
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Teng | + 2081 word(s) | 2081 | 2021-11-23 04:30:35 | | | |
| 2 | Lindsay Dong | Meta information modification | 2081 | 2021-11-24 02:49:46 | | |
Video Upload Options
Head and neck squamous cell carcinoma (HNSCC) represents an aggressive and heterogenous group of cancers whose pathologies remain largely unresolved. Exosomes are a subtype of extracellular vesicles secreted by a variety of cells that have begun to spark significant interest in their roles in cancer. As membranous vesicles, spanning from 30–150 nm in diameter, exosomes mediate the transport of various molecules, such as proteins, nucleic acids, and lipids, intercellularly throughout the body. In doing so, exosomes not only act to deliver materials to cancer cells but also as signals that can confer their progression. Accumulating evidence shows the direct correlation between exosomes and the aggressiveness of HNSCC.
1. Introduction
2. Exosomes
2.1. Biogenesis of Exosomes
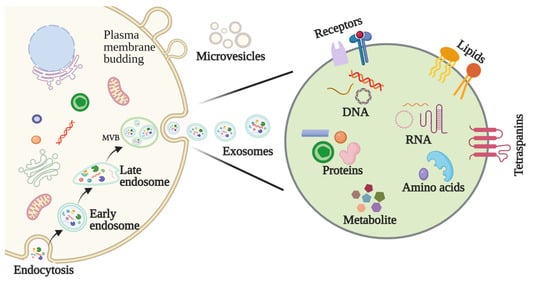
2.2. Features and Components of Exosomes
2.3. Methods for Exosome Isolation and Characterization
3. The Function of Exosomes in HNSCC
3.1. Exosomes Affect HNSCC Growth
3.2. Exosomes Are Involved in HNSCC Invasion and Metastasis
3.3. Exosomes Regulate the HNSCC Microenvironment
3.3.1. Exosomal Modulation of the Pre-Metastatic Niche (PME)
How a tumor induces PMN formation in a specific organ remains to be determined. The suppressive nature of immune cells in the TME is critical to the regulation of anti-tumor immune responses. One of the possible mechanisms is that TDEs mediate tumor PMN remodeling to establish a supportive and receptive niche to promote tumor cell colonization and metastasis. Maybruck et al. have found, however, that head and neck cancer cells can induce a suppressive phenotype in human CD8+ T cells through the release of TDEs [20]. Specifically, the group revealed through mass spectrometry that the immunoregulatory protein galectin-1 was present in these exosomes and played a key role in inducing this suppressive phenotype. The purification of exosomal RNA and subsequent CD8+ T cell suppression analysis also implicated RNAs in T cell dysfunction. This study suggests that tumor immunosuppressive exosomes could be a potential therapeutic target to preserve T cell function in anti-tumor immune responses [20].
3.3.2. Exosomal Modulation of Tumor Hypoxia
3.3.3. Exosomal Modulation of Immune Escape and Suppression
3.4. Exosomes Promote Drug Resistance in HNSCC
4. Role of Exosomes in the Diagnosis and Treatment of HNSCC
4.1. Exosomes as a Potential Biomarker in HNSCC
Early diagnosis and treatment are critical determinants in a cancer patient’s prognosis. The application of biomarkers in HNSCC detection, as well as factors such as staging, treatment efficacy, and prognosis, have consequently garnered attention in recent years [27]. Biomarkers represent a diverse range of molecules, and abnormalities in their levels or makeup can be detected in bodily fluids, like urine, saliva, and blood, as well as tumors themselves. In particular, exosomal biomarkers may be roughly divided into nucleic acids, proteins, lipids, and metabolites (Figure 2).
4.2. Exosomes as Therapeutic Targets in HNSCC
4.3. Exosomes as Drug Carriers for HNSCC Treatment
5. Prospects for Exosomes in Anticancer Therapy
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92.
- Steinbichler, T.B.; Dudas, J.; Riechelmann, H.; Skvortsova, I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181.
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514.
- Wang, X.; Guo, J.; Yu, P.; Guo, L.; Mao, X.; Wang, J.; Miao, S.; Sun, J. The roles of extracellular vesicles in the development, microenvironment, anticancer drug resistance, and therapy of head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 35.
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer. 2019, 18, 52.
- Kalluri, R.; Lebleu, V. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- McBride, J.D.; Rodriguez-Menocal, L.; Badiavas, E.V. Extracellular Vesicles as Biomarkers and Therapeutics in Dermatology: A Focus on Exosomes. J. Investig. Dermatol. 2017, 137, 1622–1629.
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312.
- Li, S.P.; Lin, Z.X.; Jiang, X.Y.; Yu, X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551.
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307.
- Ludwig, N.; Yerneni, S.S.; Azambuja, J.H.; Razzo, B.M.; Hinck, C.S.; Pietrowska, M.; Hinck, A.; Whiteside, T.L. Abstract B34: TGF-β-rich tumor-derived exosomes promote a proangiogenic phenotype in HNSCC. Clin. Cancer Res. 2020, 26, B34.
- Wang, X.; Qin, X.; Yan, M.; Shi, J.; Xu, Q.; Li, Z.; Yang, W.; Zhang, J.; Chen, W. Loss of exosomal miR-3188 in cancer-associated fibroblasts contributes to HNC progression. J. Exp. Clin. Cancer Res. 2019, 38, 151.
