Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Piyush Kumar Gupta | + 4409 word(s) | 4409 | 2021-11-24 07:39:48 | | | |
| 2 | Catherine Yang | -4 word(s) | 4405 | 2021-11-25 05:11:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gupta, P. Integrated Microbial Fuel Cell System. Encyclopedia. Available online: https://encyclopedia.pub/entry/16352 (accessed on 07 February 2026).
Gupta P. Integrated Microbial Fuel Cell System. Encyclopedia. Available at: https://encyclopedia.pub/entry/16352. Accessed February 07, 2026.
Gupta, Piyush. "Integrated Microbial Fuel Cell System" Encyclopedia, https://encyclopedia.pub/entry/16352 (accessed February 07, 2026).
Gupta, P. (2021, November 24). Integrated Microbial Fuel Cell System. In Encyclopedia. https://encyclopedia.pub/entry/16352
Gupta, Piyush. "Integrated Microbial Fuel Cell System." Encyclopedia. Web. 24 November, 2021.
Copy Citation
Microbial fuel cell is an exclusive treatment for the management of contaminated water and energy production based on microbe–electrode interaction. Although the technology has developed significantly in recent years, it is still far from being employed in the practical field. Scalability problems, including external and internal resistance, size challenges, and cathode problems, are the reasons for its confined efficiency. MFCs appear to have the potential to be integrated into other processes, such as sediment MFCs (SMFCs), desalination cells (DS), membrane biofilters, and constructed wetlands (CWs).
microbial fuel cell
1. Integrated MFC-Capacitive Deionization (MFC-CDI) System
Several technologies were integrated with MFC to attain maximum benefit in the form of improved wastewater effluent and energy production. Capacitive deionization (CDI) was one of the recent technologies assimilated with MFC through diverse assemblies.
The integrated MFC-CDI system was tested to associate deionization through energy production and sewage water treatment [1]. CDI is a potential deionization alternative that can be used to remove salt from an aqueous solution to increase freshwater sources [2]. This process depends on the electrosorption that accompanies the charge separation for accumulation and releases considerable quantities of ions with the help of porous carbon electrodes. The mechanism starts with the electrostatic segregation of the ions from the water. This is followed by the adsorption of the ions in the electrode–solution interface. The initiation of the procedure requires a potential gradient (usually less than 1.2 V) to be applied among the electrodes. As the potential difference required is very low, continuous flow MFCs were employed to improve the function of the CDI for the elimination of electrolytes. This integration of MFC-CDI can be used in the post-treatment of the effluent containing ionic pollutants such as PO43− and NO3−. Here, the MFC is used as a secondary treatment for NH+4 and the removal of organic carbon, along with electricity generation, whereas CDI is used to desalinate and decontaminate wastewater effluent [3], as illustrated in Figure 1.
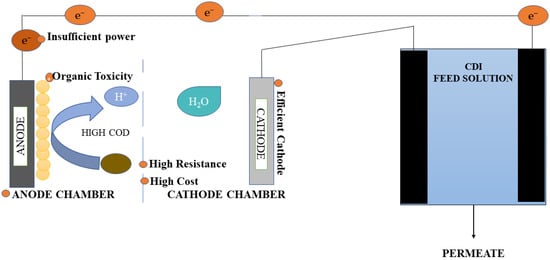
Figure 1. MFC-capacitive deionization (MFC-CDI) system.
The integration of dual MFCs in the corresponding assembly was used to power CDI in another setup, which resulted in a potential of 0.49 V. This aided in the removal of the multi-ionic species from the polluted water and also helped to gain an improved COD elimination of up to 90% [2]. An H-type MFC was functioned in batch mode and was used to supply exterior power to the CDI for less quantity of salt (60 mg/mL) treatment [4]. A salt elimination rate of 35.6 mg/L h was observed in this setup. The key factors influencing the desalination efficiency were ascertained by examining five different circuit configurations [5]. These factors were the internal resistance of the CDI and MFC, the capacitance of the CDI, and the open-circuit voltage of the MFC.
2. Membrane-Based MFCs and Membrane Bioreactor-Microbial Fuel Cells
MFCs were integrated with different types of bioreactors to improve the quality of the wastewater (Figure 2). One such method was the traditional activated sludge process integrated with an MFC [6]. The aeration tank was utilized as the cathodic compartment, and the catalyst was the developing aerobic biofilm. The biofilm is an inexpensive alternative for the catalyst and helps in reducing the overall cost of operating this integrated system. The continuous flow process was maintained by a clarifier, which was tailed to the aeration tank. However, extra costs were incurred due to the installation of the clarifier and the sludge pumping. As this integrated system faced obstacles during industrial scale-up, another setup integrating the MFCs with a sequencing batch reactor (SBR) was introduced. This approach was explored to estimate COD removal and energy production. This setup consisted of a biocathode-equipped membrane-less MFC combined with an SBR. This helps in the retrieval of energy as electricity from the aeration, hence reducing the operating cost for SBR operation [7].
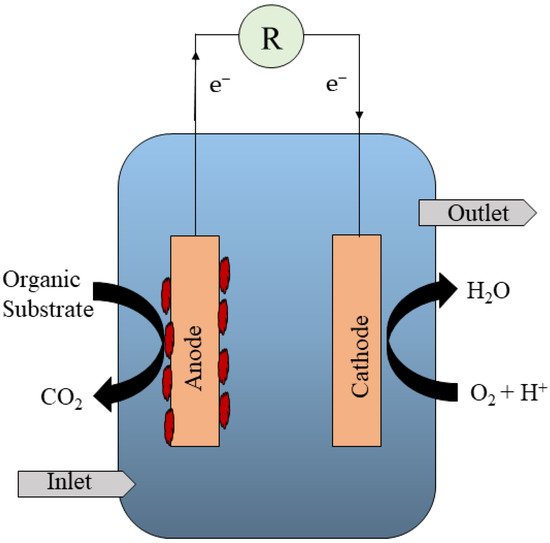
Figure 2. Representation of a typical MFC-integrated MBR reactor.
