Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | V.A. Bogdanovskaya | + 2690 word(s) | 2690 | 2021-11-16 03:34:23 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bogdanovskaya, V. Modified Carbon Nanotubes for Oxygen Reduction Reaction. Encyclopedia. Available online: https://encyclopedia.pub/entry/16290 (accessed on 07 February 2026).
Bogdanovskaya V. Modified Carbon Nanotubes for Oxygen Reduction Reaction. Encyclopedia. Available at: https://encyclopedia.pub/entry/16290. Accessed February 07, 2026.
Bogdanovskaya, V.a.. "Modified Carbon Nanotubes for Oxygen Reduction Reaction" Encyclopedia, https://encyclopedia.pub/entry/16290 (accessed February 07, 2026).
Bogdanovskaya, V. (2021, November 23). Modified Carbon Nanotubes for Oxygen Reduction Reaction. In Encyclopedia. https://encyclopedia.pub/entry/16290
Bogdanovskaya, V.a.. "Modified Carbon Nanotubes for Oxygen Reduction Reaction." Encyclopedia. Web. 23 November, 2021.
Copy Citation
In order to develop highly efficient and stable catalysts for oxygen reduction reaction (ORR) that do not contain precious metals, it is necessary to modify carbon nanotubes (CNT) and define the effect of the modification on their activity in the ORR.
carbon nanotubes
modification
oxygen electrochemical reduction
electrochemically active surface area
corrosion resistance
1. Introduction
Carbon nanotubes (CNT) are one of the most studied carbon materials (CM) for electrocatalysts [1]. They are characterised by high strength, large specific surface area, stability in alkaline and acidic media. However, much attention is paid to changing their electronic properties in order to increase chemical stability and catalytic activity [2]. Electronic properties can be tailored by modifying the CNT surface, including the formation of oxygen-containing functional groups [3] and/or introducing heteroatoms into the CNT structure [4]. Treating CNT with strong acids or alkalis leads to the formation of oxygen functional groups on the surface, such as carbonyl, carboxyl, hydroxyl, ketone and alcohol, enhancing electrocatalytic properties of CNT [5][6]. In works [7][8], calculations of a -COOH group charge on the nanotubes surface, performed by the DFT method, showed that the charges on the atoms of the functional group were as follows: +0.4 on the carbon atom, (−0.3) and (−0.3) on oxygen atoms and +0.2 on the hydrogen atom. The formation of this group on the CNT boundary results in transferring electron density from the C atom of a -COOH group to the nanotube surface. In addition, the conductivity of the system increases. The carboxyl groups, showing an effect similar to nitrogen-containing ones, are the most active in oxygen reduction reactions (ORR) [7][8].
Atoms as nitrogen, phosphorus and sulphur are the most widely studied CM dopants [9][10][11]. Sizes of N and C atoms are comparable, making the doping process (carbon substitution) readily implemented [12]. The significant difference in electronegativity between N (3.0) and C (2.55) gives rise to the electron density shift from carbons to neighbouring nitrogen atoms. According to [13], the N atom in the carbon structure is highly negative (−0.277); however, it is compensated by three neighbouring carbon atoms with a positive charge. The presence of N atoms with a lone electron pair increases the electron-donating capability of CM. This ensures O2 chemisorption in an orientation favourable for weakening (breaking) the O-O bond [14]. The neighbouring C and N atoms are characterised by Lewis basicity which makes them centres active to ORR [14][15]. In addition, nitrogen can take several configurations in the structure of CNT, as a result of the formation bonds with various atoms [12][16]. In this regard, pyridine nitrogen has one double and one single bond with carbon atoms in the benzene ring. Pyrrole nitrogen has two single bonds with C and with external hydrogen. Graphite nitrogen forms one double bond with carbon and two single bonds, one of them belongs to the neighbouring ring [12][16]. These differences in the configuration of the doping nitrogen provide its activity in interaction with oxygen. It was shown that pyridine N with a lone electron pair which does not participating in the bond formation with C atom plays an important role in increasing the CM activity in ORR [13]. According to the literature [17], depending on the nitrogen configuration in the carbon structure, both an increase and a decrease in the material electrical conductivity can occur. Graphite nitrogen is considered one of the most important active components because of its significant contribution to electrical conductivity [17]. The graphite nitrogen is bonded to three carbon atoms, one of which belongs to the neighbouring benzene ring. Moreover, three valence electrons of the N atom form σ-bonds, the fourth electron fills the p-state, and the fifth electron forms the π *-state, creating the p-doping effect that improves the conductivity of carbon materials. The pyridine nitrogen has a more complicated effect due to the distinct position of the N atom. It was reported [18] that pyridine nitrogen at the edges of the carbon matrix provides an additional electron in the delocalised π-system, which increases the conductivity of carbon materials. However, pyridine nitrogen in the basal planes generates many surface defects, leading to an increase in localised electronic states in the carbon matrix, which has negative effects on the electrochemical characteristics [19].
