
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrada Serafim | + 1544 word(s) | 1544 | 2021-11-16 10:01:53 | | | |
| 2 | Vicky Zhou | Meta information modification | 1544 | 2021-11-26 01:57:36 | | |
Video Upload Options
Computed tomography (CT) proved to be a reliable, nondestructive, high-performance machine, enabling visualization and structure analysis at submicronic resolutions. CT allows both qualitative and quantitative data of the 3D model, offering an overall image of its specific architectural features and reliable numerical data for rigorous analyses. The precise engineering of scaffolds consists in the fabrication of objects with well-defined morphometric parameters (e.g., shape, porosity, wall thickness) and in their performance validation through thorough control over their behavior (in situ visualization, degradation, new tissue formation, wear, etc.).
1. Introduction
Basing their work on Röntgen’s X-rays [1] and W.H. Oldendorf’s findings regarding the radiodensity discontinuities of two different materials [2], G.H. Hounsfield and A.M. Cormack developed the first functional medical CT scanner [3]. As a medical device, the use of CT scanners raises great concerns regarding the damage caused by the absorbed X-ray. Therefore, the device is equipped with a low tube voltage (approximately 75 KeV [4]) so that low scanning times and adequate slice thickness (usually between 0.4 and 10 mm [5]) result in images with good resolution and sufficient contrast to permit the visualization of different forms of tissue. CT scanners are used for the purposes of diagnosis and control in oncology [6][7], dentistry [8][9] or orthopedics [10].
Although initially exclusively used in the medical field [11], nowadays, CT scanners are being exploited in a variety of nondestructive measurements. However, given the difference between the areas of application, the characteristics of the equipment are also different. Since no live subjects are imaged, the X-ray tube has a much higher voltage (up to 800 kV [12]), the scanning chamber is smaller, and the resolution and accuracy of the generated images can be easily adjusted by moving the object closer to the source [13]. As a result, the field of application for CT scanners has enlarged, comprising metrology [13][14], quality control [15][16], and even forensic studies [17][18][19][20][21][22] or paleontology [23][24][25][26].
