Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ashraf S. S Gorgey | + 2804 word(s) | 2804 | 2021-11-24 09:03:21 | | | |
| 2 | Dean Liu | Meta information modification | 2804 | 2021-11-25 02:08:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gorgey, A.S. Electrical Stimulation After Spinal Cord Injury. Encyclopedia. Available online: https://encyclopedia.pub/entry/16349 (accessed on 07 February 2026).
Gorgey AS. Electrical Stimulation After Spinal Cord Injury. Encyclopedia. Available at: https://encyclopedia.pub/entry/16349. Accessed February 07, 2026.
Gorgey, Ashraf S.. "Electrical Stimulation After Spinal Cord Injury" Encyclopedia, https://encyclopedia.pub/entry/16349 (accessed February 07, 2026).
Gorgey, A.S. (2021, November 24). Electrical Stimulation After Spinal Cord Injury. In Encyclopedia. https://encyclopedia.pub/entry/16349
Gorgey, Ashraf S.. "Electrical Stimulation After Spinal Cord Injury." Encyclopedia. Web. 24 November, 2021.
Copy Citation
Electrical stimulation is used to enhance the skills of reaching, grasping, standing, and walking, among other activities of daily living.
rehabilitation
exercise
neurological
spinal cord injuries
1. Introduction
Disability following spinal cord injury (SCI) is primarily determined by the level and completeness of the injury. The higher the level of injury, the less sensory and motor activity available for physical tasks, including those related to activities involved with daily living. Likewise, complete injuries limit physical activity abilities more than incomplete injuries. However, other factors can compromise the level of physical functioning, including dramatic changes in body composition and muscle tone. One therapeutic approach with the potential to address the myriad of sensory, motor, and autonomic impairment characteristics of SCI is electrical stimulation. Both non-invasive and invasive applications of electric currents to the body have been developed and studied over the past four decades, resulting in considerable evidence and guidance to support the clinical use of electrical stimulation.
2. Optimizing Electrical Stimulation Training Parameters
Fornusek et al. investigated the differing effects between high and low-cadence FES cycling. One leg performed FES cycling at a 10 rev/min−1 cadence, while the other leg performed a cadence of 50 rev/min−1 [1]. Both legs significantly increased in thigh girth, demonstrating muscle hypertrophy. However, after six weeks of training three times per week, the low-cadence leg demonstrated a significantly greater girth circumference and electrically evoked isometric torque. A similar observation was noted following 6 months of FES cycling on muscle volume, trabecular bone parameters, and biomarkers of bone turnover in adults with SCI. Participants in the low-cadence group cycled at a maximal average torque of 2.9 ± 2.8 Nm and the high-cadence group cycled at a maximal average torque of 0.8 ± 0.2 Nm. Low-cadence cycling showed greater decreases in bone-specific alkaline phosphatase. N-telopeptide decreased 34% following low-cadence cycling, suggesting a decrease in bone resorption. Both groups increased muscle volume (low-cadence cycling by 19%, high-cadence cycling by 10%). Low-cadence cycling resulted in a 7% non-significant increase in the apparent trabecular number and a 6% decrease in the apparent trabecular separation in the distal femur without noticeable changes in the high-cadence group [2].
Gregory and Bickel described the motor fiber recruitment pattern with electrical stimulation as non-selective and asynchronous, resulting in the early onset fatigue of the stimulated muscle groups [3]. Deley et al. studied the comparison of variable electrical frequencies and continuous frequency training in 10 individuals with SCI and found significantly less training fatigue with variable frequency training than continuous training [4]. This may indicate that interval training options are more effective for individuals with SCI. Gorgey et al. investigated the effects of three different pulse widths (200 µs, 350 µs, and 500 µs) during FES cycling over three weeks [5]. There was no difference in the peak oxygen uptake (VO2 peak) or energy expenditure (EE). However, exercising at 350 µs resulted in a greater difference between FES cycling and resting than at 200 µs. Gorgey et al. summarized that FES cycling at 350 µs may be better for EE. However, Gorgey also found that 500 µs elicited an autonomic dysreflexia response, while the lower levels did not. The authors also studied the effects on peak torque and muscle fatigue at different frequencies that ranged from 20 to 100 Hz. They noted that peak torque dropped by more than 50% after an acute bout of FES cycling and remained down by more than 20% following 72 h. of cycling, suggesting low frequency fatigue [6]. Based on these findings, the authors suggested to space the frequency of the training to twice weekly especially in chronic persons with less training experience in FES cycling [6].