As a rule, oxygen-containing groups do not incorporate into the structure of a CM but bond to a carbon atom through a carbon atom. This is probably one reason for the less significant effect of oxygen-containing groups on the properties of the CNT than that of nitrogen [7][8].
Hydrophilic–hydrophobic properties and charge are important characteristics of the surface of a catalytic material [20]. The active centres on the surface of the N-doped CM increase its wettability, enhancing hydrophilic properties [19]. It was shown [21] that for nanoporous carbon doped with nitrogen, the adsorption of water molecules depends mainly on the quantity of nitrogen atoms, not on their configuration. According to a study [20], the mass activity of hydrophilic samples in ORR is much higher with onset potentials and half-wave shifting towards a positive direction. This indicates a high density of accessible active sites and their high dispersion owing to the highly hydrophilic surface of the CM. Consequently, using simultaneously hydrophilic materials, the amount of doping nitrogen and the value of a specific surface area can lead to the cumulative effect of increasing activity. However, the influence of surface hydrophilicity on the catalytic activity of the material has been rarely studied [20]. Thus, understanding the influence of surface hydrophilicity on electrocatalytic efficiency in ORR, calls for further research.
2. Modified Carbon Nanotubes for Oxygen Reduction Reaction
The surface of the initial CNT is chemically inert [22][23]; therefore, to activate it, functionalisation and/or doping are necessary to form active centres. Functionalisation of CNT with alkali yields a small number of oxygen-containing hydroxyl functional groups (532.3–532.8 eV) on the surface, as shown in Table 1.
Table 1. Structural characteristics of surface and pH of studied CNT.
| No. | Catalyst Treatment Conditions |
Element/at.% | SBET, m2/g | Sspec., m2/g C8H18//H2O |
ζ-Potential in H2O |
pH in Water from 6.5 in 30 min |
|---|---|---|---|---|---|---|
| 1 | CNTNaOH | O/2.18 | 297 | 333.5//49.2 | −1.47 | 8.2 |
| 2 | CNTNaOH-N | O/10.08 N/1.15 |
269 | 268.8//154.2 | −14.3 | 9.5 |
| 3 | CNTHNO3 | О/15.4 N/1.14 |
300 | 342.6//145.6 | −41 | 5.7 |
| 4 | CNTHNO3-N | O/12.84 N/1.98 |
309 | 241.8//196 | −23.9 | 7.7 |
Upon treating with nitric acid, several types of functional groups are formed on the surface: ketone/carbonyl (531.0–531.9 eV), hydroxyl and carboxyl (532.3–532.8 eV) (Table 1). Furthermore, the total oxygen content on the surface increases from 2.18 at.% for CNT-1 to 15.43 at.% for CNT-3. Moreover, after treatment with nitric acid, the surface of CNT-3 contains 1.14 at.% of nitrogen, mainly in the pyrrole form (399.7–400.6 eV) (Table 1). As a result of nitrogen doping, mainly pyrrole (0.77 at.%) and pyridine (398.1–398.6 eV, 0.38 at.%) nitrogen forms occur on the CNT-2 surface (Figure 1). On the surface of CNT-4, 0.91 at.% pyrrole nitrogen and 0.54 at.% graphitized N (400.7 eV) are present. Figure 1 shows the surface composition of modified nanotubes analysed by X-ray photoelectron spectroscopy (XPS).
