
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Hooshmand | + 3436 word(s) | 3436 | 2020-08-12 11:15:55 | | | |
| 2 | Catherine Yang | Meta information modification | 3436 | 2020-08-17 06:08:30 | | |
Video Upload Options
Biomedical waste management is getting significant consideration among treatment technologies, since insufficient management can cause danger to medicinal service specialists, patients, and their environmental conditions. The improvement of waste administration protocols, plans, and policies are surveyed, despite setting up training programs on legitimate waste administration for all healthcare service staff. Most biomedical waste substances do not degrade in the environment, and may also not be thoroughly removed through treatment processes. Therefore, the long-lasting persistence of biomedical waste can effectively have adverse impact on wildlife and human beings, as well. Hence, photocatalysis is gaining increasing attention for eradication of pollutants and for improving the safety and clearness of the environment due to its great potential as a green and eco-friendly process. In this regard, nanostructured photocatalysts, in contrast to their regular counterparts, exhibit significant attributes such as non-toxicity, low cost and higher absorption efficiency in a wider range of the solar spectrum, making them the best candidate to employ for photodegradation. Due to these unique properties of nanophotocatalysts for biomedical waste management, we aim to critically evaluate various aspects of these materials in the present review and highlight their importance in healthcare service settings.
1. Introduction
Biomedical waste management has recently risen as one of the major challenges that developing countries are confronting. The amount of biomedical waste produced has considerably increased as the worldwide populace has expanded, and accessible assets are not sufficient to deal with it [1]. Disposal and post treatment of the waste produced in the healthcare system may indirectly cause health hazardous through the release of pathogens and toxic pollutants into the environment. Discarding the untreated healthcare wastes in landfills, if the landfill is not properly constructed, can lead to the contamination of surface, drinking and ground water resources. Additionally, treatment of healthcare wastes with chemical disinfectants can result in the release of chemical substances into the environment if those substances are not handled, stored and disposed of in an environmentally sound manner. Incineration of waste has also been widely practiced; however, insufficient incineration or the incineration of inappropriate materials pollute the air and generate ash residue. Lack of knowledge about the health-related hazards of healthcare waste, inadequate training in proper waste management, absence of waste management and sufficient disposal systems, insufficient financial and human resources and the low priority given to the topic are the most common problems associated with healthcare waste. Many countries either do not have appropriate regulations, or do not enforce them.
Successful and efficient management of biomedical waste requires the use of different treatment practices and techniques, such as incineration, autoclave, hydroclave, and microwave treatments [2]. It is essential that every single applied innovation assures both the environment and public health protection [3]. Waste management and waste engineering advances have turned out to be extremely vital because of the significant increase in rate and diversity, both in the quality and quantity of the waste that is being produced every day, so using the most financially plausible strategies has become even more crucial than before [4]. Since waste products cannot be completely eradicated, the choice of waste treatment has become specifically important as its management.
The other commonly used methods to treat biomedical waste are mechanical treatments such as granulation, pulverization, shredding, grinding, mixing, agitation, and crushing. This type of treatment has the advantage of reducing the bulk volume of the waste materials by 60 percent or more. Although mechanical treatment does not remove the pathogens or disinfect equipment, it reduces the waste volume to facilitate further treatment or disposal. Equipment involved in mechanical treatment includes but is not limited to crushers, millers, shattering machines and splinterers. These treatment methods can alter the appearance of the waste, which can be useful in reducing the psychological impact of the waste on human observers. In addition to reducing the volume of bulk disposal, mechanical treatment can increase the surface area of the solid pieces before subsequent chemical or heat treatment.
Chemical disinfection, such as through the use of chlorine compounds, has been widely used to eliminate the microorganisms in medical waste, as well as oxidizing hazardous chemical constituents. For instance, chlorine bleach has been used to disinfect swimming pools and reduce the risk of disease transmission. Another example of chemical disinfectant compound is ethylene oxide treatment, which is used to disinfect materials and sometimes to treatment of medical waste. Ethylene oxide (EtO) treatment is used to sterilize the equipment that will be frequently used. This disinfectant chemical is not cost effective for use on equipment or treatment of waste that will be disposed of in a landfill. EtO gas can be used to kill microorganisms and disinfect products during packaging processes.
