Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Songbai Xue | + 2615 word(s) | 2615 | 2021-11-22 10:16:52 | | | |
| 2 | Catherine Yang | Meta information modification | 2615 | 2021-11-29 08:36:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xue, S. Sn on Ag-Based Brazing Filler Metals. Encyclopedia. Available online: https://encyclopedia.pub/entry/16483 (accessed on 07 February 2026).
Xue S. Sn on Ag-Based Brazing Filler Metals. Encyclopedia. Available at: https://encyclopedia.pub/entry/16483. Accessed February 07, 2026.
Xue, Songbai. "Sn on Ag-Based Brazing Filler Metals" Encyclopedia, https://encyclopedia.pub/entry/16483 (accessed February 07, 2026).
Xue, S. (2021, November 29). Sn on Ag-Based Brazing Filler Metals. In Encyclopedia. https://encyclopedia.pub/entry/16483
Xue, Songbai. "Sn on Ag-Based Brazing Filler Metals." Encyclopedia. Web. 29 November, 2021.
Copy Citation
Ag-based brazing filler metals containing Sn have been widely applied in many engineering fields. By summarizing the effects of Sn on the melting temperature, wettability and microstructure, and mechanical properties of the filler metals, the Sn element can significantly decrease the melting point and improve the wettability, and proper addition of Sn can optimize the microstructure and improve the comprehensive properties of the filler metals, while excessive addition of Sn will form brittle IMCs and decrease the mechanical properties of the filler metals.
Sn element
Ag-based filler metals
melting characteristics
wettability
microstructure
mechanical properties
1. Influences of Sn on the Melting Characteristics of Ag-Based Brazing Filler Metals
The melting characteristics of the brazing filler metal are the important performance index, and affect not only the brazing temperature, but also the wettability, as well as the properties of the brazed joint [1][2]. The melting temperature of the Ag filler metals decreases with the increasing Ag content, and the filler metals with high Ag show excellent flow and spreading properties, but the material cost is very high. Through decreasing the content of Ag, the price of the Ag filler metal can be reduced, but the decrease in Ag will increase the melting point and raise the melting temperature range, which has a negative effect on the brazing properties of the filler metal [3].
The influences of the Sn content on melting characteristics of Ag60Cu brazing filler metals are presented in Table 1 [4][5]. It was seen that the addition of Sn could decrease both the liquidus temperature and the solidus temperature of the Ag60Cu filler metals. When the content of Sn was lower than 8%, the melting temperature range of the filler metals enlarged with the increasing Sn content. When the Sn content was higher than 8%, the melting temperature range tended to be stable, but still was much greater than the filler metals without Sn. In some other studies [6][7], similar conclusions were also reached; i.e., the addition of Sn could continuously decrease the melting point of the Ag-Cu filler metals. The obvious decrease in both the solidus temperature and the liquidus temperature of the filler metals was mainly attributed to the fact that the melting point of Sn is only 232 °C, as shown in Figure 1 [8], and Sn could partly dissolve into the phases of the filler metals [9].
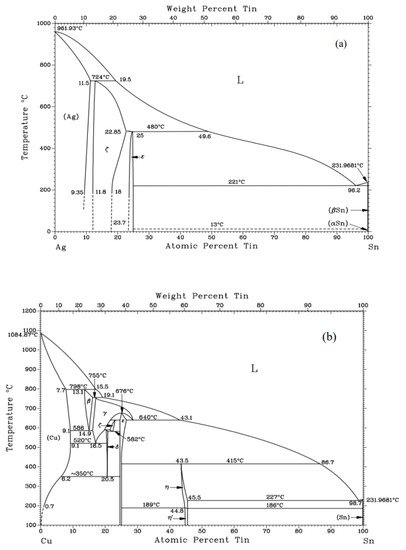
Figure 1. (a) Ag-Sn phase diagram; (b) Cu-Sn phase diagram [8].