Natural materials have been scanned using CT with the aim of using the obtained information for the fabrication of implantable scaffolds with similar structural features [27][28][29]. CT is often coupled with additive manufacturing techniques in order to obtain scaffolds that would ensure the best possible outcome in terms of architecture, mechanical properties, tissue ingrowth, and so on [29][30].
2. CT Imaging of Scaffolds with Biomedical Applications
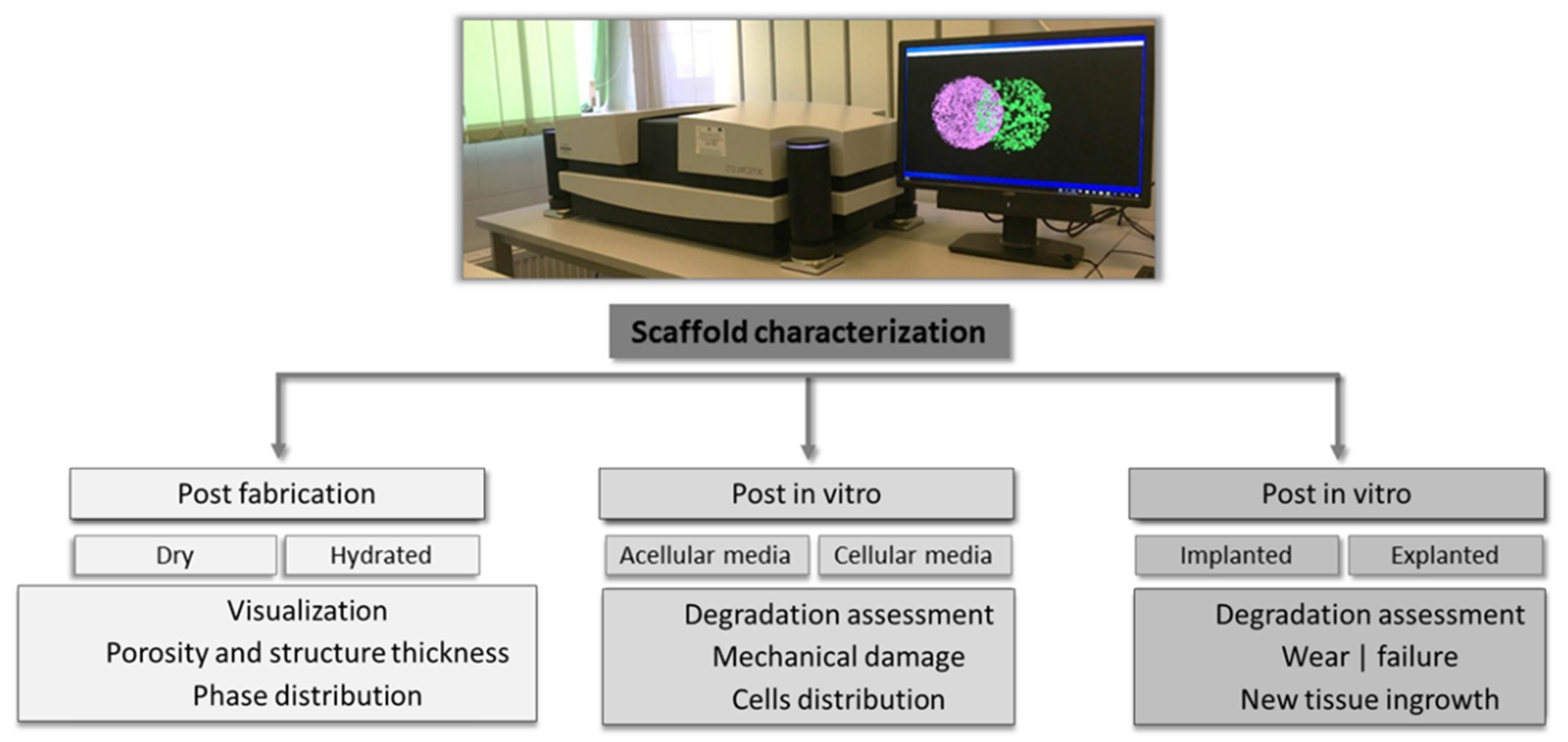 Figure 1. Types of scaffolds’ characterizations and corresponding assessments performed using CT scanners.
Figure 1. Types of scaffolds’ characterizations and corresponding assessments performed using CT scanners.3. Limitations and Perspectives of the CT Technique
References
- Röntgen, W.C. On a New Kind of Rays. Nature 1896, 53, 274–276.
- Oldendorf, W.H. The quest for an image of brain: A brief historical and technical review of brain imaging techniques. Neurology 1978, 28, 517.
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): I. Description of system. Br. J. Radiol. 1973, 46, 1016–1022.
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT–a digital 3D microstructural voyage into scaffolds: A systematic review of the reported methods and results. Biomater. Res. 2018, 22, 1–11.
- Liguori, C.; Frauenfelder, G.; Massaroni, C.; Saccomandi, P.; Giurazza, F.; Pitocco, F.; Marano, R.; Schena, E. Emerging clinical applications of computed tomography. Med. Devices Evid. Res. 2015, 8, 265–278.
- Cyran, C.C.; Paprottka, P.M.; Eisenblätter, M.; Clevert, D.A.; Rist, C.; Nikolaou, K.; Lauber, K.; Wenz, F.; Hausmann, D.; Reiser, M.F.; et al. Visualization, imaging and new preclinical diagnostics in radiation oncology. Radiat. Oncol. 2014, 9, 1–15.
- Stieb, S.; Kiser, K.; van Dijk, L.; Livingstone, N.R.; Elhalawani, H.; Elgohari, B.; McDonald, B.; Ventura, J.; Mohamed, A.S.R.; Fuller, C.D. Imaging for Response Assessment in Radiation Oncology: Current and Emerging Techniques. Hematol. Oncol. Clin. N. Am. 2020, 34, 293–306.
