cancer 2020, 30, 932–938.
- Garganese, G.; Tagliaferri, L.; Fragomeni, S.M.; Lancellotta, V.; Colloca, G.; Corrado, G.; Gentileschi, S.; Macchia, G.; Tamburrini, E.; Gambacorta, M.A.; et al. Personalizing Vulvar Cancer Workflow in COVID-19 Era: A Proposal from Vul.Can MDT. J. Cancer Res. Clin. Oncol. 2020, 146, 2535–2545.
- Chow, L.; Tsui, B.Q.; Bahrami, S.; Masamed, R.; Memarzadeh, S.; Raman, S.S.; Patel, M.K. Gynecologic Tumor Board: A Radiologist’s Guide to Vulvar and Vaginal Malignancies. Abdom. Radiol. 2021.
- Kim, K.W.; Shinagare, A.B.; Krajewski, K.M.; Howard, S.A.; Jagannathan, J.P.; Zukotynski, K.; Ramaiya, N.H. Update on Imaging of Vulvar Squamous Cell Carcinoma. AJR Am. J. Roentgenol. 2013, 201, W147–W157.
- Paño, B.; Sebastià, C.; Ripoll, E.; Paredes, P.; Salvador, R.; Buñesch, L.; Nicolau, C. Pathways of Lymphatic Spread in Gynecologic Malignancies. Radiographics 2015, 35, 916–945.
- Protzel, C.; Alcaraz, A.; Horenblas, S.; Pizzocaro, G.; Zlotta, A.; Hakenberg, O.W. Lymphadenectomy in the Surgical Management of Penile Cancer. Eur. Urol. 2009, 55, 1075–1088.
- Standring, S.; Borley, N.R.; Gray, H. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th anniversary ed.; Churchill Livingstone/Elsevier: Edinburgh, UK, 2008.
- Daseler, E.H.; Anson, B.J.; Reimann, A.F. Radical Excision of the Inguinal and Iliac Lymph Glands; a Study Based upon 450 Anatomical Dissections and upon Supportive Clinical Observations. Surg. Gynecol. Obs. 1948, 87, 679–694.
- Collarino, A.; Donswijk, M.L.; van Driel, W.J.; Stokkel, M.P.; Valdés Olmos, R.A. The Use of SPECT/CT for Anatomical Mapping of Lymphatic Drainage in Vulvar Cancer: Possible Implications for the Extent of Inguinal Lymph Node Dissection. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2064–2071.
- Nikolić, O.; Sousa, F.A.E.; Cunha, T.M.; Nikolić, M.B.; Otero-García, M.M.; Gui, B.; Nougaret, S.; Leonhardt, H. ESUR Female Pelvic Imaging Working Group Vulvar Cancer Staging: Guidelines of the European Society of Urogenital Radiology (ESUR). Insights Imaging 2021, 12, 131.
- Griffin, N.; Grant, L.A.; Sala, E. Magnetic Resonance Imaging of Vaginal and Vulval Pathology. Eur. Radiol. 2008, 18, 1269–1280.