The MFC combined systems were used to examine different types of wastewaters. For example, a combined system of an up-flow anaerobic sludge blanket reactor, MFC, and biological aerated filter (UASB-MFC-BAF) was employed to treat molasses water [8]. In this arrangement, all three components complement each other by filling up the gaps where the others lack. Here, the UASB was used primarily for sulfate reduction and removal of COD. The resultant sulfide is oxidized by the use of the MFC, along with the production of energy. The phenolic compounds were decolorized and degraded in the BAF. The azo dye, Acid Orange 7 (AO-7), underwent decolorization in the MFC-integrated setup and was denatured into less poisonous complexes. The AO-7 was degraded by a reduction process in the MFC into the constituent aromatic amines [9]. Another system was devised for the degradation of Congo red, where the integration of the MFC and a catalytic oxygen reactor (COR) was used. In this system, Congo red was degraded with the help of oxidants in the existence of a catalyst [7].
These mesh membranes had the advantage of being simply stripped and washed without affecting the anode chamber [7]. A different study came up with a similar combination but using commercial cathode membranes (stainless steel net altered using a polypyrrole film treated with 9,10-anthraquinone-2-sulfonic acid (AQS)). This added to fouling alleviation and the electrocatalytic denaturation of the pollutants via the bioelectricity that is generated [10].
Several sorts of MBRs (fluidized bed, hollow fiber, tubular) were combined with MFCs. An MFC–tubular MBR system was assembled by integrating a biocathode-equipped MFC with a tubular membrane. Here, the MFC was functioning as a biosensor to monitor the COD in actual time. The combination of individual modules was used to attain contaminated water treatment and energy retrieval [11]. The MFC–MBR combination also helped in reducing the biofouling. This was achieved by merging a hollow fiber-membrane-based bioreactor with an MFC. The anodic chamber was an anaerobic compartment that was absorbed into the MBR, while the aerobic chamber of the MBR was used as the cathodic compartment, and in between these compartments [1], the module of the hollow fiber sheath was mounted. The introduction of an electrical field between both the electrodes improves the bacterial movement, which further increases the efficiency and superiority of the wastewater treatment. The electric field also helps in reducing membrane fouling by changing the properties of the sludge and impedes the adhering of negatively charged foulants to the membrane. A system assembled from the integration of an MFC with an electronic MBR (EMBR) was reported. In this setup, the cost was reduced by replacing the expensive proton exchange membrane (PEM) with a quartz silt compartment [12]. The bioelectricity produced in the EMBR was used to successfully hamper the membrane fouling. The electrolysis procedure was magnificently merged with the MFC for a broad spectrum of applications, such as methyl-red-contaminated water and algal pollution management [13]. The electrolysis pretreatment positively enhanced the MBR degradability and removal of chlorophyll a in these systems.
Another successful integration was achieved, which was composed of an MFC and hydrogen bioreactors. Here, the effluent from the hydrogen bioreactor was used as an influent in the MFC for the production of energy and the treatment of sewage water [14]. It was detected that the H2 yield was inversely proportional to the organic loading rate (OLR), with the maximum value of hydrogen production reached (2.72 mol H2/mol glucose) when the OLR was lowest (4 g/L d). It also gained the highest energy output of 4200 mW/m3. By combining this method with solid–liquid separation, it was possible to produce value-added biochemicals [15]. Using this combined system, 214 L of methane and 37.7 L of hydrogen were obtained. An amount of 0.3 kg of solid particles/kg of the effluent was acquired using an efficient solid–liquid fractionation process. The solid mass obtained thus displayed features of fertilizers. The supernatant was utilized by the MFC for generating electricity; this supplied the energy demands of the solid–liquid separation and reduced the organic content of the wastewater.
When a tubular membrane was installed in the MFC-MBR-integrated setup [16], a power output of 40 mW/m2 was attained. The setup ran for 30 days, and it was capable of achieving 94% elimination of organic matter and 80% removal of ammonia nitrogen. Another setup was assembled where an EMBR was integrated with an MFC, and the PEM was substituted with a quartz sand compartment [12]. This system achieved remarkable results with regard to the elimination of ammonia nitrogen (93%), phosphorus (50%), and organic matter (97%). The generation of electricity was also improved simultaneously. The MFC-MBR setup was examined as a sensor for an MBR. The competence of the system as a sensor was determined based on an undeviating relationship with a COD of up to 1 g/L. One more MFC-EMBR integration was used for methyl red decolorization and the simultaneous generation of electricity [17]. This system was able to achieve a COD elimination efficacy of 89.3% and 100% decolorization efficiency.
To explore the influence of implementing a process control on petroleum refinery wastewater treatment, a two-stage MFC-MBR-integrated system was built by Zhao et al. [18]. Chemical oxygen demand (COD), ammonium nitrogen (NH4+-N), and total nitrogen (TN) extraction efficiency in the MFC-MBR system were 96.3%, 92.4%, and 86.6%, respectively, compared with 74.7%, 71.2%, and 64.7% in the control system. Further, the use of this system as a biosensor was examined.
The two-stage combined MFC-aerobic membrane bioreactor (MBR)-based wastewater treatment method along with the use of ruthenium/activated carbon (Ru/AC) as a cathode catalyst was studied in another experiment by Bhowmick et al. [19]. The maximum volumetric power density and coulombic efficiency of the MFC-MBR system with Ru/AC as a cathode catalyst (2.7 Wm–3 and 12.8 ± 1.2%, respectively) were almost 1.4 and 1.5 times higher than those of the control MFC-MBR system without Ru/AC (2.0 Wm−3 and 8.2 ± 0.6%). Furthermore, these integrated MFC-MBR systems removed more than 96% of chemical oxygen demand (COD) from synthetic wastewater with an initial COD of roughly 1 g·L–1. This integrated MFC-MBR system has a lot of potential for being developed into a full-scale application that can provide efficient wastewater treatment and bio-energy recovery.