Microwave radiation has recently been employed to treat wastewater sludge and to generate heat for treating medical waste. This method of waste treatment can be employed either on-site or mobile by using treatment vehicles. To enhance the efficacy of the microwave treatment and reduce the volume of the final product, the waste will go through a shredding process first. In the case of using dry waste, the waste is wetted with water and then introduced into the microwave chamber, as this method of disinfection is effective only when the waste is damp. Therefore, the microwave treatment units are usually supplied with a humidifier. Although the whole disinfection time is determined by the manufacturer and experience of the operators, approximately 20 min per each batch is required. Microwave should not be confused with irradiation such as gamma rays (from radioactive elements) or electrons, as these two methods are completely different.
Gamma irradiation is a means of sterilization by exposing waste to gamma rays, as it breaks down bacterial DNA. To generate gamma rays, radioactive isotopes of cobalt are employed, this is the same radiation source used for the radiation treatment of cancer. However, in cancer treatment, the radiation is intended to kill the malignant cells, whereas to sterilize equipment or treat waste, pathogens are targeted. In contrast, the ultraviolet (UV) radiation used to treat wastewater is not capable of killing microbes so much as it is able to break down chemicals. The efficiency of irradiation as a means of sterilization is highly dependent on the total energy delivered, but even then, this method of treatment suffers from the shadowing effect, which means that waste surfaces facing the radiation source are more sterile than the waste on the shaded side. Therefore, waste with odd shapes, and the sides of contaminated surfaces facing away from the cobalt source, may not be adequately exposed to the radiation. Heat treatment, by contrast, brings every piece of waste to an adequate temperature for sterilization, if done properly.
Although vitrification—the means production of glass—has rarely been used, it could be an effective treatment for medical waste. The high temperature kills pathogens and some combustible material via burning or pyrolysis, which results in an off-gas. The remaining by-product is encapsulated in glass, which has a very low diffusivity. However, this method of disinfection might become dangerous if significant quantities of the encapsulated hazardous material leach out of the glass. Ultimately, the vitrified waste can be disposed of in a landfill with confidence. Despite the development of plasma treatment as an alternative to incineration for medical waste treatment, it has not been widely implemented [5].
To decrease the expense of waste treatment through cost-effective strategies, various methods based on the exploitation of sunlight have been proposed for both solid and liquid waste management [6]. Among these, photocatalysis is a remarkable technique with a variety of applications, including the debasement of different contaminations in wastewater [7], antibacterial functions [8], cleansing of air [9], and generation of hydrogen [10]. The photocatalytic procedure is attracting more focus in the field of ecological and environmental safety, as there is a need to achieve the utmost degradation of contaminants attainable under states of mild pressure and temperature. The significant highlight of these procedures is the incorporation of cost-effective near-UV (from 400 nm down to 300 nm) light, with sunlight as an alternative source of irradiation. The term photocatalysis refers to a chemical reaction using light in the presence of a catalyst that assimilates light quanta and is associated with the chemical transformations of the reactants [11].
The optimal treatment system depends on many factors, such local conditions, availability of resources including technical expertise, waste characteristics and volume, relevant national regulations and safety requirements, technical requirements for installation, operation and maintenance of the treatment system, environmental factors and cost considerations. Nonetheless, waste management systems can be changed and improved only within the financial and technical capacity of a given health-care system, which may then require making small decisions towards an incremental improvement, as well as planning for the attainment of long-term improvements, once certain conditions have been met.
Nanophotocatalysts have been widely used to treat waste in the field of environmental and ecological safety, as they have numerous benefits, such as those of low cost, superb stability, high photocatalytic activity, innocuousness to humans, etc. [12]. Different methods, including ion exchange microorganisms and adsorption, have been used to treat sewage. However, these methods are restricted due to their complex technology, high cost, risk of second contamination, and poor degradation effectiveness [13]. Compared to other methods for biomedical waste management, nanophotocatalysts are considered one of the most particular strategies with respect to energy consumption, environmental and ecological issues. The advantages of the photocatalysis strategy are as follows [14]: (a) photocatalysis offers a decent alternative to the traditional energy-concentrated treatment techniques (e.g., ultrafiltration and reverse osmosis) with the capability of using pollution-free and renewable solar energy; (b) it prompts the creation of innocuous products, in contrast to traditional treatment methods in which pollutants only transfer from one phase to another; (c) the procedure can be used for the decimation of an assortment of risky and hazardous compounds in various wastewater streams; (d) it requires less chemical input and can be operated under mild reaction conditions with modest reaction time; (e) minimum generation of secondary waste; (f) it can also be applied to a solid phase (soil), gaseous phase (hydrogen generation), and aqueous treatments. The dependency of the photocatalytic activity on the following criteria has hampered their application [15]: (a) charge separation; (b) interfacial charge transfer needs to be improved; (c) charge carrier recombination can be inhibited.