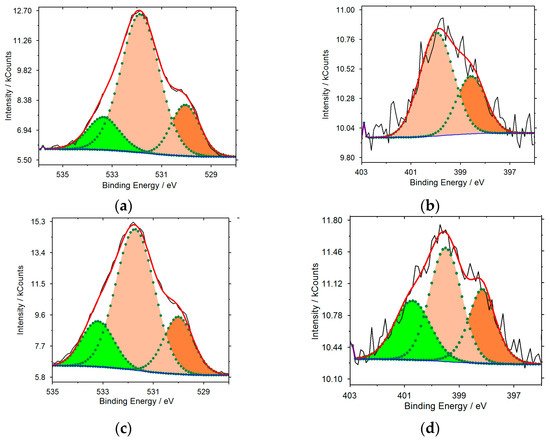
Figure 1. O1s (a,c) and N1s (b,d) X-ray spectra recorded on CNT-2 (a,b) and on CNT-4 (c,d).
For CNT-1 and CNT-3, the surface values determined by the BET and MSP using octane are similar are close. It should be noted that the hydrophilic surface is significantly larger in nanotubes treated with nitric acid. One possible reason is the large number of different oxygen-containing groups in CNT-3. For in CNT-1, there are only OH− on the surface; while in CNT-3, there are mainly carboxyl groups, which cause an electron density shift similar to formation of pyridine nitrogen, its consistent with [7][8].
Possibly, this is due to the fact that carboxyl groups are the most active in ORR in alkaline media [24]. One can assume that a significant number of carboxyl groups on the surface, where hydrogen has a charge of +0.2 and carbon of +0.4 [7][8], leads to the adsorption of hydroxide ions. In this case, the pH of the CNT-3 solution shifts slightly towards lower values (Table 1). On the contrary, on the surface of CNT-1, there are only weakly bound groups OH− that can be desorbed and shift the pH of water towards higher values, as seen in the experiment. Accordingly, the zeta potential tends to zero. CNT-3 has the highest zeta potential value among the studied samples; this is associated with the adsorption of hydroxide ions from water. In alkaline solutions containing a large quantity of OH− ions, their adsorption on the negatively charged surface of carbon material is hindered; hence, oxygen is adsorbed and reduced on the electrode. This agrees with the fact that during desorption of hydroxide ions from the surface into the water, the zeta potential decreases (tends to zero in the case of CNT-1), and during the adsorption of OH− it increases (CNT-3). As a result of the modification, the electron density and electron-donor properties increase on CNT-3.
The change in the hydrophobic–hydrophilic properties is associated with CNT functionalisation and doping and is characterised by the amount of oxygen- and nitrogen-containing groups (Table 1). For CNT functionalised with alkali and acid, surface composition differs significantly. CNT-1 is characterised by the lowest hydrophilic surface area, compared with other samples, that comprises <15% of Ssp measured by octane. CNT doped with nitrogen have the largest hydrophilic surface and the higher content of N atoms on the surface, the larger hydrophilic surface area (81% of Ssp for CNT-4). Figure 2 shows the pore volume as a function of the logarithm of their radius when measured using octane and water. For all studied CNT, the water curve is shifted towards larger radii than the octane curve. This indicates a weaker wettability with water than with octane [25]; i.e., the average value of a wetting angle between water and all CNT samples exceeds zero. Measured in water and compared in the micro and mesopore region, the curves show that doping with N atoms results in an increase in the volume of hydrophilic pores with a radius of over 100 nm (Figure 2, Curves 2 and 4). Obtained for octane, the pore volume of these nanotubes, as well as the surface composition, is similar. This indicates that introducing N into the CNT structure leads to a decrease in the pore volume (regardless of the functionalisation type) and an increase in the proportion of hydrophilic pores. The functionalisation affects hydrophilicity only in the case of pores with a larger radius (Figure 2, Curves 1, 3 and 1′, 3′).