| Sn/wt % | Solidus/°C | Liquidus/°C | ΔT/°C |
|---|---|---|---|
| 0 | 778.7 | 814.1 | 35.4 |
| 2 | 756.3 | 809.9 | 53.6 |
| 4 | 694.4 | 797.6 | 103.2 |
| 5 | 680 | 767 | 87 |
| 8 | 604.9 | 776.7 | 171.8 |
| 10 | 602 | 718 | 116 |
| 15 | 580 | 680 | 100 |
| 20 | 521 | 640 | 119 |
| 25 | 514 | 609 | 95 |
Generally, Ag-based brazing filler metals with lower than 30% Ag content are considered to be low-Ag filler metals, and those with over 30% Ag content are classified as high-Ag filler metals. Through addition of Zn into the Ag-Cu alloys, the Ag-Cu-Zn series brazing filler metals with a lower melting temperature can be obtained, but the saturated vapor pressure of Zn is high, and the addition of Zn should not be excessive. Therefore, the addition of Sn is important for further reducing the Ag content without affecting the temperature of the brazing filler metals. Some research results on the influences of Sn content on melting characteristics of the Ag-Cu-Zn series brazing filler metals are listed in Table 2 [10][11][12]. It was found that the solidus temperatures and liquidus temperatures of both the high-Ag and the low-Ag Ag-Cu-Zn brazing filler metals decreased with the increasing content of Sn. The melting temperature range of the low-Ag filler metals increased gradually with the increasing Sn content, while that of the high-Ag filler metals showed an opposite trend.
Table 2. Melting characteristics of some Ag-Cu-Zn series brazing filler metals with different contents of Sn [10][11][12].
| No. | Ag% | Cu% | Zn% | Sn% | Ni% | Temperature/°C | Melting Temperature Range/°C |
|---|---|---|---|---|---|---|---|
| 1 | 20 | Bal | 38 | 1.5 | 1.3 | 763.9–803.7 | 39.8 |
| 20 | Bal | 32 | 2.5 | - | 735–782 | 47 | |
| 20 | Bal | 36.5 | 4 | 1.2 | 696.9–787.5 | 70.6 | |
| 20 | Bal | 32 | 4.5 | - | 737–771 | 34 | |
| 20 | Bal | 32 | 6.5 | - | 670–750 | 80 | |
| 20 | Bal | 32 | 7.5 | - | 656–738 | 82 | |
| 2 | Bal | 20 | 12 | - | - | 665–760 | 95 |
| Bal | 20 | 16 | 5 | - | 612–678 | 66 | |
| Bal | 22 | 18 | 8 | - | 590–635 | 45 |
The traditional Ag-Cu-Zn-Cd brazing filler metals have superior comprehensive properties among all the Ag filler metals, and were widely used. However, the addition of the toxic Cd into the brazing filler metals has been prohibited, since the concept of green environmental protection strikes a deep chord in the hearts of the people. Through a large quantity of investigations, it was found that only the In and Sn elements can replace Cd in Ag-based filler metals, and the price of In is high. Therefore, the Ag-Cu-Zn-Sn filler metals are recognized as a substitute for the Ag-Cu-Zn-Cd filler metals [13].
To reveal the effects of Sn on the melting characteristics of Ag-Cu-Zn-Sn filler metal, Wang et al. prepared a thin Sn layer on the BAg34CuZnSn (Ag34-Cu36-Zn27.5-Sn2.5) filler metals following a process of electric cleaning, water washing, activation, water washing, brush plating, and water washing. Table 3 presents the melting characteristics of the BAg34CuZnSn filler metals with different weight ratios of the brush-plated Sn layer to the BAg34CuZnSn alloy [14]. It is obvious that with the increasing Sn content, the solidus temperature and the liquidus temperature of the filler metals showed a linear decreasing trend, and the melting temperature range decreased gradually; whereas, compared with the method of adding the Sn element into the filler metals through smelting, the brush-plated Sn has less of an effect on the melting characteristics.