Although approaches to removing pollutants based on nanostructured catalytic membranes, nanosorbents and nanophotocatalyst are eco-friendly and efficient, in order to purify the waste, they require more energy and sufficient investment. There are many challenges involved in biomedical waste treatment; some precautions are required to keep hazardous waste away from ecological and health issues. New modern equipment for waste treatment is required to be flexible, low cost and efficient for commercialization purposes. Recently, with advancements in nanomaterials such as nanophotocatalysts, nanomotors, nanomembranes, nanosorbents and imprinted polymers, the decontamination of biomedical waste has been effectively revolutionized.
However, there has not been a systematic characterization of the risk and hazards related to nanomaterials, and there is a lack of safety regulations for using such catalysts. Overall, nearly all nanocatalysts have toxic effects both in vitro and in vivo at certain concentrations. For instance, ROS generation and cell signaling perturbations are widely accepted causes of nanotoxicity. Furthermore, the toxicity of nanoparticles is also determined by factors such as particle size and surface functionalization [16]]. Although to some degree, the toxicity and adverse effects of commonly used nanocatalysts have been realized, a comprehensive investigation is still required [17].
This review aims to introduce different types of nanophotocatalysts and provide the main principles, mechanisms, and operating parameters of the photocatalysis process and emphasize its importance in biomedical waste management in detail (see Figure 1).
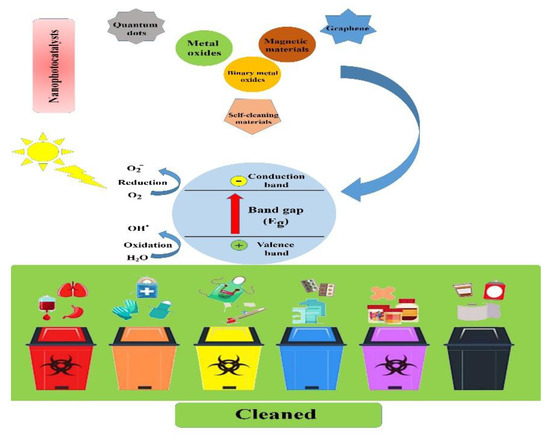
Figure 1. Schematic illustration showing the main types of nanophotocatalysts and the process by which biomedical and biological waste materials are cleaned.
2. Types and Characteristics of Nanophotocatalysts
Nano-sized photocatalyst particles demonstrate a significantly intensified reactivity compared to larger particles or bulk materials due to their large surface area [18][19]. Novel nanophotocatalysts have been developed with the ability to exploit solar energy to synthesize organic compounds under controlled conditions [20]. Nanophotocatalysts are mainly classified as surface plasmon resonance-mediated, metal-organic charge-transfer-based, and semiconductor-based nanophotocatalysts, capable of driving various organic reactions photocatalytically. Accordingly, they can be categorized as graphene semiconductors, composites of two semiconductors, core–shell composites, non-metal-doped semiconductor materials, and metal-modified semiconductors.
Metal-free catalysis, such as with graphitic carbon nitride nanocomposites, has also been used in photodegradation of aqueous phase organic pollutants [21]; likewise, polymeric graphitic carbon nitride-based Z-scheme photocatalytic systems, magnetic graphitic carbon nitride photocatalyst, and carbon quantum dot-supported graphitic carbon nitride have been respectively employed for sustainable photocatalytic water purification [22], degradation of oxytetracycline antibiotic [23], and photodegradation of 2,4-dinitrophenol [24].
