
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mariana Palma-Tenango | + 2808 word(s) | 2808 | 2021-07-09 06:14:21 | | | |
| 2 | Catherine Yang | -2 word(s) | 2806 | 2021-11-22 02:59:39 | | |
Video Upload Options
Mexico is the center of origin of the species popularly known as toronjil. its use and commercialization for traditional Mexican medicine make it the most important member of the Agastache genus in Mexico. The species Agastache mexicana divides into two subspecies, based on anatomical characteristics and chemical composition: red lemon balm, Agastache mexicana Linton & Epling subspecies mexicana, and white toronjil, Agastache mexicana subspecies xolocotziana Bye, E.L. Linares & Ramamoorthy.
1. Introduction
Lamiaceae is the eighth most diverse plant family in Mexico and 5.5% of the species worldwide are found in this country. This family contains a wide range of aromatic plants possessing agronomical, pharmacological, and commercial potential. MexicanAgastachebelongs to this family and its use and commercialization for traditional Mexican medicine make it the most important member of theAgastachegenus in Mexico The speciesAgastache mexicanadivides into two subspecies, based on anatomical characteristics [1] and chemical composition [2]: red lemon balm,Agastache mexicanaLinton & Epling subspecies mexicana, and white toronjil
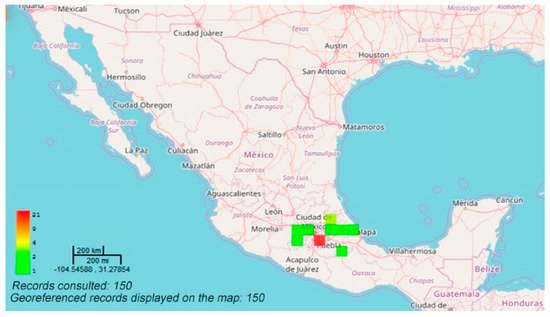
The species is distributed in the states of Guanajuato, Mexico, Michoacán, Puebla, Querétaro, Hidalgo, Veracruz, Chihuahua, Morelos, and Tlaxcala, as well as in Mexico City (Figure 1). The species concentrates in the volcanic axis of Central Mexico [1][3].
2. A Holistic Approach to Agastache mexicana Usage
Systems biology analysis allows the understanding of different biological elements and their interactions with non-biological elements, such as the environment or human impacts (for example, the analysis of various traditional medicine systems like traditional Chinese medicine) [5]. Systems biology, in tandem with reverse pharmacology, may allow discovering new active biological compounds [6].
Life science studies relying on systems biology and holistic approaches shy away from reductionist views and incorporate biological effects and their interaction with the environment [5][6]. A biological system contains numerous components interacting in a vast variety of combinations. Once the components and interactions of a system are known, a system’s behavior may be understood [7].
We used systems biology principles for a holistic analysis of different components within the lemon balm plant system and its environment. This system’s insights are derived from a general vision that includes the system components’ relationships and interactions. This approach may provide new collaborative information, fresh insights, and research prospects for the species.
It is a perennial herb. Plants of both subspecies have a typical Lamiaceae morphology: opposite, petiolate leaves, a four-angled stem, and numerous trichomes [8]. The three types of epidermal appendages described for the leaf are observed, but uniseriate the base chromosome number is 9.
Various plants from theAgastachegenus are used for bee forage and honey production [9][10]. Toronjil is a honey plant; its flowers produce nectar for bee collection [11].
The basal and middle part of the stem is purple. The form of the leaves is ovate-lanceolate, measuring 4.4 to 6.3 cm long and 2.1 to 2.5 cm wide The petiole is 1 cm long [1], and the corolla is purplish red to red [1][12]. The seeds measure approximately 4 to 5 mm.
The petiole is 1 cm long [1]. Inflorescences end in ramifications of interrupted whorls of cymes with numerous flowers. The calyx is 1.0 to 1.3 cm long and the corolla is white and approximately 2.4 cm long. Its anther is approximately 1 mm long.
