Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Awanish Mishra | + 1695 word(s) | 1695 | 2021-11-12 07:33:11 | | | |
| 2 | Dean Liu | Meta information modification | 1695 | 2021-11-29 09:37:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mishra, A. Diagnosis of Epilepsy. Encyclopedia. Available online: https://encyclopedia.pub/entry/16487 (accessed on 04 March 2026).
Mishra A. Diagnosis of Epilepsy. Encyclopedia. Available at: https://encyclopedia.pub/entry/16487. Accessed March 04, 2026.
Mishra, Awanish. "Diagnosis of Epilepsy" Encyclopedia, https://encyclopedia.pub/entry/16487 (accessed March 04, 2026).
Mishra, A. (2021, November 29). Diagnosis of Epilepsy. In Encyclopedia. https://encyclopedia.pub/entry/16487
Mishra, Awanish. "Diagnosis of Epilepsy." Encyclopedia. Web. 29 November, 2021.
Copy Citation
Epilepsy is one of the most common neurological disorders, characterized by recurrent seizures, resulting from abnormally synchronized episodic neuronal discharges.
epilepsy
diagnosis
1. Introduction
Epilepsy is a common neurological disorder, characterized by the tendency of recurrent seizures, which may take a variety of forms and result in abnormally synchronized neuronal discharges. Seizure types depend on the specific brain circuit being affected. Epilepsy affects 0.5–1% of the population, corresponding approximately to 70 million people worldwide. It may be genetic in origin (idiopathic) or may develop after brain damage, such as trauma, stroke, infection, tumor growth, or other structural or metabolic causes [1][2][3][4][5].
Epilepsy treatment is highly demanded since the continuous abnormal neuronal discharges and subsequent recurrent seizures may cause damage to various brain regions, leading to the development of several neurological or other disorders, such as neurodegeneration, motor impairment, abnormal hormone release (such as ACTH, prolactin, FSH, TSH, etc.), psychosis, etc. [6]. Hence, prompt, and proper diagnosis of epilepsy is essential for effective treatment. Traditional diagnostic methods include electroencephalography (EEG), structural and functional neuroimaging (CT scan, MRI, PET, SPECT, etc.), and blood tests for the detection of the abnormal electrical activity of the brain or the potential identification of specific serum biomarkers. Although these methods are very helpful in detecting abnormal electrical discharges in the brain or identifying potential causes of epilepsy, they might lead to false positive or false negative results [7]. Epilepsy can be difficult to be diagnosed, even for experienced clinicians. Upon clinical suspicion, the gold standard diagnostic method of epilepsy is EEG, which can detect abnormal electrical discharges in the brain. However, these abnormalities may or may not be associated with epilepsy. Some other conditions, such as infections, CNS medications (psychotic drugs such as antidepressants, antipsychotic, antiparkinsonian, etc.) as well as trauma and excessive stress can affect the results of EEG. Additionally, non-epileptic seizures (NES) due to psychological and physiological paroxysmal events, such as epileptic manifestations, can considerably complicate diagnosis [8].
The detection of serum biomarkers is not a reliable strategy since they may be associated with other non-epileptic medical conditions, raising concerns about their specificity. For instance, the levels of antidiuretic hormone, which is a biomarker of hypothalamic activity, may be reduced or elevated in response to drugs or alcohol and may increase in response to emotional and physical exercise or pain [9]. Hence, this hypothalamic hormone cannot be used as a specific serum biomarker. In addition, prolactin which is secreted from the hypothalamus and is sometimes used as a biomarker of epilepsy can be often related to other conditions, such as milk production, breast development, and pituitary tumors [9].