Table 3. Melting characteristics of BAg34CuZnSn brazing filler metals with different contents of the brush-plated Sn layer [14].
| Weight Ratio of the Sn Layer to the BAg34CuZnSn | Solidus/°C | Liquidus/°C | Melting Temperature Range/°C |
|---|---|---|---|
| 0 | 700.0 | 731.0 | 31.0 |
| 0.5 | 697.5 | 724.0 | 26.5 |
| 1.0 | 693.0 | 717.0 | 24.0 |
| 1.5 | 690.0 | 712.0 | 22.0 |
| 2.0 | 686.0 | 705.5 | 19.5 |
2. Influences of Sn on the Wettability of Ag-Based Brazing Filler Metals
The wettability of the brazing filler metals is crucial to the gap-filling ability and quality of the brazed joints, and is usually measured through the gap-filling test and the spreading test [9]. It has been revealed that the wettability of the Ag60Cu brazing filler metal with 4 wt % of Sn was close to that of the Ag72Cu filler metal [5], and both the two filler metals showed a superior spreading property on the Cu substrate. Figure 2 shows the capillary rise heights of the Ag60Cu-xSn filler metals in gaps with different widths [4]. In the figure, it can be seen that when the gap width was lower than 0.1 mm, the capillary rise heights of the filler metals with the increasing contents of Sn were almost the same, demonstrating that the content of Sn had little influence on the capillary rise height.
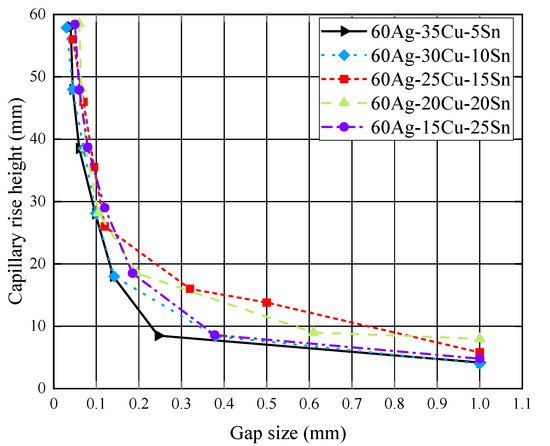
Figure 2. Capillary rise heights of Ag60Cu-xSn brazing filler metals in gaps with different widths [4].
Figure 3 shows the influence of Sn on the spreading performance of the BAg20CuZn (Ag20-Cu44-Zn36) filler metals bearing 1.5% In. As in the figure, with increasing Sn content, the spreading area of the filler metal showed an an increasing trend [15]. When the content of Sn increaseds from 1.5 wt % to 3.0 wt %, the spreading area increased from 207.7 mm2 to 320.6 mm2, indicating that the wetting property of the filler metal had been greatly improved. The reason was that the flowability of the liquid metal could be measured by its viscosity, and higher viscosity corresponded to worse flowability, while the viscosity was inversely proportional to the superheated degree of the liquid metal [16]. Therefore, at the same brazing temperature, the brazing filler metal, with a lower melting temperature, had a higher superheating degree, resulting in lower viscosity and higher flowability. On the contrary, when the melting temperature of the filler metal increased, the flowability decreased [17][18].
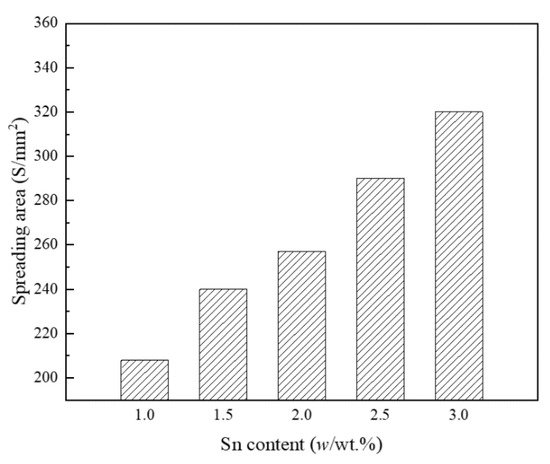
Figure 3. Variations in the wetting area of BAg20CuZnIn filler metals with increasing Sn content [15].
In another study, the Sn layers were plated on BAg34CuZnSn filler metals by the combination of electroplating and thermal diffusion; the variation of the wetting area with the change in the Sn content is shown in Figure 4 [19], in which it can be seen that the wettability of the filler metals increased with the increasing content of the surface-plated Sn. Some other reports investigated the wettability of BAg35CuZnSn filler metals with electroless-plated Sn [20] and BAg34CuZnSn filler metals with brush-plated Sn [14], and the same conclusion was reached, demonstrating that the Sn element is effective in optimizing the wettability of Ag-Cu-Zn-Sn brazing filler metals.