3. FO-MFC Integration
Osmotic MFCs (OsMFCs) are a result of the integration of the MFC with the FO membrane [20]. This setup has also been examined in numerous research studies. Forward osmosis membranes were employed in the MFC to eliminate salt while simultaneously achieving COD subtraction and generation of bioelectricity [21]. The design of the setup included both internal and external placements of the membrane. When the membrane is placed internally, it is either installed between the electrodes as a separator or submerged in the anode/cathode compartment as a purification unit used to divide the draw and feed solutions [22]. The water passes from the anode to the cathode compartment via the FO membrane because of the osmotic pressure gradient, which leads to the dilution of saltwater. In the externally placed setup, the MFC and the membrane unit can work independently.
4. Integration of MFCs with Dark Fermentation
A hybrid procedure of dark fermentation, microbial fuel cells, and microbial electrolysis cell could convert crude glycerol, a waste by-product obtained during biodiesel manufacturing, to bioenergy (MEC). This combined technique addresses the thermodynamic constraint of dark fermentation, where complete breakdown of crude glycerol is challenging [23]. Large percentages of unutilized organic metabolites remain in the dark fermenter’s effluent, which can be used to produce power in MFCs or hydrogen in microbial electrolysis cells. The greatest H2 generation of 332 mL/L (output of 0.55 mol H2/mol glycerol) was attained when crude glycerol with an initial carbon oxygen demand concentration of 7610 mg/L was used in dark fermentation. After 50% dilution, the sewage was deteriorated in MFCs to reach a power output of 92 mW/m2 and a carbon oxygen demand elimination of 49% [24].
MFCs were also integrated with dark fermentation (DF) for H2 generation via an anaerobic hydrogen production process. The waste from the hydrogen fermentation is acidic and was reacted further in the MFCs to increase the entire electricity generation and facilitate COD exclusion efficiency [12]. This system was examined to acquire marketable chemicals and fuels, such as biofertilizers, hydrogen, and methane, for bioelectricity [15].
An innovative integrated single-stage dark-fermentation-MFC method was designed in an experiment to produce biohydrogen and power from wastewater treatment at the same time. The integrated system’s biohydrogen gas was converted into energy using a proton-exchange membrane fuel cell (PEMFC). The impact of hydraulic retention time (HRT) on biohydrogen and electricity production was also investigated. At an 8 day HRT, the greatest volumetric biohydrogen production rate (VHPR) was 0.44 L H2/L·d (0.66 L H2/g COD removed), with an electricity output of 530 mV (100 mW/m2). The bacteria discovered on the anode of the integrated system were Chryseobacterium, Azotobacter, Bacillus, Enterococcus, Citrobacter, and Methanobacterium, per an 16S rRNA gene-based investigation. The PEMFC employed to generate voltage from biohydrogen produced by the integrated system was capable of achieving a maximum voltage of 459 mV (367 mW) and a maximum cell efficacy of 44% (fuel consumption of 1.5 × 105 mol/h) [25].
A hybrid approach of dark fermentation (DF) and MFC known as sDFMFC was examined in the study for simultaneous H2 and electricity production from Saccharina japonica in a single reactor. Due to simultaneous H2/carboxylic acid (CA) synthesis by DF and electricity production by MFC, the energy recovery of the sDFMFC was significantly improved. A time course of CA concentration in sDFMFC validated the coproduction of H2 and power. With an H2 yield of 110 mL/g-VS and a maximum power density of 1.82 W/m2, S. japonica provided good energy recovery of 17.3%. The sDFMFC demonstrated a diversified microbial population for optimal organic substrate microbial conversion. sDFMFC could be a promising single reactor process for producing H2 and energy from a variety of biomass feedstocks while maintaining the individual DF and MFC processes’ efficiency [26].
5. Sediment Microbial Fuel Cells
MFCs can be integrated into a variety of technologies, including sediment microbial fuel cells, which have the same arrangement as a conventional MFC. The anode (anaerobic) can be immersed in the sediment, and the cathode (aerobic) can be found at the top part of the SMFC, where oxygen acts as a terminal electron acceptor (Figure 3). More intriguingly, it was not discovered until 2001 that bacteria can donate their electrons exogenously. By embedding an MFC in an oceanic sediment, Reimers et al. [27] investigated the feasibility of extracellular electron transmission. They explored exoelectrogenic electron transport by inserting an electrode in a marine sediment and harvesting energy from it. The anode component of SMFCs was inserted into the sediment (anaerobic) region of the ocean, while the cathode was placed at the water surface. Exoelectrogenic microbes are already active in the sediment, according to Reimers et al., and can give electrons to the electrode in anaerobic environments. The electrons from the sediment can be transmitted to the cathode, which already contains dissolved oxygen from the surface water and can act as a terminal electron acceptor. From the initial SMFCs, they were able to attain an energy output of 50 mW/m2. The aerobic and anaerobic portions of all categories of SMFCs were configured based on the surface and sediment, respectively.
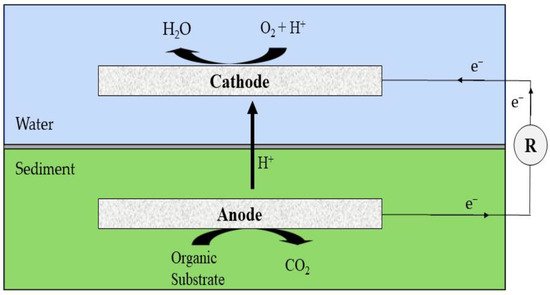
Figure 3. A typical integrated sediment MFC.