2. Diagnostic Techniques
Epilepsy in children remains poorly diagnosed and contributes to school dropout, mental retardation, and poor quality of life. The current diagnostic pattern mostly relies on the conventional EEG recording. Antiepileptics provide satisfactory control only in up to 60% of patients with epilepsy, with the rest remaining refractory. In these refractory epileptic cases, surgical removal of lesions remains a putative approach for satisfactory seizure control. For ablation surgery, the success depends on the extent of effective demarcation of the epileptic lesion area. In these cases, the use of advanced diagnostic techniques or a combination of other diagnostic tools, such as CT, MRI, PET/SPECT, MEG, MRS, etc., is required. Different diagnostic tools for epilepsy have been illustrated in Figure 1 and discussed herein.
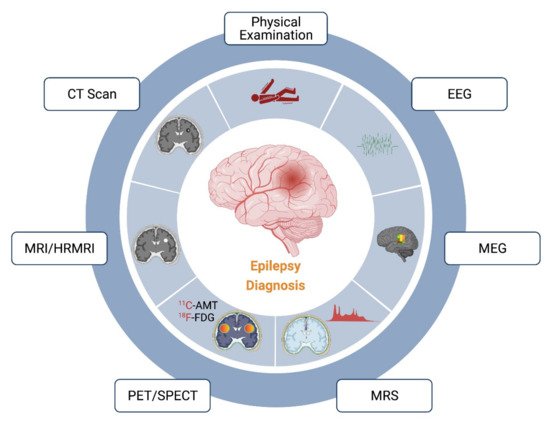
Figure 1. Current methods and techniques available for the diagnosis of epilepsy. The main diagnosis of epilepsy relies majorly in the physical examination followed by EEG analysis. However, the identification of the epileptic locus might be ambiguous with these methods. Therefore, the use of more elaborative techniques, such as CT, MRI/HRMRI, PET/SPECT, MRS, and MEG, is recommended for demarcation of the epileptic foci.
3. Biomarkers Associated with Diagnosis of Epileptogenesis
The identification of reliable biomarkers is the most practical solution for developing an economically feasible diagnostic technique [10][11]. It will provide a suitable screening tool that can identify potential subjects most likely to develop epilepsy due to genetic and structural deformities and help in its reduction at an early stage [12]. The FDA-NIH Joint Leadership Council, in 2015, Developed the Biomarkers, Endpoints, and other Tools (BEST, 2016) for better understanding and use of biomarker terminology. According to BEST, “A biomarker is a characteristic that is measured as an indicator of normal biologic processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. Biomarkers may have molecular, histologic, radiographic, and physiologic characteristics.” The BEST biomarkers are divided into six categories: (a) risk or susceptibility biomarkers, (b) diagnostic biomarkers, (c) monitoring biomarkers, (d) prognostic biomarkers, (e) predictive biomarkers, and (f) safety biomarkers.
Biomarkers in epilepsy can be broadly divided into two types, i.e., “prognostic” (indicates epilepsy after a brain insult) and “diagnostic” (indicates ongoing epileptogenesis at that time). These biomarkers can serve various objectives, including (a) the use of risk biomarkers for the identification of a given epilepsy syndrome, e.g., genetic biomarkers, (b) prognostic biomarkers to predict the likelihood of accruing epilepsy, e.g., at a 2-year timepoint after traumatic brain injury (TBI), and (c) diagnostic biomarkers to identify ongoing epileptogenesis, even without the precise timing that the earlier brain insult occurred [10].
3.1. miRNAs as Biomarkers of Epileptogenesis
miRNAs (micro RNAs) are a special kind of RNA, controlling the post-translational gene expressions of various genes. About 60% of all gene expressions are directly controlled by miRNAs [13]. A single miRNA can affect the expression of several genes in a single pathway or a single gene in multiple pathways [14]. As an example, genetic deletion of miR-128 in mice resulted in the upregulation of more than a thousand mRNA transcripts, amongst which 154 were its predicted targets [15]. The effect of miRNAs in humans with epilepsy was first studied in 2010, reporting the upregulation of miR-146 expression in patients with TLE and hippocampal sclerosis [16].