Among different kinds of photocatalysts, metal oxide semiconductors, including TiO2, ZnO, α-Fe2O3, and WO3 are the most suitable ones, since they are photocorrosion resistant and have a wide band gap energy. TiO2 is currently used as the most efficient photocatalyst, and is widely applied in wastewater treatment, since it can promote the oxidation of organic compounds while being thermally stable, non-toxic, cost-effective, and chemically and biologically inert. Structural and surface properties, including surface area, porosity, crystal composition, particle size distribution, and band gap energy, are able to affect the photocatalytic activity of the catalyst [25]. The following characteristics typically represent nanophotocatalysts with specific favorable benefits over bulk materials [26]. First, their large surface area to volume ratio results in a high particle fraction, and subsequently a high division of active sites on the catalyst surface. Second, their valence band–conduction band energy gap strongly depends on the size of the nanoparticles [27].
Moreover, changing the size of the nanocatalyst makes it possible to adjust the absorbance wavelength. Additionally, the optical and electronic characteristics of the nanocatalyst can be modified by tuning their sizes [28]. Due to all these favorable properties, nanophotocatalysts have been employed in a wide range of reactions, for instance, in organic synthesis, splitting water to hydrogen fuel generation, inactivation of cancer cells, and dye degradation [29][30]. Primary studies on the development of nanophotocatalysts have mainly focused on their degradation capabilities of pollutants and dyes. Given these properties, there is an increasing interest in the use of nanophotocatalysts as catalysts for different organic reactions, providing options in green chemistry as alternatives to the regular techniques used in research laboratories and industry, which apply thermal energy to achieve the same goals [31].
The efficacy rate in photocatalysis methods is primarily dependent on the size, shape, crystal structure and surface area of the photocatalyst, as well as the morphology, which can also act as a significant factor affecting the final degradation throughput. The amount of catalyst is also directly proportional to the overall rate of photocatalytic reaction. Disinfection efficiency of the photocatalyst can be improved by an increase of its doses [32]. The pH of the solution is another effective factor, as it determines the photocatalyst surface charge properties. Furthermore, various pH values may impact the efficiency of the disinfection process, values ranging from 6.0 to 8.0 have shown the highest impact. Different studies have evidenced that the microorganisms have pH sensitivity at around 6.5 and 8.0, the range in which photocatalytic activity has been demonstrated to be excellent. This is due to the fact that as pH moves away from neutral, the effectiveness of the overall process declines when pH reaches 7 [33]. The optimum range of reaction temperature which primarily depends on the activation energies of the materials in the photocatalytic reaction [34], the light intensity, which mainly influences the degradation rate of photocatalytic reaction [35], and the nature and concentration of pollutants [32] can also affect the performance of nanophotocatalysts. Furthermore, inorganic ions such as iron, magnesium, copper, zinc, phosphate, bicarbonate, chloride, sulfate and nitrate can change the rate of photocatalytic degradation of the organic pollutants since they can be adsorbed onto the nanophotocatalyst surface [36].
3. Biomedical Applications of Nanophotocatalysts
As photocatalysts have a superior capability in the deactivation of various destructive microorganisms, they could reasonably be used as alternatives to conventional techniques (e.g., chlorination), which can generate harmful and undesirable by-products [37]. Photocatalysis is a flexible and successful procedure that can be adopted in numerous cleansing applications in both air and water frameworks [38]. Furthermore, photocatalytic surfaces have been used on account of their self-sanitizing attributes. Photocatalytic applications have been recently developed specifically in the contexts of environmental health and indoor air, plant protection, effluents, wastewater and drinking water disinfection, dye removal, the pharmaceutical and food industries, laboratories and hospitals, and biological and medical applications. Due to the low energy consumption and feasible accessibility to solar energy and decreased treatment time, the overall cost for photodegradation of hazardous compounds and pollutants is lower, and hence beneficial [39]. Applications of nanophotocatalysts are summarized in Figure 3. With respect to the title of the study, we focus on biomedical applications of nanophotocatalysts in the following sections.
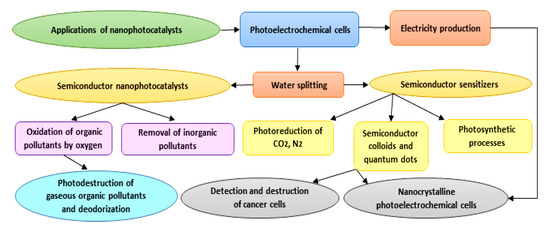
Figure 3. Diagram showing different applications proposed for nanophotocatalysts.