Different types of sediment MFCs, such as benthic MFCs (BMFCs), floating-macrophyte-dependent MFCs (FMFCs), and soil-based MFCs (SL-MFCs), were developed with time [7]. BMFCs are also an advanced use of SMFCs in an aquatic environment, especially for bioremediation of contaminants, such as sulfur and organic matter in the sediments. The contaminants in the BMFC sediment aid as a source of energy for bacteria, and the oxidation of contaminants in the sediment and the decrease in oxygen at the surface produce power [28][29]. As previously stated, constant monitoring of produced voltage and retention of power from BMFCs can also be used as a monitoring system in the sea [30].
The system design of FMFCs is also influenced by those of sediment MFCs and plant MFCs (PMFCs). Plants release rhizodeposits (organics and nutrients) in the sediment of PMFCs. The microorganisms use secretory rhizodeposits as a fuel in the sediment and oxygen as a reductant at the surfaces and subsequently generate energy. Mohan et al. [31] introduced FMFCs to remove excessive volatile fatty acids (VFAs) and residual organic matter from an H2 bioreactor’s outflow. COD elimination was around 86.67%, and VFA removal was around 72.32%, respectively. The power production was shown to accelerate as COD increased, reaching 224.93 mA/m2 in some cases.
6. Integration of MFC with MDC
Desalination is another approach for the treatment of contaminated water and potable water production. Distillation, electrodialysis, and reverse osmosis are the methods used in desalination [32]. It is believed that desalination is not applicable in all circumstances due to the high energy requirements and maintenance cost [20]. MFCs have the potential to be integrated into desalination cells due to their advantages in the synthesis of renewable energy, such as CH4, H2, and electric power. In 2009, Cao along with his co-workers [33] described a new type of desalination system known as microbial desalination cell, which is based on the movement of ions from water in accordance to the electron supplied by microbes. A desalination cell MFC with three chambers was constructed (as shown in Figure 4. An anion exchange and cation exchange membrane, as well as a central compartment, were included in the three-chambered DS-MFCs. In the anode chamber, bacteria decompose contaminants and yield power, while negatively charged ions pass through a membrane from the central chamber to the anodic chamber. The cathode utilizes protons in the same way as that in typical MFCs, and a positive charge from the central compartment is transferred to the cathodic compartment. Water in the central compartment is desalinated, and contaminated water is treated at the same time [34].
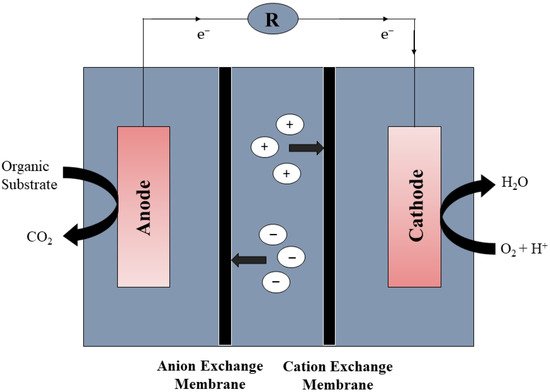
Figure 4. General representation of MDC-MFCs.
For synthetic wastewater and saline sewage treatment, Zhang and He [35] devised an osmotic MFC with an FO membrane coupled with a microbial desalination cell (MDC). The research achieved an energy output of 0.160 kW h/m3 and an electrical conductance decline of 95.9%. Due to dilution and desalination, the system was demonstrated to be an excellent technology for salt elimination. Zhang and He [35] used 105 L MDC to test the scaling up of the system. The research revealed that several feeding inlets could improve the current production. The current production improved from 670 to 2000 mA with a salt elimination of 3.7 to 9.2 kg/h when external power was applied (m3 day). Sevda and Abu-Reesh [36] employed organics from petroleum effluent for an MFC and used osmotic MFCs with up-flow microbial desalination cell for marine treatment.
7. Integrated Constructed Wetland MFC
Yadav (2010) introduced a hybrid method that combines an MFC with a CW to improve the treatment effectiveness of the CW while reducing the required landmass. It has found wide acceptance from scientists all over the world over the last 7–8 years. Because the majority of the component in the CW is anaerobic, it has low treatment efficiency (less electron acceptor). As a result, the primary purpose of integrating the MFC into the CW is to improve the CW’s treatment potential (Figure 5). However, power generation has recently been discovered to be another CW resource [37].
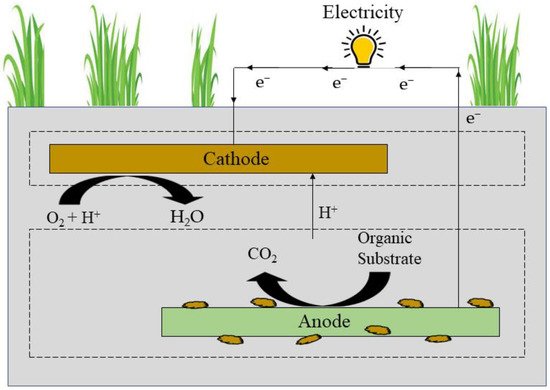
Figure 5. A typical representation of constructed wetland MFCs.
An up-flow constructed wetland-MFC system with various fillers was designed for the removal of Cr (VI) and simultaneous production of energy. These fillers, such as bio-ceramic (CW-MFC1), zeolite (CW-MFC2), calcite (CW-MFC3), and volcanic rock (CW-MFC4), are able to absorb contaminants over a long period of time. All systems removed over 93% COD, and the rate of Cr (VI) removal was as follows: CW-MFC4 (99.0%) > CW-MFC2 (95.5%) > CW-MFC3 (89.7%) > CW-MFC1 (72.2%). Due to abundance of organic substance absorbed by microbes in the filler layer, which weakens the action of anodic microbes, bio-ceramic is a simple way to immobilize microorganisms, which as a filler (CW-MFC1) showed the lowest removal rate of Cr (VI) in the CW-MFC1 system. Furthermore, calcite, which has a reduced surface area but a less porous structure than volcanic rock and zeolite, was unfavorable to microbial life, resulting in decreased Cr (VI) removal in the bottom layer of the CW-MFC3. The CW-MFC system’s output voltage and maximum energy density were in the subsequent order: CW-MFC3 > CW-MFC 4 > CW-MFC2 > CW-MFC1. As a result, using volcanic rock as fillers was the greatest option, as it permitted for the most Cr (VI) removal (99.0%) and the ideal power production (0.595–0.019 V output voltage, 0.462 W/m3 power density). Furthermore, due to the extensive Cr(VI) stress, microbial diversity in the cathode was higher than that in the anode, and Acetoanaerobium and Exiguobacterium were the leading genera in the anode and cathode, respectively [38].