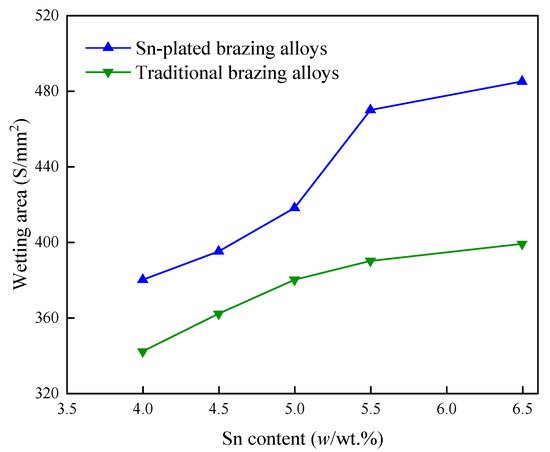
Figure 4. Variations in the wetting area of BAg34CuZnSn brazing filler metal with the increasing content of plated Sn [19].
3. Influences of Sn on the Microstructure of Ag-Based Brazing Filler Metals
The properties and processing technologies of brazing filler metals are determined by their microstructure [21]. Therefore, it is particularly important to reveal the effect of Sn addition on the microstructure of Ag-based filler metals. Figure 5 shows scanning electron microscope (SEM) images of Ag60Cu filler metals with different contents of Sn that reveal that the microstructure of the filler metals changed obviously with the increasing Sn content [5]. The energy-dispersive X-ray spectrometry (EDS) analysis of the phases of the filler metals showed that the Ag60Cu filler metals were composed mainly of the Cu-rich phase with a Ag solute element (α-Cu) and a Ag-Cu eutectic. With the addition of 2% or 4% of Sn, the filler metals were composed of the α-Cu, the Ag-rich phase with a Cu solute element (α-Ag), and some Ag-Cu-Sn ternary eutectic. With the addition of 8% Sn, a Cu-rich phase with a Sn solute element (β-Cu) appeared. As the α-Cu and α-Ag were ductile phases but the β-Cu was a brittle phase, and a large amount of Cu6Sn5 intermetallic compound (IMC) was produced, the forming properties of the Ag60Cu filler metal with 8% of Sn deteriorated sharply. Liu et al. attempted to add Sn and In with a total content of 20% to Ag58Cu22 filler metals, and found that the filler metals had good forming properties only when the content of Sn was lower than 12%, while the filler metals became too brittle to be formed when the content of Sn was higher than 15% [22].
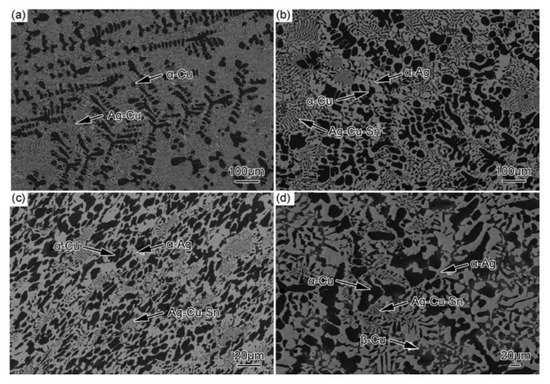
Figure 5. Microstructures of Ag60Cu-xSn filler metals containing (a) 0%, (b) 2%, (c) 4%, and (d) 8% Sn [5].
The major phases in Ag-Cu-Zn filler metals include the Ag-based solid solution, the Cu-based solid solution, the Cu-Zn IMC, etc. When the content of Sn in Ag-Cu-Zn filler metals is lower than 4%, the alloys are composed mainly of the Cu-based solid solution, the Cu-Zn IMC, and the needlelike or bulk Ag-based solid solution. With increasing Sn content, the large blocky structure consisted mainly of the Ag-based solid solution, a small portion of Cu-Zn IMC increased obviously (see Figure 6), and the forming properties of the filler metals were optimized accordingly [11]. When the content of Sn in the Ag-Cu-Zn filler metal exceeded 4.5%, the filler metals were composed mainly of the Cu-rich phase, the Ag-rich phase, the Cu-Zn IMC, the Cu5.6Sn IMC, the Cu40.5Sn11 IMC, and Cu3P compounds. Meanwhile, coarse dendrites with a certain directionality appeared in the filler metal, resulting in an obvious decrease in the performance of the filler metals [17]. Figure 7 shows the microstructure of the Cu-20Ag-Zn-xSn alloys with 6.5% and 7.5% Sn, in which more Sn bronze phases appeared, and the segregation was more serious with increasing Sn content, which led to a gradual increase in the brittleness of the filler metals, as the Sn bronze was a brittle phase [10].