Bochkezanian et al. used a different approach to the use of NMES investigating the effects of high-intensity strength training performed under isometric conditions using a lower frequency (30 Hz) and a higher pulse width (1000 µs) for 12 weeks on five adults with chronic SCI [7]. The participants were seated with the hip and knee joint angles at 85 and 90 degrees, respectively. Electrical stimulation was delivered to the legs separately every 20 s, starting at an electrical stimulation intensity of 30 mA and then increasing by 10 mA incrementally until a plateau in observed maximal peak twitch or 99 mA was reached. The results showed that after 12 weeks, the muscle strength and quadriceps cross-sectional area were significantly increased and subjective measures of spasticity were also ameliorated [7].
While no conclusions concerning optimal specific protocols have been determined, the evidence does support that, for NMES, higher intensity resistance training produces strong muscle contractions that result in significant gains in muscle strength and muscle mass in individuals with SCI [7]. Concerning FES-LCE, evidence has demonstrated that a lower cycling cadence and greater muscle stimulus can increase lean mass. The addition of nutritional counseling has also demonstrated benefits for battling obesity [8][9][10][1].
3. Neuromodulation of the Spinal Cord
The lumbosacral spinal cord contains a neural circuitry that can process proprioceptive and cutaneous information to generate cyclic motor patterns such as stepping [11]. The important aspect is that CPGs can spontaneously fire in the absence of supraspinal input, and they have a high level of automaticity [12]. The activity of CPGs is under the full control of the cerebral cortex, subcortical centers, and the brain stem. The rhythmicity of the CPGs is likely to induce a delicate balance between the extensors/flexors muscle groups that vary in a phase-dependent manner during the gait cycle [13]. Gerasimenko et al. successfully produced the stepping pattern while individuals were in a gravity-eliminated position by using non-invasive transcutaneous electrical stimulation to the spinal cord [12]. Surface electrodes were placed over the vertebrae of the lower thoracic and lumbosacral spinal cord. The electrical stimulation synapsed with interneurons that then stimulated neurons in the CPG circuitry.
The repeated activation of the sensory and motor systems through activity-based therapies has become an accepted strategy of enhancing neuroplasticity and restoring overground ambulation [14][15]. The mechanisms targeted to improve functional recovery after SCI are: (1) the modulation of axonal sprouting and synapse formation in spared and reorganized neural tissue, promoting axonal growth, (2) regeneration into and beyond non-neural lesion cores via placement of neural-stem-cell-derived grafts, (3) providing new relay neurons that can support host axon growth, and (4) the modulation of the sensory input steering circuit reorganization [15][16]. Individuals with complete SCI are limited due to the lack of intact excitable neural pathways. However, when excitable neural pathways remain intact after injury, plasticity-enhancing physical activities fostered by electrical stimulation are possible [14][15][16].
To highlight the possibilities of neuroplasticity after SCI, we reported on two individuals who participated in a series of electrical stimulation training protocols reported by [16]. The first participant was a 26-year-old male initially diagnosed with T6 motor and sensory complete SCI, and the second a 37-year-old male with T3 motor and sensory complete SCI. Injuries were sustained three and six years, respectively, before implantation with epidural stimulation. To confirm that physical activity alone would not restore motor function, both participants underwent 6 months of physical rehabilitation, including 60 motor training sessions over 22 weeks with rehabilitation specialists [17][18]. Each training session consisted of 15 min of stretching to the lower extremities, 45 min of locomotor training on a bodyweight-supported treadmill, and 30 min of balance and task-specific training. Afterward, a 16-contact epidural electrode array was inserted in the dorsal midline of the dura from T11 to L1. The participants were tested with FES attempting multiple motor tasks, including attempted standing, flexion, and extension leg movements while supine or side-lying. A variety of stimulation parameters were tested to optimize the stimulation during this time. Three weeks after the epidural implantation, the participants were tested in a side-lying position with the top leg suspended. Both participants were able to initiate, maintain, and terminate the electrically enabled leg activities [17][18].