Early functional studies have revealed that miRNAs are linked to seizure development, neuroinflammation, and changes in neuronal microstructure. For example, miR-134, which regulates LIM domain kinase 1, plays a vital role in altering the number and volume of dendritic spines on excitatory neurons [17][18]. On the other hand, miR-146a, miR-221, and mir-222 control immune response through the targeting of IL-1β and cell adhesion molecules [16][19][20]. miRNAs can also control cell differentiation, proliferation, and migration, which play a critical role in the epileptogenic pathway [21].
There is emerging evidence that miRNAs can serve as potential biomarkers of brain injuries, including epilepsy. A pool of brain-expressed micro RNAs may leak into the extracellular fluid from controlled exoplasm release or damage or even disruption of the BBB, allowing their passage into the blood. These miRNAs form a stable complex with blood proteins or get encapsulated in extracellular vesicles, remaining in the circulation for some time after their release [22]. Thus, a molecular biomarker of epilepsy is of great importance since it may enable diagnosis, assessing the risk of developing epilepsy, monitoring, and treatment (Figure 2).
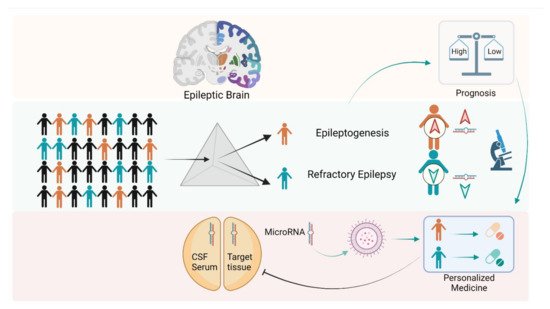
Figure 2. Role of microRNAs in diagnosis and personalized medicine in epilepsy. microRNAs (miRs) have been recognized as important tools for assessing the diagnosis of epileptic foci in patients. Changes in the expression levels of various circulatory/tissue specific miRs may differentiate epileptic phenomena such as epileptogenesis or drug responsive/drug-resistant epilepsy. Further, expression of a specific RNA may be altered using nucleic acid-based approaches (miR mimics, anti-miR, short hairpin RNA, small interfering RNA, aptamers, antisense oligonucleotide) for pursuing personalized medicine approaches to patients with epilepsy.
Early animal studies suggested that a specific miRNA profile exists for different types of brain injuries, including epilepsy. A study has identified a set of circulating miRNAs, which includes the upregulated expression of miR-146 (a miRNA already linked with epileptogenesis) in blood [23]. In another study, a set of upregulated miRNAs’ expression was identified in the serum, which was observed to induce neuroinflammation, the dysregulation of protein synthesis, and neurodegeneration in epilepsy [24]. In that same study, two more miRNAs, miR-15a-5p and miR-194-5p, were identified to be downregulated in serum [24]. A set of downregulated miRNAs, including miR-301a-3p, miR-194p, miR-301a-3p, miR-30b-5p, and miR-4446-3p, were also detected in a different study serving as biomarkers of drug-resistant epilepsy [25]. Additionally, miR-323a-5p was found upregulated in serum as well as in the cerebral cortex of focal cortical dysplasia and drug-resistant epilepsy patients [21]. For temporal lobe epilepsy and mTLE-HS, several studies have identified a wide range of circulating miRNAs to be dysregulated, which can serve as a potential biomarker for epileptogenesis [26][27][28][29][30].
The evidence of miRNAs as a potential biomarker of epilepsy is largely preclinical, and clinical evidence with significant results is still missing. miRNAs have great potential to be served as a potential biomarker, but more research is required to solidify their application in real life.
3.2. Genetic Biomarkers
A few genetic biomarkers have been identified that indicate an increased risk of structural epileptogenesis. Two markers associated with post-stroke epilepsy are mutations in CD-40-1C/T or Rs671 genes [31].