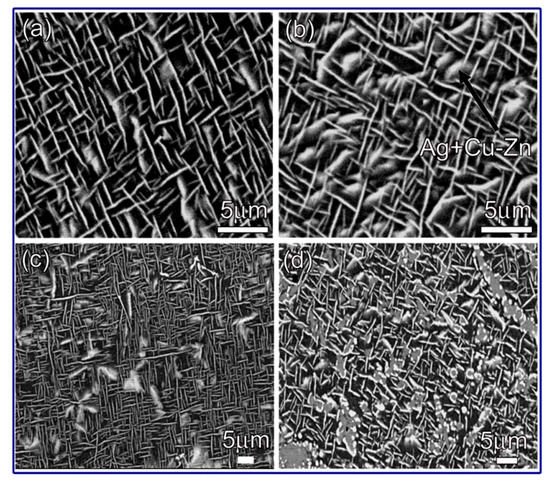
Figure 6. BSE images of Ag-Cu-Sn filler metals with (a) 1.5%, (b) 2.2%, (c) 3%, and (d) 4% Sn [11].
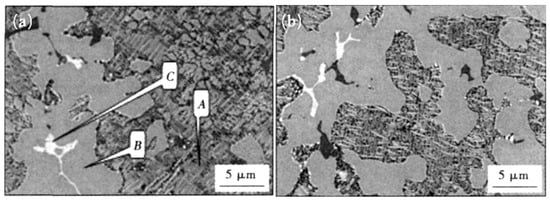
Figure 7. Microstructures of Cu-20Ag-Zn-xSn with (a) 6.5% and (b) 7.5% Sn [10].
The XRD spectrum and interfacial morphology of the BAg34CuZnSn filler metal with a brush-coated Sn layer are shown in Figure 8 [23]. As exhibited in Figure 8a, the crystalline grains of the brush coated Sn showed an obvious preferred orientation of (200) and (112). In Figure 8b, it can be seen that the bonding interface between the filler metal and the Sn coating was flat and compact, and the Sn coating was uniform in microstructure, with no defects such as pores and inclusions. The results demonstrated that the plating method could decrease the internal stress between the Sn coating and the filler metal substrate, and improveds the bonding between the coating and the filler metal, making the bonding between the filler metal and the Sn coating dense, and without defects. In this way, forming of the AgCuZnSn filler metal with high Sn content could be realized.
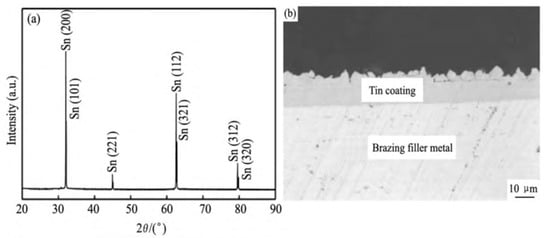
Figure 8. Analysis results of BAg34CuZnSn filler metals with a plated Sn coating: (a) XRD spectrum of the Sn coating; (b) interface morphology [23].
4. Influences of Sn on the Mechanical Properties of Joints Brazed by Ag Filler Metals
During the service process of brazed devices, the mechanical properties of the brazed joints are the key factors that affect the reliability of the joints [24]. At present, investigations of mechanical properties of brazed joints focus mainly on the shear strength and the tensile strength. The shear strength of Cu/Cu joints brazed by using Ag60Cu filler metals with different contents of Sn is shown in Figure 9. As shown in the figure, when the content of Sn was 5%, the average shear strength of the brazed joints was 210 MPa. When the content of Sn increased to 10%, the shear strength increased by 9%. In contrast, when the Sn content was over 10%, the shear strength of the brazed joints decreased [4], because the volume ratio of the brittle Cu-Sn IMCs increased with the increasing Sn content [25].