8. Integration of Microbial Fuel Cells with Microalgae
The concept of microalgae MFC is dependent on substantial MFC and microalgae research. A combination of algae and MFCs will be a unique technology that converts solar energy to electric energy through the metabolic processes of photosynthetic microbes (Figure 6) [39].
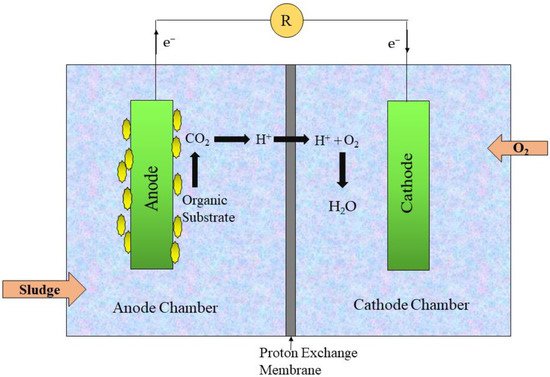
Figure 6. Illustration of algae-assisted cathode in MFC.
Likewise, MFCs are used in algal bioreactors, and the bases of microbial-fuel-cell-combined algal bioreactors are solar energy; hence, the existence of algal biomass turns solar energy into chemical energy. Furthermore, the accessible algae transform chemical energy into electrical energy. Photosynthetic fuel cells or solar cells are MFCs that have been integrated into algal bioreactors. Microbes oxidize organics at the anodic compartment, and algae release oxygen at the cathode to meet the need for aeration in the alternative arrangement of algal MFCs. As a result, it is regarded as passive aeration from algae, which provides an oxidant to the cathode for the anode’s reaction accomplishment [40].
Furthermore, the oxygen generated by Chlorella vulgaris during photosynthesis in the cathodic chamber of MFCs could act as the terminal electron acceptor. Therefore, the integrated system can be used to sequester carbon dioxide, produce oxygen, and remove nitrogen from contaminated water [41].
In another technique, dead microalgae were employed as the substrate in the anode compartment of MFCs, while viable algae were introduced in the cathode chamber. When the conductive cathode was fixed with multiple strains of algae, such as C. vulgaris, Dunaliella tertiolecta, and Synechocystis sp., the energy production of single-chambered photosynthetic MFCs was detected. When irradiated with 10 W/m2 of white light, an MFC containing Synechococcus sp. had a maximum energy output of 10.3 mW/m2. A serial arrangement of four MFCs can produce enough energy to run a commonly available tiny digital clock (currently around 10 mA and a potential of 2 V) [42][43].
An anoxic MFC was generated using dissolved CO2 as an electron acceptor and a photobiocathode that was activated. The MFC achieved an energy output of 750 mW/m2 after this biocathode efficiently fixed CO2 [44]. The MFC was used in conjunction with a tubular PHB as the cathode chamber, which was colonized with C. vulgaris. The amount of oxygen produced by C. vulgaris and the amount of power produced by the MFC were both light-dependent. Under inconsistent illumination, an MFC with an algal biocathode attained the highest energy output of 24.4 mW/m2, which was 2.8 times greater than the MFC with an abiotic cathode [45].
During the anaerobic digestion of Taihu blue algae, biohydrogen employing migrating ammonia as a nitrogen supply and biogas upgrading using hydrogen collected at the biocathode in an integrated bioelectrochemical system (BES) were examined by Wu et al. [46]. The use of an integrated BES allowed for simultaneous ammonia reduction and biogas upgrading. It was observed that under a 0.4 V applied voltage, ammonia utilization and hydrogen production achieved 73.67% and 202.87 mL, respectively. As a result, performing ammonia mitigation, hydrogen production, and biogas upgrading all at the same time with BES appears to be an effective approach.
9. Anaerobic-Anoxic-Oxic (AO/O) Integrated with MFC
An MFC was also integrated into an anaerobic-anoxic-oxic (AO/O) system, resulting in a hybrid system with a total volume of 1 m3 (Figure 7) for treatment of domestic wastewater and power generation at the same time [47]. During its 1-year operation, a maximum current density of 3.6 mW/m3 and a COD removal of 95% were reached under an HRT of 18 h, a temperature of 8–23 °C, and a recirculation ratio of 200%. Tang et al. [48] combined an MFC with a constructed wetland (CW), resulting in an energy recovery of 0.448 W/m3 and a COD reduction of 92% while treating dewatered alum sludge.
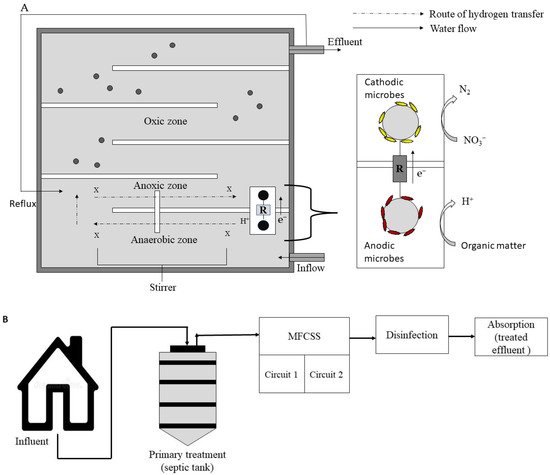
Figure 7. (A) Integrated MFC-AA/O system for wastewater treatment; (B) integrated MFC septic tank for wastewater treatment.