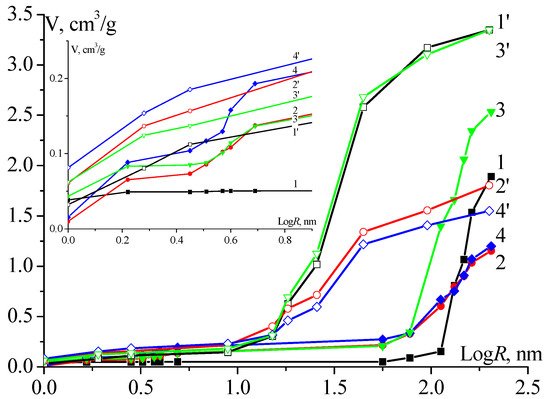
Figure 2. Integral curves of specific pore volume distribution V with respect to effective radii R (porometric curves) obtained with water (1, 2, 3, 4) and octane (1′, 2′, 3′, 4′) for CNT-1(1, 1′), CNT-2(2, 2′), CNT-3(3, 3′), and CNT-4 (4, 4′). The insert in the figure corresponds to the porometric curves in the area of micropores.
According to the data in Table 1 and Figure 2, the growth of oxygen-containing groups (mainly carboxyl) results in increase of the zeta potential and hydrophilic surface area. Upon subsequent nitrogen doping of functionalised CNT, the total pore volume measured with octane decreases, as well as the fraction of the volume of hydrophilic pores. For CNT-2 and CNT-4 the volume of hydrophilic pores is 1.2 cm3/g, while the maximum volume of hydrophilic pores is 2.5 cm3/g (CNT-3). The electrochemically active surface of CNT-3 is significantly higher than that of CNT-1 due to the large volume of hydrophilic pores (as will be shown further).
Electrical conductivity is one of the most important characteristics of electrode materials. The electrical conductivity of modified CNT is presented in Table 2.
Table 2. Electrical conductivity and electrochemical characteristics of modified CNT.
| No. | Catalyst Treatment Conditions |
1/2Q, C/g |
Е1/2, V ½ilim.theoretical |
ikin, mA/cm2 //E, V (+0.05 V from Estat) |
n, Number of Electrons | i, mA/cm2 (at 0.2 V) |
κspec, S/cm | ρ, g/cm3 * |
|---|---|---|---|---|---|---|---|---|
| in 0.1М KOH | in 0.5М H2SO4 | |||||||
| 1 | CNTNaOH | 21.5 | 0.64 | 0.12//0.75 | 1.6 | 0.124 | 0.234 | 0.407 |
| 2 | CNTNaOH-N | 61.2 | 0.81 | 0.7//0.83 | 2.6 | 1.895 | 0.283 | 0.90 |
| 3 | CNTHNO3 | 79.5 | 0.71 | 0.15//0.81 | 1.8 | 0.450 | 0.17 | 0.58 |
| 4 | CNTHNO3-N | 62.0 | 0.82 | 0.9//0.83 | 3 | 2.329 | 0.212 | 0.85 |
CNT doped with nitrogen have the highest electrical conductivity. The high electrical conductivity for CNT-1 can be explained by lower (almost twice) material density than that of other CNT. Since the resistance was measured at a constant height between the electrodes (0.01 cm), the compaction of CNT with lower density is more prominent than that of CNT with high density. This leads to a higher number of contacts between neighbouring nanotubes and, consequently, increasing electrical conductivity. In the case of CNT-3, the low electrical conductivity (Table 2) is probably associated with the surface defects formed during harsh oxidation and lower compaction than that of CNT-1. For CNT-2 and CNT-4, the electrical conductivity amounts to 0.283 and 0.212 S/cm, respectively.