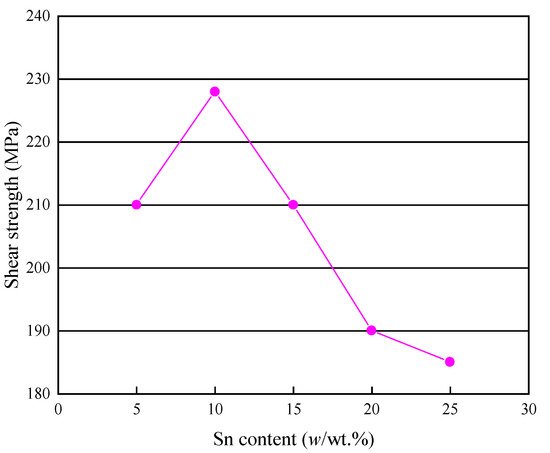
Figure 9. Shear strengths of Cu/Cu joints brazed by using Ag60Cu filler metals with different contents of Sn [4].
Figure 10 shows the shear strength of the stainless steel/stainless steel joints brazed by using Ag-Cu-Zn filler metals with different contents of Sn [11]. As shown in the figure, the shear strength increased firstly and then decreased with the increasing Sn content in the filler metals, because the alloying of Sn could decrease the brazing temperature of the filler metals, which made grain coarsening less likely to occur in the joining zone of the substrate material, and the lower temperature could decrease the residual stress. In addition, the addition of Sn improved the strength of the Ag-based filler metals through solid solution strengthening, and the introduction of Sn formed a Cottrell atmosphere that pinned the dislocation and hindered the dislocation slip [26], thus the joints’ strength was improved. However, when the content of Sn was too high, a brittle structure appeared in the brazing filler metals and sharply decreased the joints strength. Similarly, some other reports also obtained the same test results [10]. Wang et al. plated the Sn coating on BAg45CuZn [27] and BAg50CuZn filler metals [28], and found that when the content of Sn was too high, the brittle phases that appeared in the filler metals were Cu5Zn8, Cu41Sn11, Cu3Sn, and Ag3Sn, and the brazed joints fractured mainly in a brittle mode, with a small ductile fracture.
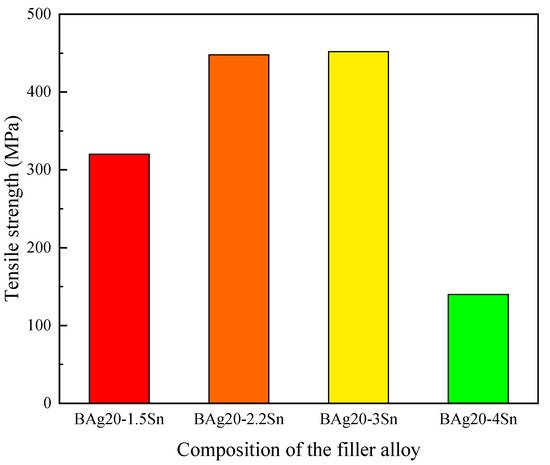
Figure 10. Tensile strengths of stainless steel/stainless steel joints brazed by using Ag-Cu-Zn filler metals with different contents of Sn [11].
The tensile strength of the stainless steel/stainless steel joints brazed by using Ag-Cu-Zn-Sn filler metals were higher than 300 MPa and could satisfy the application requirements in most cases [29]. Figure 11 shows the tensile strength of the 316LN stainless steel joints brazed using a BAg34CuZnSn filler metal with a plated Sn coating, and a filler metal of the same composition but manufactured through the traditional melting and drawing processes [30]. At the same Sn content, the joints brazed using the traditional filler metal were a little higher, because the Sn distribution in the traditional filler metal was more uniform, and the Sn mainly played the role of solid solution strengthening and precipitation strengthening, with Ag3Sn and Cu41Sn11 as the main strengthening phases [12]. In contrast, the Sn coated on the Ag filler metal strengthened mainly through an aging strengthening mechanism. The Sn was relatively uniform in the filler metal, but there was local segregation, and the strengthening phases were mainly Ag3Sn and Cu3Sn [28]. When the content of Sn was 5.5%, the brazed joints showed the highest tensile strength.