Notably, Valladares Linares et al. [49] established the feasibility and long-term viability of a linked septic-tank-MFC-disinfection system for residential wastewater treatment. As shown in Figure 7, raw influent from a five-person residence flowed by gravity to a 1300 L septic tank, then to a 700 L Aquox® MFC comprising two stacks of 9 MFC units, and lastly to a sodium hypochlorite disinfection system. An ultralow power consumption unit made up of capacitors and microcontrollers gathered and stored energy from the MFC. Because no external energy was required, the system was declared sustainable and viable, and the treated effluent met the local discharge standard.
10. Other Integrated MFCs
In this system, the potential of COD removal was 53.2%, and the supreme energy output reached was 1410.2 mW/m2. The absolute denaturation of the azo dye AO-7 was obtained through the integration of an MFC and an anaerobic bioreactor [9]. The dual-staged technique was used to achieve a decrease in ecological toxicity and reach 90% COD removal along with the simultaneous generation of bioelectricity. An MFC-COR setup packed with a granular catalyst was used to obtain degradation of the azo dye, Congo red [7]. An amount of 90% of the dye was observed to have degraded in 3 days in a chargeless mixture, and the maximum power density reached was 808.3 mW/m3.
In a plug flow environment, Feng et al. [50] combined a number of MFCs in a stacked configuration. With a total volume of 250 L, the flow was horizontal. While treating residential wastewater, the maximum energy obtained in each module was 0.435–0.010 A, with carbon oxygen demand and total nitrogen (TN) elimination rates of 79 ± 67% and 71 ± 68%, respectively. Moreover, MFC has been applied to a number of other procedures, such as electro-Fenton reactions, to improve treatment efficiency. The strategy for integrating MFCs to improve electro-Fenton reactions is to add hydroxyl ions to the Fenton reactions to speed up the processes. Many studies have shown the generation of H2O2 at the cathode of MFCs. The generation of H2O2 at the cathode can be accomplished using two or four electron transmission processes, whereas in electro-Fenton reactions, ferrous ions are oxidized to ferric ions using oxidants (Figure 8).
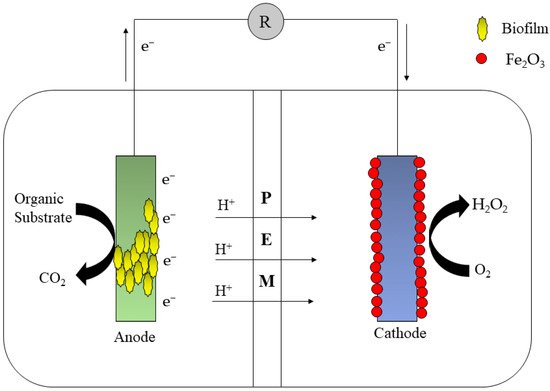
Figure 8. MFC integrated with bio-electro-Fenton.
During the operation, the highest COD elimination of 90%, a nitrate reduction of 76.8%, and an energy output of 3.4 mW/m2 were attained. Composting was eventually performed with solid waste [51]. A three-column MFC stacking structure that can be easily plugged in a septic tank to yield an energy output of 142 mW/m2 was devised. This system is predicted to have a daily power consumption of 24 W/h, which is enough to run a 6 W LED bulb for 4 h. Similarly, 15 MFCs were stacked in a 2.44 m3 septic tank and linked to a power management circuit, finally discharging the highest current of 1.98 mA and power of 4.51 mW [52].
References
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alex. Eng. J. 2016, 55, 427–443.
- Liu, J.; Wang, X.; Wang, Z.; Lu, Y.; Li, X.; Ren, Y. Integrating Microbial Fuel Cells with Anaerobic Acidification and Forward Osmosis Membrane for Enhancing Bio-Electricity and Water Recovery from Low-Strength Wastewater. Water Res. 2017, 110, 74–82.
- Lu, Y.; Bian, X.; Wang, H.; Wang, X.; Ren, Y.; Li, X. Simultaneously Recovering Electricity and Water from Wastewater by Osmotic Microbial Fuel Cells: Performance and Membrane Fouling. Front. Environ. Sci. Eng. 2018, 12, 5.
- Yuan, L.; Yang, X.; Liang, P.; Wang, L.; Huang, Z.-H.; Wei, J.; Huang, X. Capacitive Deionization Coupled with Microbial Fuel Cells to Desalinate Low-Concentration Salt Water. Bioresour. Technol. 2012, 110, 735–738.
- Ren, L.; Ahn, Y.; Logan, B.E. A Two-Stage Microbial Fuel Cell and Anaerobic Fluidized Bed Membrane Bioreactor (MFC-AFMBR) System for Effective Domestic Wastewater Treatment. Environ. Sci. Technol. 2014, 48, 4199–4206.
- Cha, J.; Choi, S.; Yu, H.; Kim, H.; Kim, C. Directly Applicable Microbial Fuel Cells in Aeration Tank for Wastewater Treatment. Bioelectrochemistry 2009, 78, 72–79.
- Liu, X.-W.; Wang, Y.-P.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zeng, R.J.; Li, F.; Dong, F.; Wang, S.-G.; Tong, Z.-H.; et al. Integration of a Microbial Fuel Cell with Activated Sludge Process for Energy-Saving Wastewater Treatment: Taking a Sequencing Batch Reactor as an Example. Biotechnol. Bioeng. 2011, 108, 1260–1267.
- Wang, A.; Sun, D.; Cao, G.; Wang, H.; Ren, N.; Wu, W.-M.; Logan, B.E. Integrated Hydrogen Production Process from Cellulose by Combining Dark Fermentation, Microbial Fuel Cells, and a Microbial Electrolysis Cell. Bioresour. Technol. 2011, 102, 4137–4143.