3.3. Molecular Analysis
Two biomarkers have been identified in epileptogenesis diagnosis. Increased plasma levels of high-mobility group box 1 protein have been reported after unilateral hippocampal electrical stimulation-induced status epilepticus [32]. A 1.5-fold increase in cortisol levels in a 3 cm scalp hair sample was reported in a 6–12-year-old child with benign childhood epilepsy syndromes when the sample was analyzed within 24 h of the first seizure [33]. Generally, under normal physiological conditions, cortisol accumulation in hair takes several weeks [34][35][36]. Therefore, these studies suggest increased hair cortisol levels and dysfunction of the hypothalamic–pituitary–adrenal axis is a potent indicator of epileptogenesis.
3.4. Molecular Profiling after Surgery
It had been reported that there is a relative reduction in ZNF852, CDCP2, PRRT1, FLJ41170, and 7RNA probes in patients who become seizure-free, after lobectomy for interactable TLE patients [37]. An amplified hippocampal myoinositol/total creatine ratio is a potential diagnostic biomarker for epileptogenesis in the case of lithium–pilocarpine-induced status epilepticus [38]. Another potential biomarker of diagnosis of status epilepticus is upregulation of miR-451a or miR-21p, or downregulation of miR-19b in the CSF [26]. The SE unit involved patients with focal SE, nonconvulsive SE, and generalized tonic-clonic SE, although, after TBI or stroke, the diagnosis of nonconvulsive SE becomes difficult [26]. Risk biomarkers, including a CD1 background or carrying an APP/PS1 mutation, indicate a greater susceptibility towards epilepsy [39][40]. These groups need better surveillance and care.
References
- Singh, T.; Joshi, S.; Williamson, J.M.; Kapur, J. Neocortical injury–induced status epilepticus. Epilepsia 2020, 61, 2811–2824.
- Mishra, A.; Goel, R.K. Modulatory Effect of Serotonergic System in Pentylenetetrazole-Induced Seizures and Associated Memory Deficit: Role of 5-HT1A and 5-HT2A/2C. J. Epilepsy Res. 2019, 9, 119–125.
- Sharma, P.; Wright, D.K.; Johnston, L.A.; Powell, K.L.; Wlodek, M.E.; Shultz, S.R.; O’Brien, T.J.; Gilby, K.L. Differences in white matter structure between seizure prone (FAST) and seizure resistant (SLOW) rat strains. Neurobiol. Dis. 2017, 104, 33–40.
- Mishra, A.; Goel, R.K. Chronic 5-HT3 receptor antagonism ameliorates seizures and associated memory deficit in pentylenetetrazole-kindled mice. Neuroscience 2016, 339, 319–328.
- Shorvon, S.D. The causes of epilepsy: Changing concepts of etiology of epilepsy over the past 150 years. Epilepsia 2011, 52, 1033–1044.
- Shorvon, S.D.; Andermann, F.; Guerrini, R. (Eds.) The Causes of Epilepsy: Common and Uncommon Causes in Adults and Children; Cambridge University Press: Cambridge, UK, 2011.
- Xu, Y.; Nguyen, D.; Mohamed, A.; Carcel, C.; Li, Q.; Kutlubaev, M.A.; Anderson, C.S.; Hackett, M.L. Frequency of a false positive diagnosis of epilepsy: A systematic review of observational studies. Seizure 2016, 41, 167–174.
- Smith, D.; Defalla, B.A.; Chadwick, D.W. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. QJM 1999, 92, 15–23.
- Vilar, L.; Vilar, C.F.; Lyra, R.; Freitas, M.D.C. Pitfalls in the Diagnostic Evaluation of Hyperprolactinemia. Neuroendocrinology 2019, 109, 7–19.
- Engel, J., Jr.; Pitkänen, A.; Loeb, J.A.; Dudek, F.E.; Bertram, E.H., 3rd; Cole, A.J.; Moshé, S.L.; Wiebe, S.; Jensen, F.E.; Mody, I.; et al. Epilepsy biomarkers. Epilepsia 2013, 54 (Suppl. S4), 61–69.
- Pitkänen, A.; Engel, J., Jr. Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics 2014, 11, 231–241.