- Hol, C.; Hellén-Halme, K.; Torgersen, G.; Nilsson, M.; Møystad, A. How do dentists use CBCT in dental clinics? A Norwegian nationwide survey. Acta Odontol. Scand. 2015, 73, 195–201.
- Vandenberghe, B. The crucial role of imaging in digital dentistry. Dent. Mater. 2020, 36, 1–11.
- Anderson, P.A.; Morgan, S.L.; Krueger, D.; Zapalowski, C.; Tanner, B.; Jeray, K.J.; Krohn, K.D.; Lane, J.P.; Yeap, S.S.; Shuhart, C.R.; et al. Use of Bone Health Evaluation in Orthopedic Surgery: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 517–543.
- Villarraga-Gómez, H.; Herazo, E.L.; Smith, S.T. X-ray computed tomography: From medical imaging to dimensional metrology. Precis. Eng. 2019, 60, 544–569.
- Ametova, E.; Probst, G.; Dewulf, W. X-ray Computed Tomography Devices and Their Components. In Industrial X-ray Computed Tomography; Carmignato, S., Dewulf, W., Leach, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319595733.
- Kruth, J.P.; Bartscher, M.; Carmignato, S.; Schmitt, R.; De Chiffre, L.; Weckenmann, A. Computed tomography for dimensional metrology. CIRP Ann.-Manuf. Technol. 2011, 60, 821–842.
- Tan, Y.; Kiekens, K.; Welkenhuyzen, F.; Angel, J.; De Chiffre, L.; Kruth, J.-P.; Dewulf, W. Simulation-aided investigation of beam hardening induced errors in CT dimensional metrology. Meas. Sci. Technol. 2014, 25, 64014.
- Pejryd, L.; Beno, T.; Carmignato, S. Computed tomography as a tool for examining surface integrity in drilled holes in CFRP composites. Procedia CIRP 2014, 13, 43–48.
- Kastner, J.; Plank, B.; Salaberger, D.; Sekelja, J. Defect and Porosity Determination of Fibre Reinforced Polymers by X-ray Computed Tomography. In Proceedings of the 2nd International Symposium on NDT in Aerospace, Hamburg, Germany, 22–24 November 2010; pp. 1–12.
- Cecchetto, G.; Amagliani, A.; Giraudo, C.; Fais, P.; Cavarzeran, F.; Montisci, M.; Feltrin, G.; Viel, G.; Ferrara, S.D. MicroCT detection of gunshot residue in fresh and decomposed firearm wounds. Int. J. Leg. Med. 2012, 126, 377–383.
- Cecchetto, G.; Giraudo, C.; Amagliani, A.; Viel, G.; Fais, P.; Cavarzeran, F.; Feltrin, G.; Davide Ferrara, S.; Montisci, M.; Ferrara, S.D.; et al. Estimation of the firing distance through micro-CT analysis of gunshot wounds. Int. J. Leg. Med. 2011, 125, 245–251.
- Pounder, D.J.; Sim, L.J. Virtual casting of stab wounds in cartilage using micro-computed tomography. Am. J. Forensic Med. Pathol. 2011, 32, 97–99.
- Thali, M.J.; Taubenreuther, U.; Karolczak, M.; Braun, M.; Brueschweiler, W.; Kalender, W.A.; Dirnhofer, R. Forensic microradiology: Micro-computed tomography (Micro-CT) and analysis of patterned injuries inside of bone. J. Forensic Sci. 2003, 48, 1336–1342.
- Sakuma, A.; Saitoh, H.; Suzuki, Y.; Makino, Y.; Inokuchi, G.; Hayakawa, M.; Yajima, D.; Iwase, H. Age estimation based on pulp cavity to tooth volume ratio using postmortem computed tomography images. J. Forensic Sci. 2013, 58, 1531–1535.
- Azmi, N.A.; Heo, C.C.; Shafini, N.; Mahmud, M. Age estimation of forensically important blowfly, Chrysomya megacephala (Diptera: Calliphoridae) pupae using micro-computed tomography imaging. Trop. Biomed. 2019, 36, 640–653.