The Figure 3a,b shows CV of the studied CNT. The values of SEAS are given in Table 2. The electrochemical activity of modified CNT in ORR was investigated using 0.1 M KOH and 0.5 M H2SO4 electrolytes (Figure 3c,d). According to the polarisation curves the studied CNT exhibit high catalytic activity in an alkaline electrolyte.
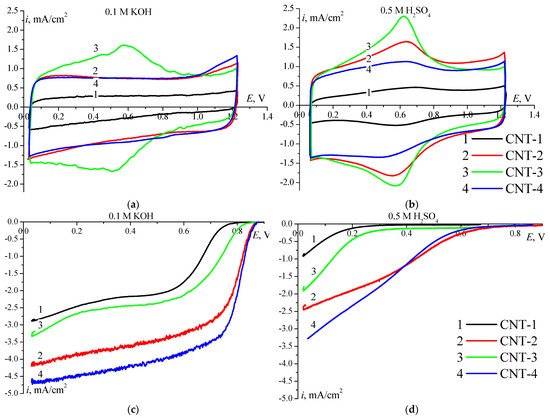
Figure 3. CV on modified CNT in 0.1 M KOH (a) and in 0.5 M H2SO4 (b) electrolyte solution. Ar-saturated, 100 mV/s, 0.150 mg/cm2. Polarization curves of О2 reduction on modified CNTs in 0.1 M KOH (c) and in 0.5 M H2SO4 (d) electrolyte solution, 5 mV/s, 1500 rpm, 0.150 mg/cm2.
The shape of the obtained curves suggests the reaction mechanism. In the acidic electrolyte, functionalised CNT-1 and CNT-3 are not active in ORR since the entire surface of negatively charged carbon material is filled with hydrogen adsorbed from the solution. The activity of nitrogen-doped CNT in ORR is slightly higher. The presence of nitrogen leads to a decrease in the volume and surface of pores, moreover significant part of which is hydrophobic and provide oxygen supply to the active centres. The polarisation capacity of this type of CNT is higher than that of CNT-1 (Figure 3). Due to the high electrical conductivity of CNT-2 and CNT-4, the activity in ORR in 0.5 M H2SO4 is higher than that on CNT-1 and CNT-3. In addition, the polarisation curve is positively shifted by ~0.300 V. The most probable factors influencing the activity are the low SEAS value of CNT-1 and the actual electrical conductivity; CNT-3 shows the lowest electrical conductivity among the studied samples. The overall reason for the low activity of CNT in an acidic electrolyte comprises blocking the surface for oxygen adsorption by adsorbed hydrogen. Modified CNT, primarily doped with nitrogen, exhibit high activity in an alkaline electrolyte. A high negative value of the zeta potential and a shift in pH towards lower values (Table 1) can be explained by the adsorption of OH− ions on the surface and are observed only for CNT-3. In this regard, the high activity of CNT-3 in the ORR stems from the higher SEAS value than that of CNT-1 (Figure 3). In an alkaline electrolyte, the entire SEAS remain accessible. OH− ions do not adsorb on a negatively charged surface and do not hinder the oxygen adsorption and subsequent reduction. Thus, CNT modified with oxygen- and nitrogen-containing groups are ORR catalysts in an alkaline medium. In an alkaline electrolyte, CNT-1 is characterised by the lowest catalytic activity in the ORR due to the low content of heteroatoms on the surface and a small hydrophilic surface area. An increase in the activity of CNT-3 with respect to CNT-1 is associated with an increase in the number of oxygen-containing groups on its surface and the presence of nitrogen-containing groups increasing the hydrophilic and electrochemically active surface area (Table 2), as well as a high negative value of surface zeta-potential. The nitrogen-doped CNT are characterised by the highest activity in ORR, due to the presence in the surface composition of carboxyl groups, as well as pyridine and pyrrole nitrogen groups, ensuring a large hydrophilic surface area. The half-wave potential values are 0.81V and 0.82V for CNT-2 and CNT-4, respectively, being close to the E1/2 values for Pt-catalysts [26]. In the case of CNT doped with nitrogen atoms, the current increases in the kinetic potential range, namely, 0.7 and 0.9 mA/cm2 at 0.83 V for CNT-2 and CNT-4, respectively (Table 2).