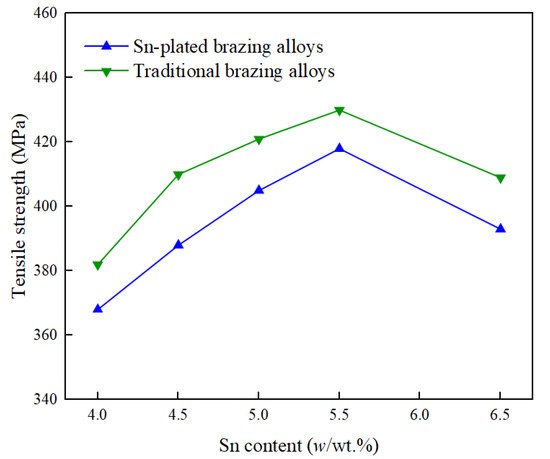
Figure 11. Tensile strengths of 316LN stainless steel joints brazed using BAg34CuZnSn filler metals with a plated Sn coating and a filler metal of the same composition but manufactured through the traditional melting and drawing processes [30].
References
- Long, W.M.; Zhang, G.X.; Zhang, Q.K. In situ synthesis of high strength Ag brazing filler metals during induction brazing process. Scr. Mater. 2016, 11, 41–43.
- Apel, M.; Laschet, G.; Böttger, B.; Berger, R. Phase field modeling of microstructure formation, DSC curves, and thermal expansion for Ag-Cu brazing fillers under reactive air brazing conditions. Adv. Eng. Mater. 2014, 16, 1468–1474.
- Wang, H.; Xue, S.B. Effect of Ag on the properties of solders and brazing filler metals. J. Mater. Sci. Mater. Electron. 2016, 27, 1–13.
- Basri, D.K.; Sisamouth, L.; Farazila, Y.; Miyazawa, Y.; Ariga, T. Brazeability and mechanical properties of Ag-Cu-Sn brazing filler metals on copper-brazed joint. Mater. Res. Innov. 2014, 18, 429–432.
- Shi, L.; Cui, L.; Zhou, F.; Gu, X.L.; He, P. Influence of Sn on microstructure and performance of electric vacuum Ag-Cu filler metal. J. Mater. Eng. 2016, 44, 54–59.
- Winiowski, A.; Rózanski, M. Impact of tin and nickel on the brazing properties of silver filler metals and on the strength of brazed joints made of stainless steels. Arch. Metall. Mater. 2013, 58, 1007–1011.
- Liu, W.; Zheng, M.; Wang, X.R.; Fan, Z.H.; Yu, D.K.; Chen, R.; Shen, H.Y.; Guo, J.Y.; Guo, B.; He, P. Processing and characterization of Ag-Cu-Sn brazing alloy prepared by a mechanical alloying method. J. Mater. Eng. Perform. 2018, 27, 1148–1153.
- Murray, J.L. ASM Handbook: Alloy Phase Diagrams; ASM International: Geauga County, OH, USA, 1992.
- Yun, D.H. Influence of Tin and Gallium on Microstructure and Properties of Ag–Cu–Zn Brazing Filler Metal. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2014.
- Li, Z.Y.; Liu, B.; Feng, J.C. Optimum design of cadmium-free silver-based filler metal contained 20% Ag. Trans. China Weld. Inst. 2008, 8, 5–8.
- Wang, X.R.; Yu, D.K.; He, Y.M.; Huang, S.H.; Chen, R. Effect of Sn content on brazing properties of Ag-based filler alloy. Mater. Sci. 2013, 3, 16–21.
- Li, M.G.; Sun, D.Q.; Qiu, X.M.; Liu, J.B.; Miao, K.; Wu, W.C. Effects of silver based filler metals on microstructure and properties of laser brazed joints between TiNi shape memory alloy and stainless steel. Sci. Technol. Weld. Join. 2007, 12, 183–188.