- Rahman, I.A.; Adcock, K.; Garwood, R.J. Virtual Fossils: A New Resource for Science Communication in Paleontology. Evol. Educ. Outreach 2012, 5, 635–641.
- Cunningham, J.A.; Rahman, I.A.; Lautenschlager, S.; Rayfield, E.J.; Donoghue, P.C.J. A virtual world of paleontology. Trends Ecol. Evol. 2014, 29, 347–357.
- Keklikoglou, K.; Faulwetter, S.; Chatzinikolaou, E.; Wils, P.; Brecko, J.; Kvaček, J.; Metscher, B.; Arvanitidis, C. Micro-computed tomography for natural history specimens: A handbook of best practice protocols. Eur. J. Taxon. 2019, 522, 1–55.
- Lewis, D. The fight for control over virtual fossils. Nature 2019, 567, 20–23.
- Drol, C.J.; Kennedy, E.B.; Hsiung, B.K.; Swift, N.B.; Tan, K.T. Bioinspirational understanding of flexural performance in hedgehog spines. Acta Biomater. 2019, 94, 553–564.
- du Plessis, A.; Broeckhoven, C.; Yadroitsev, I.; Yadroitsava, I.; le Roux, S.G. Analyzing nature’s protective design: The glyptodont body armor. J. Mech. Behav. Biomed. Mater. 2018, 82, 218–223.
- Frank, M.B.; Naleway, S.E.; Wirth, T.S.; Jung, J.Y.; Cheung, C.L.; Loera, F.B.; Medina, S.; Sato, K.N.; Taylor, J.R.A.; McKittrick, J. A protocol for bioinspired design: A ground sampler based on sea urchin jaws. J. Vis. Exp. 2016, 2016, 1–8.
- Helguero, C.G.; Amaya, J.L.; Komatsu, D.E.; Pentyala, S.; Mustahsan, V.; Ramirez, E.A.; Kao, I. Trabecular Scaffolds’ Mechanical Properties of Bone Reconstruction Using Biomimetic Implants. Procedia CIRP 2017, 65, 121–126.
- Gabrielson, K.; Maronpot, R.; Monette, S.; Mlynarczyk, C.; Ramot, Y.; Nyska, A.; Sysa-Shah, P. In Vivo Imaging with Confirmation by Histopathology for Increased Rigor and Reproducibility in Translational Research: A Review of Examples, Options, and Resources. ILAR J. 2018, 59, 80–98.
- Vielreicher, M.; Schürmann, S.; Detsch, R.; Schmidt, M.A.; Buttgereit, A.; Boccaccini, A.; Friedrich, O. Taking a deep look: Modern microscopy technologies to optimize the design and functionality of biocompatible scaffolds for tissue engineering in regenerative medicine. J. R. Soc. Interface 2013, 10, 20130263.
- Online, V.A.; Basu, B.; Swain, S.K.; Sarkar, D. Cryogenically cured hydroxyapatite–gelatin nanobiocomposite for bovine serum albumin protein adsorption and release. RSC Adv. 2013, 3, 14622–14633.
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900.
- Liao, J.; Tian, T.; Shi, S.; Xie, X.; Ma, Q.; Li, G.; Lin, Y. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017, 5, 1–15.
- Selaru, A.; Dragusin, D.-M.; Olaret, E.; Serafim, A.; Steinmüller-Nethl, D.; Vasile, E.; Iovu, H.; Stancu, I.-C.; Dinescu, S.; Costache, M. Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications. Materials 2019, 12, 2933.
- Shkarina, S.; Shkarin, R.; Weinhardt, V.; Elizaveta, M.; Kluger, P.J.; Loza, K.; Epple, M.; Ivlev, S.I.; Baumbach, T.; Surmeneva, M.A.; et al. 3D biodegradable scaffolds of polycaprolactone with silicate-containing hydroxyapatite microparticles for bone tissue engineering: High-resolution tomography and in vitro study. Sci. Rep. 2018, 8, 1–13.
- Krause, M.; Hausherr, J.M.; Burgeth, B.; Herrmann, C.; Krenkel, W. Determination of the fibre orientation in composites using the structure tensor and local X-ray transform. J. Mater. Sci. 2010, 45, 888–896.