Gill et al. determined the effects of interleaved epidural stimulation on the progression of stepping and overground locomotion in a person with complete SCI [19]. After implantation with a single paddle that was set at 210 µs, 25 Hz, and 5–6 volts, the participants underwent locomotor training with a 30% body weight support and a speed that was set 0.35 m/s. In week 4 session 11, till week 16 session 37, the participant still required maximum assistance at the hips and during the swing phase of the gait cycle. After switching to interleaved epidural stimulation in week 43, the combined frequency was set at 40 Hz (i.e., each paddle was set 210 µs, 20 Hz, and 3.3–3.7 volts), the participant was able to perform stepping at the treadmill with no required body weight support at a speed of 0.22 m/s, no assistance at the hips or the knees with arms supported on the treadmill bars for balance. Furthermore, the participant was to restore overground stepping with a front-wheel walker and with minimal assistance at the hips [19]. The authors also investigated the use of stimulation in the caudal region of the epidural array to improve the forward reaching ability of the participants. The participants were tested with and without electrical stimulation. Both participants were able to reach forward farther with either arm when the epidural stimulation was utilized [19][20].
Finally, Wagner et al. showed that targeted epidural stimulation can restore walking in persons with SCI [21]. The authors provided the multidirectional assistance of trunk movements to provide bodyweight support during overground locomotion with targeted epidural stimulation. Targeted mapping involved the activation of the upper lumbar segments and ankle extension mapping-involved activation of the upper sacral segments. Electrophysiological mapping was used to determine optimal electrodes and amplitudes for targeting specific proprioceptive circuits through the posterior. This resulted in shifting the paradigm from continuous epidural stimulation to more a temporal paradigm that was phase-dependent and coincided with different parts of the gait cycles. With spatiotemporal-targeted epidural stimulation, all participants with complete SCI restored voluntary control of overground walking [21].
4. Combined Epidural Stimulation and Exoskeletal-Assisted Walking
Gorgey et al. demonstrated the feasibility of using exoskeletal-assisted walking with epidural stimulation in an adult with a motor and sensory complete C7 injury. The exoskeleton provides robotic technology for overground weight-bearing ambulation with different levels of stepping assistance. This allows a low-metabolic cost for persons with SCI when walking with a 100% swing phase assistance for the legs [22]. With training, the amount of assisted stepping can be reduced depending on the degree of improvements developed by the participant. Gorgey and colleagues attempted to enhance this development by combining exoskeletal-assisted walking with an epidural electrical stimulation.
After epidural stimulator implantation along the spinal segments T12–S2, the participant underwent 12 weeks of exoskeletal-assisted walking with the first two weeks having 100% support with stepping [22]. The third week, the participant was transitioned to Canadian crutches from a walker. During weeks four through eight, swing phase assistance was gradually decreased depending on the participant’s performance. By week ten, the stepping or swing phase assistance had been decreased to 35% while the participant walked for at least 45 min per session. As the assistance level was decreased from 45% to 35% during the last weeks of the training, the number of unassisted steps also increased. Overall, through the 12 weeks of training (24 sessions), the participant decreased exoskeletal assistance from 100% to 35% and improved in temporal and rhythmic patterns as determined by electromyogram testing and gait speed [22].
Dissimilar to the paddle applications that may require a laminectomy, we are currently adopting the implantation of percutaneous leads (right and left) to activate the lumbosacral neural circuitries below the level of lesion. Each lead was mounted by eight contact electrodes that covered that distance from the T11 vertebra to the proximal border L1 vertebra. Figure 1 demonstrated successful mapping in a person with C8 cervical complete SCI in an attempt to target the muscle responsible for the initiation of standing and stepping.