- Pitkänen, A.; EkolleNdode-Ekane, X.; Lapinlampi, N.; Puhakka, N. Epilepsy biomarkers—Toward etiology and pathology specificity. Neurobiol. Dis. 2019, 123, 42–58.
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105.
- Ebert, M.S.; Sharp, P.A. Roles for microRNAs in conferring robustness to biological processes. Cell 2012, 149, 515–524.
- Tan, C.L.; Plotkin, J.L.; Venø, M.T.; von Schimmelmann, M.; Feinberg, P.; Mann, S.; Handler, A.; Kjems, J.; Surmeier, D.J.; O’Carroll, D.; et al. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 2013, 342, 1254–1258.
- Aronica, E.; Fluiter, K.; Iyer, A.; Zurolo, E.; Vreijling, J.; van Vliet, E.A.; Baayen, J.C.; Gorter, J.A. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur. J. Neurosci. 2010, 31, 1100–1107.
- Jimenez-Mateos, E.M.; Engel, T.; Merino-Serrais, P.; McKiernan, R.C.; Tanaka, K.; Mouri, G.; Sano, T.; O’Tuathaigh, C.; Waddington, J.L.; Prenter, S.; et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 2012, 18, 1087–1094.
- Jimenez-Mateos, E.M.; Engel, T.; Merino-Serrais, P.; Fernaud-Espinosa, I.; Rodriguez-Alvarez, N.; Reynolds, J.; Reschke, C.R.; Conroy, R.M.; McKiernan, R.C.; deFelipe, J.; et al. Antagomirs targeting microRNA-134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine-induced status epilepticus. Brain Struct. Funct. 2015, 220, 2387–2399.
- Kan, A.A.; van Erp, S.; Derijck, A.A.; de Wit, M.; Hessel, E.V.; O’Duibhir, E.; de Jager, W.; Van Rijen, P.C.; Gosselaar, P.H.; de Graan, P.N.; et al. Genome-wide microRNA profiling of human temporal lobe epilepsy identifies modulators of the immune response. Cell Mol. Life Sci. 2012, 69, 3127–3145.
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.; van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A key regulator of astrocyte-mediated inflammatory response. PLoS ONE 2012, 7, e44789.
- Henshall, D.C.; Hamer, H.M.; Pasterkamp, R.J.; Goldstein, D.B.; Kjems, J.; Prehn, J.H.M.; Schorge, S.; Lamottke, K.; Rosenow, F. MicroRNAs in epilepsy: Pathophysiology and clinical utility. Lancet Neurol. 2016, 15, 1368–1376.
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012, 37, 460–465.
- Spain, E.; Jimenez-Mateos, E.M.; Raoof, R.; ElNaggar, H.; Delanty, N.; Forster, R.J.; Henshall, D.C. Direct, non-amplified detection of microRNA-134 in plasma from epilepsy patients. RSC Adv. 2015, 5, 90071–90078.
- Wang, J.; Yu, J.T.; Tan, L.; Tian, Y.; Ma, J.; Tan, C.C.; Wang, H.F.; Liu, Y.; Tan, M.S.; Jiang, T.; et al. Genome-wide circulating microRNA expression profiling indicates biomarkers for epilepsy. Sci. Rep. 2015, 5, 9522.
- Wang, J.; Tan, L.; Tan, L.; Tian, Y.; Ma, J.; Tan, C.C.; Wang, H.F.; Liu, Y.; Tan, M.S.; Jiang, T.; et al. Circulating microRNAs are promising novel biomarkers for drug-resistant epilepsy. Sci. Rep. 2015, 5, 10201.
- Raoof, R.; Jimenez-Mateos, E.M.; Bauer, S.; Tackenberg, B.; Rosenow, F.; Lang, J.; Onugoren, M.D.; Hamer, H.; Huchtemann, T.; Körtvélyessy, P.; et al. Cerebrospinal fluid microRNAs are potential biomarkers of temporal lobe epilepsy and status epilepticus. Sci. Rep. 2017, 7, 3328.