The corrosion stability of modified CNT was evaluated following the change in the SEAS value and the half-wave potential using the method of accelerated corrosion testing. All the studied materials are characterised by high stability within up to 1000 cycles (Figure 4). The maximum decrease in SEAS is observed at CNT-3 (13%) and may occur due to a large number of surface defects formed during the harsh oxidation with HNO3. However, more surface defects promote the incorporation of more N atoms (Table 1), which significantly increase the CNT-4 stability. Thus, functionalising CNT with nitric acid increases its hydrophilic and electrochemically active surface area due to the formation of surface defects during harsh oxidation; however, its stability decreases compared to that of CNT-1.
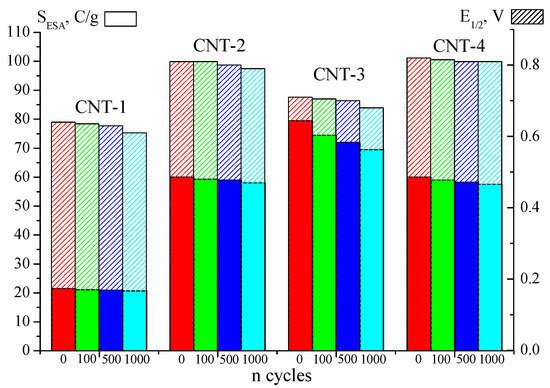
Figure 4. Variation of the SEAS and Е1/2 parameters of modified CNTs during accelerated corrosion testing in 0.1M KOH.
References
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346.
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103.
- Qian, Z.; Sun, B.; Du, L.; Lou, S.; Du, C.; Zuo, P.; Ma, Y.; Cheng, X.; Gao, Y.; Yin, G. Insights into the role of oxygen functional groups and defects in the rechargeable nonaqueous Li–O2 batteries. Electrochim. Acta 2018, 292, 838–845.
- Li, J.-C.; Hou, P.-X.; Liu, C. Heteroatom-Doped Carbon Nanotube and Graphene-BasedElectrocatalysts for Oxygen Reduction Reaction. Small 2017, 13, 1702002.
- Liang, S.; Li, G.; Tian, R. Multi-walled carbon nanotubes functionalized with a ultrahigh fraction of carboxyl and hydroxyl groups by ultrasound-assisted oxidation. J. Mater. Sci. 2016, 51, 3513–3524.
- Chen, J.; Li, C.; Lian, Y.; Chen, Y.; Chen, T.; Hu, X. Understanding the oxygen-containing functional groups on multiwall carbon nanotubes toward supercapacitors. Mater. Today Chem. 2021, 19, 100414.
- Zaporotskova, I.V.; Polikarpova, N.P.; Vil’keeva, D.E. Sensor Activity of Carbon Nanotubes with a Boundary Functional Group. Nanosci. Nanotechnol. Lett. 2013, 5, 1169–1173.
- Zaporotskov, P.A. Semiconductor Modified Structures Based on Carbon Nanotubes. Ph.D. Dissertation, National University of Science and Technology “MISIS”, Moscow, Russian, 2016. (In Russian).