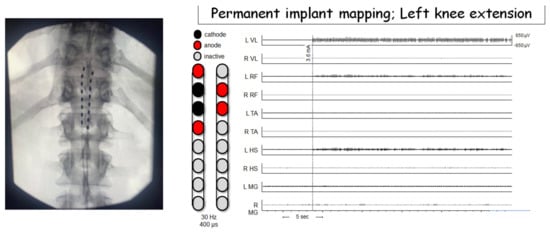
Figure 1. Epidural stimulation configuration for one participant resulting in left knee extension. All EMGs are shown on the same scale. Motor activity occurred at a stimulation amplitude of 3.6 mA. Phasic output is most prominent in knee extension muscles (L VL, L RF) with negligible activity at knee flexor muscles. Hz—hertz (stimulation frequency); µs—microseconds; L—left; R—right; VL—vastus lateralis; RF—rectus femoris; TA—tibialis anterior; HS—hamstrings; MG—medial gastrocnemius; sec—seconds; µv—microvolts.
5. Applications of Epidural Stimulation on Cardiovascular Performance
Epidural stimulation has been used for cardiovascular management in many populations for decades. The first studies showed that epidural stimulation, when delivered to the upper or mid-thoracic spinal cord, can alleviate symptoms of peripheral vascular disease [23][24] and improve baroreflex function in people with orthostatic issues [25]. Epidural stimulation was also shown to alter the response to an autonomic stressor, such as a cold pressor test [26].
Studies using animal models have provided mechanistic insight into how epidural stimulation can relieve angina pectoris [27][28], cause vasodilation [29], or also relieve orthostatic hypotension [30], supporting its use clinically. One of the ways cardiac function may be affected is that epidural stimulation seems to exhibit an indirect effect on the heart’s intrinsic nervous system [27] (Figure 2). Another way cardiovascular function is affected by epidural stimulation is via the antidromic depolarization of afferent neurons leading to the release of calcitonin gene-related peptide, causing vasodilation [29] (Figure 2). While there is some fundamental mechanistic insight into how epidural stimulation can affect cardiovascular function, there are also unique considerations for how epidural stimulation affects cardiovascular function in persons with SCI, and the mechanisms explaining subsequent effects.
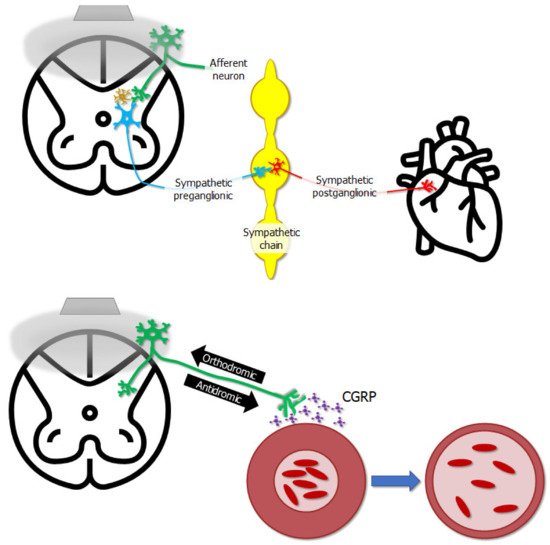
Figure 2. The gray trapezoid in the upper left represents a stimulator laying on the dorsal aspect of the spinal cord, and the gray bubble represents the electrical field emitted by the stimulator. The stimulator does not affect neurons innervating the heart directly—epidural stimulation affects the excitability of afferent neurons whose cell bodies lie in dorsal root ganglia on the dorsal aspect of the cord. These neurons in turn can affect the excitability of interneurons, represented by the gold neuron in the spinal cord, or afferent neurons can directly influence sympathetic preganglionic neurons. These in turn affect sympathetic post-ganglionic neurons, which finally innervate the heart, completing the chain of events by which epidural stimulation influences cardiac function. Electrical stimulation can potentially cause action potentials to propagate in an “antidromic” direction, i.e., the opposite direction of what is natural. In this case, action potentials in afferent neurons propagate from the periphery towards the spinal cord, as shown by the “orthodromic” arrow—however, epidural stimulation can push action potentials in the opposite “antidromic” direction, back towards the periphery. One outcome of this is the release of calcitonin gene-related peptide (CGRP) near blood vessels, causing vasodilation and altering local blood flow.