- Manda, M.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan Gum-Hydroxyapatite Composite Hydrogels for Bone Tissue Engineering Marianthi. J. Tissue Eng. Regen. Med. 2012, 6, 1–31.
- Dumont, V.C.; Mansur, H.S.; Mansur, A.A.P.; Carvalho, S.M.; Capanema, N.S.V.; Barrioni, B.R. Glycol chitosan/nanohydroxyapatite biocomposites for potential bone tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 93, 1465–1478.
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T.E.L.L.; Maria, A.; et al. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408.
- Zhou, C.; Liu, S.; Li, J.; Guo, K.; Yuan, Q.; Sun, S.J. Collagen Functionalized With Graphene Oxide Enhanced Biomimetic Mineralization and In Situ Bone Defect Repair. ACS Appl. Mater. Interfaces 2018, 10, 44080–44091.
- Bradley, R.S.; Robinson, I.K.; Yusuf, M. 3D X-Ray Nanotomography of Cells Grown on Electrospun Scaffolds. Macromol. Biosci. 2017, 17, 1–8.
- Albertini, G.; Giuliani, A.; Komlev, V.; Moroncini, F.; Pugnaloni, A.; Pennesi, G.; Belicchi, M.; Rubini, C.; Rustichelli, F.; Tasso, R.; et al. Organization of Extracellular Matrix Fibers Within Polyglycolic Acid–Polylactic Acid Scaffolds Analyzed Using X-Ray Synchrotron-Radiation Phase-Contrast Micro Computed Tomography. Tissue Eng. Part C Methods 2009, 15, 403–412.
- Columbus, S.; Krishnan, L.K.; Krishnan, V.K. Relating pore size variation of poly (E-caprolactone) scaffolds to molecular weight of porogen and evaluation of scaffold properties after degradation. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 789–796.
- Lin, Q.; Andrew, M.; Thompson, W.; Blunt, M.J.; Bijeljic, B. Optimization of image quality and acquisition time for lab-based X-ray microtomography using an iterative reconstruction algorithm. Adv. Water Resour. 2018, 115, 112–124.
- Offeddu, G.S.; Ashworth, J.C.; Cameron, R.E.; Oyen, M.L. Structural determinants of hydration, mechanics and fluid flow in freeze-dried collagen scaffolds. Acta Biomater. 2016, 41, 193–203.
- Shepherd, J.H.; Vriend, E.S.; Best, S.M.; Cameron, R.E. Analysis of structurally variable lyophilized collagen scaffolds for cell sieving using micro-CT. In Proceedings of the Micro-CT User Meeting 2018, Ghent, Belgium, 16–19 April 2018; pp. 182–186.
- Zitnay, J.L.; Reese, S.P.; Tran, G.; Farhang, N.; Bowles, R.D.; Weiss, J.A. Fabrication of dense anisotropic collagen scaffolds using biaxial compression. Acta Biomater. 2018, 65, 76–87.
- Crica, L.E.; Wengenroth, J.; Tiainen, H.; Ionita, M.; Haugen, H.J. Enhanced X-ray absorption for micro-CT analysis of low density polymers. J. Biomater. Sci. Polym. Ed. 2016, 27, 805–823.
- Suchý, T.; Šupová, M.; Bartoš, M.; Sedláček, R.; Piola, M.; Soncini, M.; Fiore, G.B.; Sauerová, P.; Kalbáčová, M.H.; Monika, Š.; et al. Dry versus hydrated collagen scaffolds: Are dry states representative of hydrated states? J. Mater. Sci. Mater. Med. 2018, 29, 20.
- Bartos, M.; Suchý, T.; Tonar, Z.; Foltán, R.; Kalbacova, M.H. Micro-CT in tissue engineering scaffolds for bone regeneration: Principles and application. Ceram.-Silik. 2018, 62, 194–199.
- Withers, P.J.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Plessis, A. Du X-ray computed tomography. Nat. Rev. Methods Prim. 2021, 1, 1–18.