- He, L.; Ping, F.; Fan, Z.; Zhang, C.; Deng, M.; Cheng, B.; Xia, J. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharmacother. 2020, 121, 109553.
- Wee, I.; Syn, N.; Sethi, G.; Goh, B.C.; Wang, L. Role of tumor-derived exosomes in cancer metastasis. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 12–19.
- Theodoraki, M.N.; Yerneni, S.S.; Brunner, C.; Theodorakis, J.; Hoffmann, T.K.; Whiteside, T.L. Plasma-derived Exosomes Reverse Epithelial-to-Mesenchymal Transition after Photodynamic Therapy of Patients with Head and Neck Cancer. Oncoscience 2018, 5, 75–87.
- Theodoraki, M.N.; Matsumoto, A.; Beccard, I.; Hoffmann, T.K.; Whiteside, T.L. CD44v3 protein-carrying tumor-derived exosomes in HNSCC patients’ plasma as potential noninvasive biomarkers of disease activity. Oncoimmunology 2020, 9, 1747732.
- Maybruck, B.T.; Pfannenstiel, L.W.; Diaz-Montero, M.; Gastman, B.R. Tumor-derived exosomes induce CD8(+) T cell suppressors. J. Immunother. Cancer 2017, 5, 65.
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905.
- Xiao, M.; Zhang, J.; Chen, W.; Chen, W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 143.
- Silva, E.D.P.; Marti, L.C.; Andreghetto, F.M.; de Sales, R.O.; Hoberman, M.; Dias, B.D.S.; Diniz, L.F.A.; dos Santos, A.M.; Moyses, R.A.; Curioni, O.A.; et al. Extracellular vesicles cargo from head and neck cancer cell lines disrupt dendritic cells function and match plasma microRNAs. Sci. Rep. 2021, 11, 18534.
- Dong, X.; Bai, X.; Ni, J.; Zhang, H.; Duan, W.; Graham, P.; Li, Y. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020, 11, 987.
- Steinbichler, T.B.; Dudas, J.; Skvortsov, S.; Ganswindt, U.; Riechelmann, H.; Skvortsova, I.I. Therapy resistance mediated by exosomes. Mol. Cancer 2019, 18, 58.
- Qin, X.; Guo, H.Y.; Wang, X.N.; Zhu, X.Q.; Yan, M.; Wang, X.; Xu, Q.; Shi, J.B.; Lu, E.Y.; Chen, W.T.; et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome. Biol. 2019, 20, 12.
- Qu, X.Y.; Li, J.W.; Chan, J.; Meehan, K. Extracellular Vesicles in Head and Neck Cancer: A Potential New Trend in Diagnosis, Prognosis, and Treatment. Int. J. Mol. Sci. 2020, 21, 8260.
- Gollin, S.M. Cytogenetic alterations and their molecular genetic correlates in head and neck squamous cell carcinoma: A next generation window to the biology of disease. Genes Chromosomes Cancer 2014, 53, 972–990.
- Ebnoether, E.; Muller, L. Diagnostic and Therapeutic Applications of Exosomes in Cancer with a Special Focus on Head and Neck Squamous Cell Carcinoma (HNSCC). Int. J. Mol. Sci. 2020, 21, 4344.
- Brand, M.; Laban, S.; Theodoraki, M.N.; Doescher, J.; Hoffmann, T.K.; Schuler, P.J.; Brunner, C. Characterization and Differentiation of the Tumor Microenvironment (TME) of Orthotopic and Subcutaneously Grown Head and Neck Squamous Cell Carcinoma (HNSCC) in Immunocompetent Mice. Int. J. Mol. Sci. 2020, 22, 247.
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2019, 9, 1703244.
- Aung, T.; Chapuy, B.; Vogel, D.; Wenzel, D.; Oppermann, M.; Lahmann, M.; Weinhage, T.; Menck, K.; Hupfeld, T.; Koch, R.; et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc. Natl. Acad. Sci. USA 2011, 108, 15336–15341.
- Datta, A.; Kim, H.; Lal, M.; McGee, L.; Johnson, A.; Moustafa, A.A.; Jones, J.C.; Mondal, D.; Ferrer, M.; Abdel-Mageed, A.B. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castra-tion-resistant prostate cancer cells. Cancer Lett. 2017, 408, 73–81.
- Di Bonito, P.; Accardi, L.; Galati, L.; Ferrantelli, F.; Federico, M. Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes. Cancers 2019, 11, 138.
- Li, Y.; Zhang, Y.; Li, Z.; Zhou, K.; Feng, N. Exosomes as Carriers for Antitumor Therapy. ACS Biomater. Sci. Eng. 2019, 5, 4870–4881.
- Cohen, O.; Betzer, O.; Elmaliach-Pnini, N.; Motiei, M.; Sadan, T.; Cohen-Berkman, M.; Dagan, O.; Popovtzer, A.; Yosepovich, A.; Barhom, H.; et al. ‘Golden’ exosomes as delivery vehicles to target tumors and overcome intratumoral barriers: In vivo tracking in a model for head and neck cancer. Biomater. Sci. 2021, 9, 2103–2114.
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008, 16, 782–790.
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2015, 5, e1071008.
- Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 2021, 20, 6–7.
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565.
- Cao, J.; Zhang, M.; Xie, F.; Lou, J.; Zhou, X.; Zhang, L.; Fang, M.; Zhou, F. Exosomes in head and neck cancer: Roles, mechanisms and applications. Cancer Lett. 2020, 494, 7–16.