Epidural stimulation has evolved in the last decade to be a promising approach to facilitate or restore motor functions lost after SCI, even in individuals with severe, motor complete injuries [14]. A critical point is that these results have been shown with epidural stimulation to the lumbar spinal cord, not to the thoracic cord as with previous cardiovascular literature. Despite this, lumbar epidural stimulation has shown many promising results for cardiovascular function in persons with SCI. For instance, even in persons with clinically complete SCI, epidural stimulation can induce favorable changes in blood pressure regulation and the baroreflex response to postural changes [31][32][33], improve heart rate variability [34] enable improved cognitive function during an orthostatic challenge [35], improve cardiac output [31], and across a period of combining physical training with epidural stimulation, can favorably remodel cardiac tissue [36]. Theoretical mechanisms explaining these changes have been proposed in the literature [31][37].
Figure 3 shows a visual model of an injured spinal cord and sympathetic structures influencing cardiovascular function without and with epidural stimulation, respectively. After SCI, brainstem neurons which help regulate cardiovascular control may be cut off from their target neural circuits in the spinal cord. While many clinically complete injuries are not anatomically complete [38], even if descending signals can get past the injury, they may be too weak to exert any influence on surviving sympathetic preganglionic neurons. If those neurons are inactive, they will not influence the sympathetic chain, and sympathetic postganglionic neuron exertion to cardiovascular organs will be reduced, thereby giving rise to cardiovascular dysregulation. However, when epidural stimulation is delivered to the lumbar spinal cord below the injury it can act through dorsal root afferents to influence interneurons in the spinal cord which can span multiple spinal segments, subsequently exerting influence on sympathetic preganglionic neurons [31][37]. Once brought into a more excitable state, sympathetic preganglionic neurons are then enabled to influence the postganglionic neurons originating in the sympathetic chain, thereby influencing cardiovascular function in ways that have been beneficial to persons with SCI.
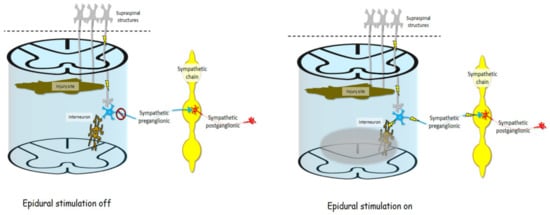
Figure 3. After SCI, axons descending from supraspinal neurons, such as those in the rostral ventrolateral medulla, may be cut off completely by the injury site, or spared connections may exert so little influence at the spinal level that sympathetic preganglionic neurons are dormant and therefore do not exert any influence on the sympathetic chain, and therefore do not influence sympathetic postganglionic neurons or organs subserving cardiovascular functions. Electrical fields delivered by epidural stimulation (gray bubble) may indirectly influence multi-segmental interneurons via dorsal root afferents (not pictured). This influence on interneurons may change the baseline excitability of sympathetic preganglionic neurons, making them more sensitive to any input from afferent or descending neurons, thereby enabling them to fire and in turn excite postganglionic neurons in the sympathetic chain, resulting in better cardiovascular regulation.
References
- Fornusek, C.; Davis, G.M.; Russold, M.F. Pilot study of the effect of low-cadence functional electrical stimulation cycling after spinal cord injury on thigh girth and strength. Arch. Phys. Med. Rehabil. 2013, 94, 990–993.
- Johnston, T.E.; Marino, R.J.; Oleson, C.V.; Schmidt-Read, M.; Leiby, B.E.; Sendecki, J.; Singh, H.; Modlesky, C.M. Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: A pilot study. Arch. Phys. Med. Rehabil. 2016, 97, 1413–1422.
- Gregory, C.M.; Bickel, C.S. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys. Ther. 2005, 85, 358–364.
- Deley, G.; Denuziller, J.; Babault, N.; Taylor, J.A. Effects of electrical stimulation pattern on quadriceps isometric force and fatigue in individuals with spinal cord injury. Muscle Nerve 2015, 52, 260–264.
- Gorgey, A.S.; Poarch, H.J.; Dolbow, D.R.; Castillo, T.; Gater, D.R. Effect of adjusting pulse durations of functional electrical stimulation cycling on energy expenditure and fatigue after spinal cord injury. J. Rehabil. Res. Dev. 2014, 51, 1455–1468.