- Avansini, S.H.; de Sousa Lima, B.P.; Secolin, R.; Santos, M.L.; Coan, A.C.; Vieira, A.S.; Torres, F.R.; Carvalho, B.S.; Alvim, M.K.; Morita, M.E.; et al. MicroRNA hsa-miR-134 is a circulating biomarker for mesial temporal lobe epilepsy. PLoS ONE 2017, 12, e0173060.
- Raoof, R.; Bauer, S.; El Naggar, H.; Connolly, N.M.C.; Brennan, G.P.; Brindley, E.; Hill, T.; McArdle, H.; Spain, E.; Forster, R.J.; et al. Dual-center, dual-platform microRNA profiling identifies potential plasma biomarkers of adult temporal lobe epilepsy. EBioMedicine 2018, 38, 127–141.
- Li, J.; Lin, H.; Sun, Z.; Kong, G.; Yan, X.; Wang, Y.; Wang, X.; Wen, Y.; Liu, X.; Zheng, H.; et al. High-Throughput Data of Circular RNA Profiles in Human Temporal Cortex Tissue Reveals Novel Insights into Temporal Lobe Epilepsy. Cell PhysiolBiochem. 2018, 45, 677–691.
- Antônio, L.G.L.; Freitas-Lima, P.; Pereira-da-Silva, G.; Assirati, J.A., Jr.; Matias, C.M.; Cirino, M.L.A.; Tirapelli, L.F.; Velasco, T.R.; Sakamoto, A.C.; Carlotti, C.G., Jr.; et al. Expression of MicroRNAs miR-145, miR-181c, miR-199a and miR-1183 in the Blood and Hippocampus of Patients with Mesial Temporal Lobe Epilepsy. J. Mol. Neurosci. 2019, 69, 580–587.
- Pitkänen, A.; Roivainen, R.; Lukasiuk, K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 2016, 15, 185–197.
- Walker, L.E.; Frigerio, F.; Ravizza, T.; Ricci, E.; Tse, K.; Jenkins, R.E.; Sills, G.J.; Jorgensen, A.; Porcu, L.; Thippeswamy, T.; et al. Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J. Clin. Investig. 2017, 127, 2118–2132.
- Stavropoulos, I.; Pervanidou, P.; Gnardellis, C.; Loli, N.; Theodorou, V.; Mantzou, A.; Soukou, F.; Sinani, O.; Chrousos, G.P. Increased hair cortisol and antecedent somatic complaints in children with a first epileptic seizure. Epilepsy Behav. 2017, 68, 146–152.
- Wester, V.L.; van Rossum, E.F. Clinical applications of cortisol measurements in hair. Eur. J. Endocrinol. 2015, 173, M1–M10.
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601.
- Stalder, T.; Kirschbaum, C. Analysis of cortisol in hair--state of the art and future directions. Brain Behav. Immun. 2012, 26, 1019–1029.
- Gallek, M.J.; Skoch, J.; Ansay, T.; Behbahani, M.; Mount, D.; Manziello, A.; Witte, M.; Bernas, M.; Labiner, D.M.; Weinand, M.E. Cortical gene expression: Prognostic value for seizure outcome following temporal lobectomy and amygdalohippocampectomy. Neurogenetics 2016, 17, 211–218.
- Pascente, R.; Frigerio, F.; Rizzi, M.; Porcu, L.; Boido, M.; Davids, J.; Zaben, M.; Tolomeo, D.; Filibian, M.; Gray, W.P.; et al. Cognitive deficits and brain myo-Inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol. Dis. 2016, 93, 146–155.
- Guo, D.; Zeng, L.; Brody, D.L.; Wong, M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS ONE 2013, 8, e64078.
- Miszczuk, D.; Dębski, K.J.; Tanila, H.; Lukasiuk, K.; Pitkänen, A. Traumatic Brain Injury Increases the Expression of Nos1, Aβ Clearance, and Epileptogenesis in APP/PS1 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 7010–7027.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.8K
Revisions:
2 times
(View History)
Update Date:
29 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