4. Future Prospects, and Concluding Remarks
Biomedical waste eradication strategies are still very limited due to low photocatalytic efficacy. Therefore, extensive assessments are highly demanded from the practical point of view [40]. The applications of TiO2 photocatalysts, for example, is restricted because of poor quantum efficacy as a result of limited absorption within only UV range (4% of sunlight). The recent advancement in the field of photocatalysis technology is investigating novel agents with higher photocatalytic performance to expand their light respond range. To address these issues, coupled semiconductor, noble metal deposition and ion modification, are the proposed methods to improve energy band and photocatalytic efficacy of explicit applications. In addition, due to concurrent photocatalytic and redox reactions, sensible photocatalytic systems can be designed for the simultaneously photocatalytic treatment of two or more contaminants [41]. However, several issues (e.g., evaluating the immobilization of photocatalysts and suspension systems) should be contemplated for further advancement. Solar-Based photocatalytic methods have shown better performance than conventional methods in the removal of tenacious organic contaminations [42]. To improve the photodegradation of wastewater, suitable surface modification technique of photocatalysts is an essential need. In addition, development of nanostructures, photoactivity of recycled photocatalysts, mechanisms of degradation, recovery of photocatalyst during treatment, and interactions between the photocatalysts and the pollutants are yet to be further improvements. To predict the kinetics, quantum yield and optimized conditions of the process, further investigations are required to verify the mathematical models for photocatalytic systems. The future improvement of nanophotocatalysts would be made by making them multifunctional and controllable enough to be subsequently transformed into nano-gadgets. To facilitate the accessibility of these innovations, extensive endeavors are expected to defeat the challenges in the future.
The implementation of the photocatalytic method for disinfection and cleansing is an adaptable and effective procedure for incapacitating a broad range of adverse microorganisms in different media. This approach is a non-toxic, safe, and cost-effective sterilization technique whose versatility enables it to be used in various purposes. Nanotechnology has shown an incredible potential to improve the effectiveness of biomedical waste treatment [43]. Therefore, the use of nanophotocatalysts has become of great interest in biomedical waste management as they can provide excellent and practical applicability. Improving the biomedical waste management; however, should start with the reduction of wastes production according to the norms, rules, and standards in each country, with respect to regulation of biomedical waste disposal in various categories. Besides, practicing the optimized models for monitoring the waste produced by hospitals, health-care centers as well as research into eco-friendly sustainable technologies, recycling and PVC-free devices will go in a long way for a safe environment. Globally, more focused research in the field of biomedical waste management required to comprehend its impact on the field of public health better.
There is an ongoing research in field of nanomaterials to design and develop nanophotocatalytic reactors. Despot of the advancements in field of nanophotocatalytic materials, further investigations are required to be done to characteristics the nanophotocatalytic materials. The major remaining challenges are strengthening the process, mass transfer limitations and high photons consumption. Therefore, the concept of using nanocomposites is ideal to resolve the issues related to electron pair recombination which can be prolonged by combining the nanocomposites with nanophotocatalytic reactor structures. The recent reactors known as microfluidic reactors open a new opportunity for intense characteristics study in reaction and synthesis phase. Microfluidic reactors are based on micro level reactants. The remarkable features of these reactors are; improved diffusion effect and great mass transfer coefficient factor, large surface-to-volume ratio, highly stable hydrodynamics, less Reynold’s flow, and informal handling which make them better candidate compared to the conventional reactors. However, implementing the photocatalysis in a larger scale and actual wastewater is still challenging. Synthesis of structures such as nanorod, nanosphere, nanoflowers, nanoflakes and nanocones with improved functional and structural properties could open a new area of study in this subject. Crucially nanophotocatalysts with excellent efficiency, inexpensive, eco-friendly and high stability are needed to be synthesized [44].
References
- Babatunde, B.; Vincent-Akpu, I.; Woke, G.; Atarhinyo, E.; Aharanwa, U.; Green, A.; Isaac-Joe, O. Comparative analysis of municipal solid waste (MSW) composition in three local government areas in Rivers State, Nigeria. Afr. J. Environ. Sci. Technol. 2013, 7, 874–881.
- Pasupathi, P.; Sindhu, S.; Ponnusha, B.S. Biomedical waste management for health care industry: A review. Int. J. Biol. Med. Res. 2011, 2, 472–486.