- Mahoney, E.; Puetz, T.W.; Dudley, G.A.; McCully, K.K. Low-frequency fatigue in individuals with spinal cord injury. J. Spinal Cord Med. 2007, 30, 458–466.
- Bochkezanian, V.; Newton, R.U.; Trajano, G.S.; Blazevich, A.J. Effects of neuromuscular electrical stimulation in people with spinal cord injury. Med. Sci. Sport. Exerc. 2018, 50, 1733–1739.
- Gorgey, A.S.; Mather, K.J.; Cupp, H.R.; Gater, D.R. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med. Sci. Sport. Exerc. 2012, 44, 165–174.
- Demchak, T.J.; Linderman, J.K.; Mysiw, W.J.; Jackson, R.; Sunn, J.; Devor, S.T. Functional Electric Stimulation Cycle Ergometry Training Effect on Lower Limb Muscles in Acute SCI Individuals. J. Sport. Sci. Med. 2005, 4, 263–271.
- Dolbow, D.R.; Credeur, D.P.; Lemacks, J.L.; Stokic, D.S.; Pattanaik, S.; Corbin, G.N.; Courtner, A.S. Electrically induced cycling and nutritional counseling for counteracting obesity after spinal cord injury: A pilot study. J. Spinal Cord Med. 2021, 44, 533–540.
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231.
- Kumru, H.; Rodríguez-Cañón, M.; Edgerton, V.R.; García, L.; Flores, Á.; Soriano, I.; Opisso, E.; Gerasimenko, Y.; Navarro, X.; García-Alías, G. Transcutaneous Electrical Neuromodulation of the Cervical Spinal Cord Depends Both on the Stimulation Intensity and the Degree of Voluntary Activity for Training. A Pilot Study. J. Clin. Med. 2021, 10, 3278.
- Knikou, M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin. Neurophysiol. 2010, 121, 1655–1668.
- Hackman, J.; Yousak, A.; Wallner, J.J.; Gad, P.; Edgerton, V.R.; Gorgey, A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J. Neurophysiol. 2021, in press.
- Jervis Rademeyer, H.; Gauthier, C.; Masani, K.; Pakosh, M.; Musselman, K.E. The effects of epidural stimulation on individuals living with spinal cord injury or disease: A scoping review. Phys. Ther. Rev. 2021, 26, 344–369.
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908.
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947.
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018, 379, 1244–1250.
- Gill, M.; Linde, M.; Fautsch, K.; Hale, R.; Lopez, C.; Veith, D.; Calvert, J.; Beck, L.; Garlanger, K.; Edgerton, R. Epidural electrical stimulation of the lumbosacral spinal cord improves trunk stability during seated reaching in two humans with severe thoracic spinal cord injury. Front. Syst. Neurosci. 2020, 14, 79.
- Calvert, J.S.; Grahn, P.J.; Strommen, J.A.; Lavrov, I.A.; Beck, L.A.; Gill, M.L.; Linde, M.B.; Brown, D.A.; Van Straaten, M.G.; Veith, D.D. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J. Neurotrauma 2019, 36, 1451–1460.
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71.
- Gorgey, A.S.; Gill, S.; Holman, M.E.; Davis, J.C.; Atri, R.; Bai, O.; Goetz, L.; Lester, D.L.; Trainer, R.; Lavis, T.D. The feasibility of using exoskeletal-assisted walking with epidural stimulation: A case report study. Ann. Clin. Transl. Neurol. 2020, 7, 259–265.
- Galley, D.; Rettori, R.; Boccalon, H.; Medvedowsky, A.; Lefebvre, J.; Sellier, F.; Chauvreau, C.; Serisé, J.; Pieronne, A. Electric stimulation of the spinal cord in arterial diseases of the legs. A multicenter study of 244 patients. J. Mal. Vasc. 1992, 17, 208–213.
- Claeys, L.G.Y. Improvement of Microcirculatory Blood Flow underEpidural Spinal Cord Stimulation in Patients withNonreconstructible Peripheral Arterial Occlusive Disease. Artif. Organs 1997, 21, 201–206.
