Head and neck squamous cell carcinoma (HNSCC) represents an aggressive and heterogenous group of cancers whose pathologies remain largely unresolved. Exosomes are a subtype of extracellular vesicles secreted by a variety of cells that have begun to spark significant interest in their roles in cancer. As membranous vesicles, spanning from 30–150 nm in diameter, exosomes mediate the transport of various molecules, such as proteins, nucleic acids, and lipids, intercellularly throughout the body. In doing so, exosomes not only act to deliver materials to cancer cells but also as signals that can confer their progression. Accumulating evidence shows the direct correlation between exosomes and the aggressiveness of HNSCC.
- exosomes
- head and neck squamous cell carcinoma
1. Introduction
2. Exosomes
2.1. Biogenesis of Exosomes
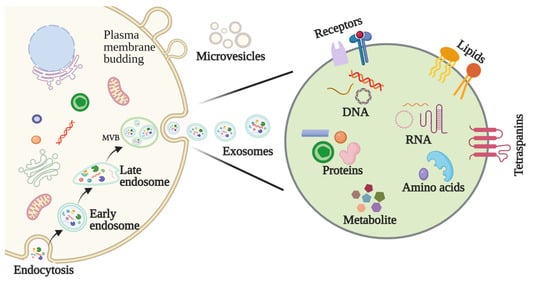
2.2. Features and Components of Exosomes
2.3. Methods for Exosome Isolation and Characterization
The isolation of pure exosomes is the first critical step in studying their mechanisms of action and their application in biomedical sciences. Various techniques have been adopted to facilitate the isolation of exosomes, including ultracentrifugation, size-based filtration, size-exclusion chromatography, polymer precipitation, and microfluidics-based isolation. Because exosome membranes are known to contain large quantities of proteins, immunoaffinity methods are also suitable for isolating exosomes [13]. Characterization of the physicochemical properties of exosomes, including their size, shape, surface charge, density, and porosity, is also important to their functional determinations. Many techniques have been routinely used to characterize exosomes, including dynamic light scattering (DLS), tunable resistive pulse sensing (TRPS), flow cytometry, electron microscopy, nanoparticle tracking analysis (NTA), and atomic force microscopy (AFM) [13]. Due to the inherent heterogeneity of exosomes and the advantages and limitations of each technique, more sophisticated techniques are necessary for the isolation and characterization of exosomes.3. The Function of Exosomes in HNSCC
3.1. Exosomes Affect HNSCC Growth
3.2. Exosomes Are Involved in HNSCC Invasion and Metastasis
3.3. Exosomes Regulate the HNSCC Microenvironment
3.3.1. Exosomal Modulation of the Pre-Metastatic Niche (PME)
How a tumor induces PMN formation in a specific organ remains to be determined. The suppressive nature of immune cells in the TME is critical to the regulation of anti-tumor immune responses. One of the possible mechanisms is that TDEs mediate tumor PMN remodeling to establish a supportive and receptive niche to promote tumor cell colonization and metastasis. Maybruck et al. have found, however, that head and neck cancer cells can induce a suppressive phenotype in human CD8+ T cells through the release of TDEs [20][42]. Specifically, the group revealed through mass spectrometry that the immunoregulatory protein galectin-1 was present in these exosomes and played a key role in inducing this suppressive phenotype. The purification of exosomal RNA and subsequent CD8+ T cell suppression analysis also implicated RNAs in T cell dysfunction. This study suggests that tumor immunosuppressive exosomes could be a potential therapeutic target to preserve T cell function in anti-tumor immune responses [20][42].
3.3.2. Exosomal Modulation of Tumor Hypoxia
3.3.3. Exosomal Modulation of Immune Escape and Suppression
3.4. Exosomes Promote Drug Resistance in HNSCC
4. Role of Exosomes in the Diagnosis and Treatment of HNSCC
4.1. Exosomes as a Potential Biomarker in HNSCC
Early diagnosis and treatment are critical determinants in a cancer patient’s prognosis. The application of biomarkers in HNSCC detection, as well as factors such as staging, treatment efficacy, and prognosis, have consequently garnered attention in recent years [27][53]. Biomarkers represent a diverse range of molecules, and abnormalities in their levels or makeup can be detected in bodily fluids, like urine, saliva, and blood, as well as tumors themselves. In particular, exosomal biomarkers may be roughly divided into nucleic acids, proteins, lipids, and metabolites (Figure 2).