- Bo Ugwuishiwu; Ip Owoh; Ij Udom; SOLAR ENERGY APPLICATION IN WASTE TREATMENT- A REVIEW. Nigerian Journal of Technology 2016, 35, 432, 10.4314/njt.v35i2.27.
- Changkook Ryu; Potential of Municipal Solid Waste for Renewable Energy Production and Reduction of Greenhouse Gas Emissions in South Korea. Journal of the Air & Waste Management Association 2010, 60, 176-183, 10.3155/1047-3289.60.2.176.
- Pei Tang; Wei Chen; Dongxing Xuan; Hiuwun Cheng; Chi-Sun Poon; Daniel C. W. Tsang; Immobilization of hazardous municipal solid waste incineration fly ash by novel alternative binders derived from cementitious waste. Journal of Hazardous Materials 2020, 393, 122386, 10.1016/j.jhazmat.2020.122386.
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012; Volume 15.
- Fraiese, A.; Naddeo, V.; Uyguner-Demirel, C.; Prado, M.; Cesaro, A.; Zarra, T.; Liu, H.; Belgiorno, V.; Ballesteros, F., Jr.; Removal of Emerging Contaminants in Wastewater by Sonolysis, Photocatalysis and Ozonation. Global NEST: the international Journal 2018, 21, 98-105, 10.30955/gnj.002625.
- Su-Eon Jin; Jun Eon Jin; Woochul Hwang; Seok Won Hong; Photocatalytic antibacterial application of zinc oxide nanoparticles and self-assembled networks under dual UV irradiation for enhanced disinfection.. International Journal of Nanomedicine 2019, 14, 1737-1751, 10.2147/IJN.S192277.
- F. Gauvin; V. Caprai; Qingliang Yu; H.J.H. Brouwers; Effect of the morphology and pore structure of porous building materials on photocatalytic oxidation of air pollutants. Applied Catalysis B: Environmental 2018, 227, 123-131, 10.1016/j.apcatb.2018.01.029.
- Yang Su; Ling Zhang; Wenzhong Wang; Dengkui Shao; Internal Electric Field Assisted Photocatalytic Generation of Hydrogen Peroxide over BiOCl with HCOOH. ACS Sustainable Chemistry & Engineering 2018, 6, 8704-8710, 10.1021/acssuschemeng.8b01023.
- Jens, H. Industrial Catalysis: A Practical Approach; Wiley-VCH: Weinheim, Germany, 2006.
- Tahir, M.B.; Kiran, H.; Iqbal, T. The detoxification of heavy metals from aqueous environment using nano-photocatalysis approach: A review. Environ. Sci. Pollut. Res. 2019, 26, 10515–10528.
- Jiang, L.; Wang, Y.; Feng, C. Application of photocatalytic technology in environmental safety. Procedia Eng. 2012, 45, 993–997.
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189.
- Rehman, S.; Ullah, R.; Butt, A.; Gohar, N. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569.
- Meysam Keshavarz; Dominic James Wales; Florent Seichepine; Mohamed E. M. K. Abdelaziz; Panagiotis Kassanos; Quan Li; Burak Temelkuran; Hongxing Shen; Guang-Zhong Yang; Induced neural stem cell differentiation on a drawn fiber scaffold—toward peripheral nerve regeneration. Biomedical Materials 2020, 15, 055011, 10.1088/1748-605x/ab8d12.
- Cuijuan Jiang; Jianbo Jia; Shumei Zhai; Mechanistic Understanding of Toxicity from Nanocatalysts. International Journal of Molecular Sciences 2014, 15, 13967-13992, 10.3390/ijms150813967.
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Label-Free SERS Quantum Semiconductor Probe for Molecular-Level and in Vitro Cellular Detection: A Noble-Metal-Free Methodology. ACS Appl. Mater. Interfaces 2018, 10, 34886–34904.
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Multiplex Photoluminescent Silicon Nanoprobe for Diagnostic Bioimaging and Intracellular Analysis. Adv. Sci. 2018, 5, 1700548.
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. In Organic Pollutants-Monitoring, Risk and Treatment; IntechOpen: Rijeka, Coratia, 2013; Volume 8, pp. 196–197.