- Yamasaki, F.; Ushida, T.; Yokoyama, T.; Ando, M.; Yamashita, K.; Sato, T. Artificial baroreflex: Clinical application of a bionic baroreflex system. Circulation 2006, 113, 634–639.
- Schultz, D.M.; Musley, S.; Beltrand, P.; Christensen, J.; Euler, D.; Warman, E. Acute cardiovascular effects of epidural spinal cord stimulation. Pain Physician 2007, 10, 677–685.
- Foreman, R.D.; Linderoth, B.; Ardell, J.L.; Barron, K.W.; Chandler, M.; Hull, S.S., Jr.; TerHorst, G.J.; DeJongste, M.J.; Armour, J.A. Modulation of intrinsic cardiac neurons by spinal cord stimulation: Implications for its therapeutic use in angina pectoris. Cardiovasc. Res. 2000, 47, 367–375.
- Eliasson, T.; Mannheimer, C.; Waagstein, F.; Andersson, B.; Bergh, C.-H.; Augustinsson, L.-E.; Hedner, T.; Larson, G. Myocardial turnover of endogenous opioids and calcitonin-gene-related peptide in the human heart and the effects of spinal cord stimulation on pacing-induced angina pectoris. Cardiology 1998, 89, 170–177.
- Croom, J.E.; Foreman, R.D.; Chandler, M.J.; Barron, K.W. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am. J. Physiol.-Heart Circ. Physiol. 1997, 272, H950–H957.
- Yanagiya, Y.; Sato, T.; Kawada, T.; Inagaki, M.; Tatewaki, T.; Zheng, C.; Kamiya, A.; Takaki, H.; Sugimachi, M.; Sunagawa, K. Bionic epidural stimulation restores arterial pressure regulation during orthostasis. J. Appl. Physiol. 2004, 97, 984–990.
- West, C.R.; Phillips, A.A.; Squair, J.W.; Williams, A.M.; Walter, M.; Lam, T.; Krassioukov, A.V. Association of epidural stimulation with cardiovascular function in an individual with spinal cord injury. JAMA Neurol. 2018, 75, 630–632.
- Harkema, S.J.; Wang, S.; Angeli, C.A.; Chen, Y.; Boakye, M.; Ugiliweneza, B.; Hirsch, G.A. Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front. Hum. Neurosci. 2018, 12, 83.
- Aslan, S.C.; Legg Ditterline, B.E.; Park, M.C.; Angeli, C.A.; Rejc, E.; Chen, Y.; Ovechkin, A.V.; Krassioukov, A.; Harkema, S.J. Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits. Front. Physiol. 2018, 9, 565.
- Legg Ditterline, B.E.; Aslan, S.C.; Wang, S.; Ugiliweneza, B.; Hirsch, G.A.; Wecht, J.M.; Harkema, S. Restoration of autonomic cardiovascular regulation in spinal cord injury with epidural stimulation: A case series. Clin. Auton. Res. 2021, 31, 317–320.
- Darrow, D.; Balser, D.; Netoff, T.I.; Krassioukov, A.; Phillips, A.; Parr, A.; Samadani, U. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J. Neurotrauma 2019, 36, 2325–2336.
- Ditterline, B.E.L.; Wade, S.; Ugiliweneza, B.; Singam, N.S.; Harkema, S.J.; Stoddard, M.F.; Hirsch, G.A. Beneficial cardiac structural and functional adaptations after lumbosacral spinal cord epidural stimulation and task-specific interventions: A pilot study. Front. Neurosci. 2020, 14, 554018.
- Squair, J.W.; Gautier, M.; Mahe, L.; Soriano, J.E.; Rowald, A.; Bichat, A.; Cho, N.; Anderson, M.A.; James, N.D.; Gandar, J. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 2021, 590, 308–314.
- Wahlgren, C.; Levi, R.; Amezcua, S.; Thorell, O.; Thordstein, M. Prevalence of discomplete sensorimotor spinal cord injury as evidenced by neurophysiological methods: A cross-sectional study. J. Rehabil. Med. 2021, 53, jrm00156.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
915
Revisions:
2 times
(View History)
Update Date:
25 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