- Fernando, E.; Keshavarz, T.; Kyazze, G. Complete Degradation of the Azo Dye Acid Orange-7 and Bioelectricity Generation in an Integrated Microbial Fuel Cell, Aerobic Two-Stage Bioreactor System in Continuous Flow Mode at Ambient Temperature. Bioresour. Technol. 2014, 156, 155–162.
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The Controversial Antibacterial Activity of Graphene-Based Materials. Carbon 2016, 105, 362–376.
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A Novel UASB-MFC-BAF Integrated System for High Strength Molasses Wastewater Treatment and Bioelectricity Generation. Bioresour. Technol. 2009, 100, 5687–5693.
- Gao, C.; Liu, L.; Yang, F. Development of a Novel Proton Exchange Membrane-Free Integrated MFC System with Electric Membrane Bioreactor and Air Contact Oxidation Bed for Efficient and Energy-Saving Wastewater Treatment. Bioresour. Technol. 2017, 238, 472–483.
- Asif, M.B.; Maqbool, T.; Zhang, Z. Electrochemical Membrane Bioreactors: State-of-the-Art and Future Prospects. Sci. Total Environ. 2020, 741, 140233.
- Ajayi, F.F.; Kim, K.-Y.; Chae, K.-J.; Choi, M.-J.; Chang, I.S.; Kim, I.S. Optimization Studies of Bio-Hydrogen Production in a Coupled Microbial Electrolysis-Dye Sensitized Solar Cell System. Photochem. Photobiol. Sci. 2010, 9, 349–356.
- Manzini, E.; Scaglia, B.; Schievano, A.; Adani, F. Dark Fermentation Effectiveness as a Key Step for Waste Biomass Refineries: Influence of Organic Matter Macromolecular Composition and Bioavailability. Int. J. Energy Res. 2015, 39, 1519–1527.
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of Carbon Mesh Anodes and the Effect of Different Pretreatment Methods on Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6870–6874.
- Mu, Y.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Decolorization of Azo Dyes in Bioelectrochemical Systems. Environ. Sci. Technol. 2009, 43, 5137–5143.
- Zhao, S.; Yun, H.; Khan, A.; Salama, E.-S.; Redina, M.M.; Liu, P.; Li, X. Two-Stage Microbial Fuel Cell (MFC) and Membrane Bioreactor (MBR) System for Enhancing Wastewater Treatment and Resource Recovery Based on MFC as a Biosensor. Environ. Res. 2022, 204, 112089.
- Bhowmick, G.D.; Ghangrekar, M.M.; Banerjee, R. Improved Wastewater Treatment by Using Integrated Microbial Fuel Cell-Membrane Bioreactor System Along with Ruthenium/activated Carbon Cathode Catalyst to Enhance Bio-energy Recovery. In Climate Impacts on Water Resources in India: Environment and Health; Pandey, A., Mishra, S.K., Kansal, M.L., Singh, R.D., Singh, V.P., Eds.; Water Science and Technology Library; Springer: New York, NY, USA, 2021; pp. 183–192. ISBN 978-3-030-51427-3.
- Savla, N.; Pandit, S.; Khanna, N.; Mathuriya, A.S.; Jung, S.P. Microbially Powered Electrochemical Systems Coupled with Membrane-Based Technology for Sustainable Desalination and Efficient Wastewater Treatment. J. Korean Soc. Environ. Eng. 2020, 42, 360–380.
- Zhang, B.; He, Z. Integrated Salinity Reduction and Water Recovery in an Osmotic Microbial Desalination Cell. RSC Adv. 2012, 2, 3265–3269.
- Rozendal, R.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effects of Membrane Cation Transport on PH and Microbial Fuel Cell Performance. Environ. Sci. Technol. 2006, 40, 5206–5211.
- Keskin, T.; Abo-Hashesh, M.; Hallenbeck, P.C. Photofermentative Hydrogen Production from Wastes. Bioresour. Technol. 2011, 102, 8557–8568.
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-Stage Conversion of Crude Glycerol to Energy Using Dark Fermentation Linked with Microbial Fuel Cell or Microbial Electrolysis Cell. New Biotechnol. 2014, 31, 179–184.
- Estrada-Arriaga, E.B.; Hernández-Romano, J.; Mijaylova-Nacheva, P.; Gutiérrez-Macías, T.; Morales-Morales, C. Assessment of a Novel Single-Stage Integrated Dark Fermentation-Microbial Fuel Cell System Coupled to Proton-Exchange Membrane Fuel Cell to Generate Bio-Hydrogen and Recover Electricity from Wastewater. Biomass Bioenergy 2021, 147, 106016.
- Gebreslassie, T.R.; Nguyen, P.K.T.; Yoon, H.H.; Kim, J. Co-Production of Hydrogen and Electricity from Macroalgae by Simultaneous Dark Fermentation and Microbial Fuel Cell. Bioresour. Technol. 2021, 336, 125269.
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting Energy from the Marine Sediment−Water Interface. Environ. Sci. Technol. 2001, 35, 192–195.
- Fadzli, F.S.; Rashid, M.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Electricity Generation and Heavy Metal Remediation by Utilizing Yam (Dioscorea Alata) Waste in Benthic Microbial Fuel Cells (BMFCs). Biochem. Eng. J. 2021, 172, 108067.
- Daud, N.N.M.; Ahmad, A.; Yaqoob, A.A.; Ibrahim, M.N.M. Application of Rotten Rice as a Substrate for Bacterial Species to Generate Energy and the Removal of Toxic Metals from Wastewater through Microbial Fuel Cells. Environ. Sci. Pollut. Res. Int. 2021, 1–12.
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; AlAmmari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135.
- Venkata Mohan, S.; Mohanakrishna, G.; Chiranjeevi, P. Sustainable Power Generation from Floating Macrophytes Based Ecological Microenvironment through Embedded Fuel Cells along with Simultaneous Wastewater Treatment. Bioresour. Technol. 2011, 102, 7036–7042.