- Xue, P.; Zou, Y.; He, P.; Pei, Y.Y.; Sun, H.W.; Ma, C.L.; Luo, J.Y. Development of low silver AgCuZnSn filler metal for Cu/steel dissimilar metal joining. Metals 2019, 9, 198.
- Wang, X.X.; Du, Q.B.; Long, W.M.; Lv, D.F. Effect of micro tin brush-electroplated coating on properties of AgCuZnSn brazing filler metals. Trans. China Weld. Inst. 2015, 36, 47–50.
- Wu, Q.Q. Influence of Indium and Stannum on Microstructure and Properties of BAg20CuZn Filler Metal. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2014.
- Wang, X.X.; Tan, Q.Y.; Xue, P.; Tang, M.Q.; Long, W.M. Phase composition and formation mechanism of diffusion transition zone for silver-based brazing alloys with tin coatings. Mater. Rep. 2017, 31, 66–69.
- Li, Z.R.; Jiao, N.; Feng, J.C.; Lu, C.H. Effect of alloying elements on microstructure and property of AgCuZnSn brazing alloy. Trans. China Weld. Inst. 2008, 3, 65–68.
- Bao, L.; Long, W.M.; Zhang, G.X.; Sui, F.F.; Li, H.; Ma, J. Effect of trace calcium on performance of AgCuZn alloy. Trans. China Weld. Inst. 2012, 33, 57–60.
- Wang, X.X.; Peng, J.; Cui, D.T. Quantitative characterization of brazing performance for Sn-plated silver alloy fillers. Mater. Res. Express 2017, 4, 126509.
- Wang, X.X.; Long, W.M.; Zhu, K.; Dong, B.W. Effect of electroless tin plating on wettability of BAg35CuZnSn brazing filler metal. Weld. Join. 2014, 9, 32–35.
- Pu, J.; Xue, S.B.; Zhang, L.; Wu, M.F.; Long, W.M.; Wang, S.Q.; Qian, S.J.; Lin, T.S. Effect of CeO2 on wettability of Ag30CuZnSn flux cored brazing filler metal and microstructure and properties of brazed joint. Mater. Rep. 2021, 35, 8134–8139.
- Liu, Z.G.; Wang, W.X.; Tang, M.; Lin, Q.Y.; Zhang, B.H. Silver based alloys with low melting point. Precious Met. 1991, 3, 17–25.
- Wang, X.X.; Peng, J.; Cui, D.T.; Tang, M.Q.; Long, W.M. Microstructure and mechanical properties of 316LN stainless steel brazed joints based on silver brazing filler metals with plating Sn. Chin. J. Rare Met. 2017, 41, 1167–1172.
- Ma, H.T.; Suhling, J.C. A review of mechanical properties of lead-free solders for electronic packaging. J. Mater. Sci. 2009, 44, 1141–1158.
- Chakravarty, I.; Gupta, S.P. Formation of intermetallics during brazing of alumina with Fe, Ni and Cr using Ag–30Cu–10Sn as filler metal. Mater. Charact. 2003, 51, 235–241.
- Lai, Z.M. Effects of Ga/In and Rare Earth Ce on Microstructures and Properties of Brazed Joint of Ag30CuZnSn Filler Metal. Ph.D. Thesis, Nanjing University of Aeronautics and Astronautics, Nanjing, China, 2011.
- Wang, X.X.; Wang, B.; Han, L.H.; Long, W.M.; Tang, M.Q. Effect of brazing alloys with electroless plated tin on microstructure and mechanical properties of brass brazed joints. Rare Met. Mater. Eng. 2018, 47, 367–370.
- Wang, X.X.; Peng, J.; Cui, D.T. Microstructure and mechanical properties of stainless steel/brass joints brazed by Sn-electroplated Ag brazing filler metals. J. Mater. Eng. Perform. 2018, 27, 2233–2238.
- Arenas, M.F.; Acoff, V.L.; Reddy, R.G. Physical properties of selected brazing filler metals. Sci. Technol. Weld. Join. 2004, 9, 423–429.
- Quantitative characterization of brazability for Sn-plated Ag brazing filler metals based on entropy model. Trans. China Weld. Inst. 2020, 41, 18–22.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
29 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