- Deng, H.; Li, Q.; Liu, J.; Wang, F. Active sites for oxygen reduction reaction on nitrogen-doped carbon nanotubes derived from polyaniline. Carbon 2017, 112, 219–229.
- Yang, H.; Miao, J.; Hung, S.; Chen, J.; Tao, H.; Wang, X.; Zhang, L.; Chen, R. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, 1501122–1501133.
- Gislasona, P.M.; Skulason, E. Catalytic trends of nitrogen doped carbon nanotubes for oxygen reduction reaction. Nanoscale 2019, 11, 18683–18690.
- Biddinger, E.J.; von Deak, D.; Ozkan, U.S. Nitrogen-Containing Carbon Nanostructures as Oxygen-Reduction Catalysts. Top. Catal. 2009, 52, 1566–1574.
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764.
- Zhang, X.; Zhang, X.; Zhao, S.; Wang, Y.Q.; Lin, X.; Tian, Z.Q.; Shen, P.K.; Jiang, S.P. Precursor modulated active sites of nitrogen doped graphene-based carbon catalysts via one-step pyrolysis method for the enhanced oxygen reduction reaction. Electrochim. Acta 2021, 370, 137712.
- Guo, D.; Shibuya, R.; Akiba, C. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365.
- Hussein, L. Towards a fine-tuning of surface chemistry in aligned carbon nanotubes induced by nitrogen plasma discharge post-treatment: A combined microscopic and spectroscopic study. RSC Adv. 2016, 6, 13088–13100.
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574–1602.
- Zhang, D.; Hao, Y.; Zheng, L.; Ma, Y.; Feng, H.; Luo, H. Nitrogen and sulfur co-doped ordered mesoporous carbon with enhanced electrochemical capacitance performance. J. Mater. Chem. A 2013, 1, 7584–7591.
- Podyacheva, O.Y.; Ismagilov, Z.R. Nitrogen-doped carbon nanomaterials: To the mechanism of growth, electrical conductivity and application in catalysis. Catal. Today 2015, 249, 12–22.
- Hao, G.-P.; Sahraie, N.R.; Zhang, Q.; Krause, S.; Oschatz, M.; Bachmatiuk, A.; Strasser, P.; Kaskel, S. Hydrophilic non-precious metal nitrogen-doped carbon electrocatalysts for enhanced efficiency in oxygen reduction reaction. Chem. Commun. 2015, 51, 17285–17288.
- Kumar, K.V.; Preuss, K.; Guo, Z.X.; Titirici, M.M. Understanding the Hydrophilicity and Water Adsorption Behavior of Nanoporous Nitrogen-Doped Carbons. J. Phys. Chem. C 2016, 120, 18167–18179.
- Wang, X.; Ouyang, C.; Dou, S.; Liu, D.; Wang, S. Oxidized carbon nanotubes as an efficient metal free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2015, 5, 41901–41904.
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O.; Novikov, V. Carbon nanotubes modified by (O, N, P) atoms as effective catalysts for electroreduction of oxygen in alkaline media. Catalysts 2020, 10, 892.
- Sang, Y.; Fu, A.; Li, H.; Zhang, J.; Li, Z.; Li, H.; Zhao, X.S.; Guo, P. Experimental and theoretical studies on the effect of functional groups on carbon nanotubes to its oxygen reduction reaction activity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 476–484.
- Vol’fkovich, Y.M.; Sosenkinz, V.E.; Nikol’skaya, N.F. Hydrophilic–Hydrophobic and Sorption Properties of the Catalyst Layers of Electrodes in a Proton-Exchange Membrane Fuel Cell: A Stage-by-Stage Study. Russ. J. Electrochem. 2010, 46, 438–449.
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O. Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms. Catalysts 2021, 11, 335.
More
Information
Subjects:
Electrochemistry
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Chemical Bond
Revision:
1 time
(View History)
Update Date:
23 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