- Zahid, M.; Savla, N.; Pandit, S.; Thakur, V.K.; Jung, S.P.; Gupta, P.K.; Prasad, R.; Marsili, E. Microbial Desalination Cell: Desalination through Conserving Energy. Desalination 2022, 521, 115381.
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152.
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Bio-Electrochemical Treatment of Distillery Wastewater in Microbial Fuel Cell Facilitating Decolorization and Desalination along with Power Generation. J. Hazard. Mater. 2010, 177, 487–494.
- Zhang, B.; He, Z. Improving Water Desalination by Hydraulically Coupling an Osmotic Microbial Fuel Cell with a Microbial Desalination Cell. J. Membr. Sci. 2013, 441, 18–24.
- Zhang, F.; He, Z. Scaling up Microbial Desalination Cell System with a Post-Aerobic Process for Simultaneous Wastewater Treatment and Seawater Desalination. Desalination 2015, 360, 28–34.
- Yadav, A.K.; Abbassi, R.; Kumar, N.; Satya, S.; Sreekrishnan, T.R.; Mishra, B.K. The Removal of Heavy Metals in Wetland Microcosms: Effects of Bed Depth, Plant Species, and Metal Mobility. Chem. Eng. J. 2012, 211–212, 501–507.
- Mu, C.; Wang, L.; Wang, L. Removal of Cr(VI) and Electricity Production by Constructed Wetland Combined with Microbial Fuel Cell (CW-MFC): Influence of Filler Media. J. Clean. Prod. 2021, 320, 128860.
- Bombelli, P.; Bradley, R.W.; Scott, A.M.; Philips, A.J.; McCormick, A.J.; Cruz, S.M.; Anderson, A.; Yunus, K.; Bendall, D.S.; Cameron, P.J.; et al. Quantitative Analysis of the Factors Limiting Solar Power Transduction by Synechocystis Sp. PCC 6803 in Biological Photovoltaic Devices. Energy Environ. Sci. 2011, 4, 4690–4698.
- Xu, L.; Zhao, Y.; Doherty, L.; Hu, Y.; Hao, X. The Integrated Processes for Wastewater Treatment Based on the Principle of Microbial Fuel Cells: A Review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 60–91.
- Kakarla, R.; Min, B. Photoautotrophic Microalgae Scenedesmus Obliquus Attached on a Cathode as Oxygen Producers for Microbial Fuel Cell (MFC) Operation. Int. J. Hydrogen Energy 2014, 39, 10275–10283.
- Kondaveeti, S.; Choi, K.S.; Kakarla, R.; Min, B. Microalgae Scenedesmus Obliquus as Renewable Biomass Feedstock for Electricity Generation in Microbial Fuel Cells (MFCs). Front. Environ. Sci. Eng. 2014, 8, 784–791.
- Rashid, N.; Cui, Y.-F.; Saif Ur Rehman, M.; Han, J.-I. Enhanced Electricity Generation by Using Algae Biomass and Activated Sludge in Microbial Fuel Cell. Sci. Total Environ. 2013, 456–457, 91–94.
- Cao, X.; Huang, X.; Liang, P.; Boon, N.; Fan, M.; Zhang, L.; Zhang, X. A Completely Anoxic Microbial Fuel Cell Using a Photo-Biocathode for Cathodic Carbon Dioxide Reduction. Energy Environ. Sci. 2009, 2, 498–501.
- Wu, X.; Song, T.; Zhu, X.; Wei, P.; Zhou, C.C. Construction and Operation of Microbial Fuel Cell with Chlorella Vulgaris Biocathode for Electricity Generation. Appl. Biochem. Biotechnol. 2013, 171, 2082–2092.
- Wu, H.; Wang, H.; Zhang, Y.; Antonopoulou, G.; Ntaikou, I.; Lyberatos, G.; Yan, Q. In Situ Biogas Upgrading via Cathodic Biohydrogen Using Mitigated Ammonia Nitrogen during the Anaerobic Digestion of Taihu Blue Algae in an Integrated Bioelectrochemical System (BES). Bioresour. Technol. 2021, 341, 125902.
- Liu, R.; Tursun, H.; Hou, X.; Odey, F.; Li, Y.; Wang, X.; Xie, T. Microbial Community Dynamics in a Pilot-Scale MFC-AA/O System Treating Domestic Sewage. Bioresour. Technol. 2017, 241, 439–447.
- Tang, C.; Zhao, Y.; Kang, C.; Yang, Y.; Morgan, D.; Xu, L. Towards Concurrent Pollutants Removal and High Energy Harvesting in a Pilot-Scale CW-MFC: Insight into the Cathode Conditions and Electrodes Connection. Chem. Eng. J. 2019, 373, 150–160.
- Valladares Linares, R.; Domínguez-Maldonado, J.; Rodríguez-Leal, E.; Patrón, G.; Castillo-Hernández, A.; Miranda, A.; Diaz Romero, D.; Moreno-Cervera, R.; Camara-chale, G.; Borroto, C.G.; et al. Scale up of Microbial Fuel Cell Stack System for Residential Wastewater Treatment in Continuous Mode Operation. Water 2019, 11, 217.
- Yang, Z.-Z.; Zhu, C.-X.; Tang, Y.-L.; Li, H.-N. Research Progresses in Microbial Fuel Cells for Antibiotic Wastewater Treatment. Chin. J. Agrometeorol. 2020, 41, 275.
- Yazdi, H.; Alzate-Gaviria, L.; Ren, Z.J. Pluggable Microbial Fuel Cell Stacks for Septic Wastewater Treatment and Electricity Production. Bioresour. Technol. 2015, 180, 258–263.
- Alzate-Gaviria, L.; García-Rodríguez, O.; Flota-Bañuelos, M.; Del Rio Jorge-Rivera, F.; Cámara-Chalé, G.; Domínguez-Maldonado, J. Stacked-MFC into a Typical Septic Tank Used in Public Housing. Biofuels 2016, 7, 79–86.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
25 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