- Anita Sudhaik; Pankaj Raizada; Pooja Shandilya; Dae-Yong Jeong; Ji-Ho Lim; Pardeep Singh; Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. Journal of Industrial and Engineering Chemistry 2018, 67, 28-51, 10.1016/j.jiec.2018.07.007.
- Saravanan, R.; Gracia, F.; Stephen, A. Basic Principles, Mechanism, and Challenges of Photocatalysis. In Nanocomposites for Visible Light-Induced Photocatalysis; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–40.
- Anita Sudhaik; Pankaj Raizada; Pooja Shandilya; Pardeep Singh; Magnetically recoverable graphitic carbon nitride and NiFe2O4 based magnetic photocatalyst for degradation of oxytetracycline antibiotic in simulated wastewater under solar light. Journal of Environmental Chemical Engineering 2018, 6, 3874-3883, 10.1016/j.jece.2018.05.039.
- Vasudha Hasija; Anita Sudhaik; Pankaj Raizada; Ahmad Hosseini-Bandegharaei; Pardeep Singh; Kirti Sharma; Carbon quantum dots supported AgI /ZnO/phosphorus doped graphitic carbon nitride as Z-scheme photocatalyst for efficient photodegradation of 2, 4-dinitrophenol. Journal of Environmental Chemical Engineering 2019, 7, 103272, 10.1016/j.jece.2019.103272.
- Saber Ahmed; Mohammad G. Rasul; Richard Brown; M.A. Hashib; Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. Journal of Environmental Management 2011, 92, 311-330, 10.1016/j.jenvman.2010.08.028.
- Radhika, N.; Selvin, R.; Kakkar, R.; Umar, A. Recent advances in nano-photocatalysts for organic synthesis. Arab. J. Chem. 2019, 12, 4550–4578.
- Poole, C.P., Jr.; Owens, F.J. Introduction to Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2003.
- Kumar, P.; Singh, P.K.; Bhattacharya, B. Study of nano-CdS prepared in methanolic solution and polymer electrolyte matrix. Ionics 2011, 17, 721–725.
- Townsend, T.K.; Browning, N.D.; Osterloh, F.E. Nanoscale strontium titanate photocatalysts for overall water splitting. ACS Nano 2012, 6, 7420–7426.
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Cell Selective Apoptosis Induced by Polymorphic Alteration of Self-Assembled Silica Nanowebs. ACS Appl. Mater. Interfaces 2017, 9, 6292–6305.
- Kaur, P.; Bansal, P.; Sud, D. Heterostructured nanophotocatalysts for degradation of organophosphate pesticides from aqueous streams. J. Korean Chem. Soc. 2013, 57, 382–388.
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027.
- Castillo-Ledezma, J.; Salas, J.S.; López-Malo, A.; Bandala, E. Effect of pH, solar irradiation, and semiconductor concentration on the photocatalytic disinfection of Escherichia coli in water using nitrogen-doped TiO2. Eur. Food Res. Technol. 2011, 233, 825.
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205.
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59.
- Wang, K.; Zhang, J.; Lou, L.; Yang, S.; Chen, Y. UV or visible light induced photodegradation of AO7 on TiO2 particles: The influence of inorganic anions. J. Photochem. Photobiol. A Chem. 2004, 165, 201–207.
- Kumar, A.; Thakur, P.R.; Sharma, G.; Naushad, M.; Rana, A.; Mola, G.T.; Stadler, F.J. Carbon nitride, metal nitrides, phosphides, chalcogenides, perovskites and carbides nanophotocatalysts for environmental applications. Environ. Chem. Lett. 2019, 17, 655–682.
- Gamage, J.; Zhang, Z. Applications of photocatalytic disinfection. Int. J. Photoenergy 2010, 2010, 764870.
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.Á.; Mangalaraja, R.V.; O’Shea, K.; Dionysiou, D.D. Industrial synthesis and characterization of nanophotocatalysts materials: Titania. Nanotechnol. Rev. 2016, 5, 467–479.
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Springer: Cham, Switzerland, 2013; ISBN 978-1-4471-5061-9.
- Kitano, M.; Matsuoka, M.; Ueshima, M.; Anpo, M. Recent developments in titanium oxide-based photocatalysts. Appl. Catal. A Gen. 2007, 325, 1–14.
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551.
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500.
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495.