The genusAgastacheincludes ornamental plants and aromatic plants that contain essential oils [8]. For example, bothA. mexicanasubspecies have therapeutic and ornamental uses [1]. Knowledge about lemon balm healing properties is cited in sources dating back to pre-Hispanic culture, such as in the De la Cruz Badiano Codex [13]. In the Nahuatl tongue,A. mexicanais known as tlalahuehuetl [3].
The holistic method to study plants with medicinal properties examines the interactions and relationships among the environment’s biological and cultural components. Rural and urban populations use this plant for in-home treatments in the form of herbal teas (infusions and decoctions) In Mexico,A. mexicanais identified for its medicinal properties against anxiety and as a sleep-promoting plant [14][13]. mexicana is preferred for wound healing, as an antispasmodic agent, and against stomach pain, whileA. mexicanassp.
Many modern drugs originated from ethnopharmacology and knowledge of traditional medicine [15]. Results from research on the medicinal effects ofA. mexicanassp. mexicana and ssp. xolocotziana support their use in traditional medicine as an anxiolytic, tranquilizer, and sedative, as well as a remedy to alleviate “nervousness” [14][16].
Many medicinal and aromatic plants are industrially sown, but most are still obtained by harvesting wild populations. The need for renewable sources and protection of plant biodiversity creates an opportunity for farmers to grow these crops [17]. In Mexico, over-harvesting of medicinal plants is counteracted by collecting seeds, cuttings, or roots to propagate the plant. Most of these collected samples are planted in small home gardens to be later sown on cultivated fields [18].
A. mexicanais a candidate species for structured cultivation as a source for active principles, extracts, essential oils, and pharmaceutical products [19]. Propagation is mainly asexual [20], through vegetative propagation, and depends on its rhizomes’ division, as seed viability is low;A. mexicanassp. A further complication arises as seed formation is hindered since harvesting occurs during flowering [12]; inflorescences are the main commercialized product. However, red lemon balm exists in wild populations, unlike white lemon balm.
A. mexicanablooms from June to November [19]. Subspecies show phenotypic differences in leaf shape, flower color, and flavor [1]. Farmers from Santiago Mamalhuazuca (State of Mexico) have empirically gathered knowledge that the xolocotziana subspecies is more susceptible to extreme temperature and humidity. No technological packages based on crop physiology, detailing handling on its phenological stages, leading to higher biomass yields or providing information on bioactive production per cultivation area, exist forA. mexicanacultivation.
Empirical observations have detected that mexican markets sold a different subspecies from the typicalA. mexicanasubspecies mexicana. The commercialization of botanical products promotes the cultural exchange of traditional knowledge and the exploitation of natural resources. Studies illustrate the influence popular markets have on the demand for plants with novel applications. Attention should also focus on the dangers of overcollection of wild species in response to increasing demand and supporting natural habitats’ conservation [18].
White and red lemon balm are commercially sown and traded in various Mexican regions, including Hidalgo, Mexico, Morelos, Puebla, and Veracruz. Inflorescence bundles or dried plants are distributed through different regional sales channels in the State of Mexico, Southeast Puebla, Morelos, and Mexico City [21].
TheAgastachegenus produces various volatile and non-volatile secondary metabolites, mainly phenylpropanoids and terpenoids. A. mexicanacontains terpenoid compounds like monoterpenes (limonene, pulegone), sesquiterpenes (β-caryophyllene), diterpenes (breviflorine), triterpenes (ursolic, corosolic, maslinic acids); phenolic and phenylpropanoid compounds like flavones (acacetin) and flavonoids (tilianin, hesperitin); carboxylic acids (9-hexadecenoic acid, butanoic acid); and soluble sugars (glucose, sucrose) [8]. Subspecies mexicana and xolocotziana share common compounds, but have different chemical profiles [14][2].
The chemical composition of essential oils is influenced by the subspecies, environmental conditions of the crop, harvest time and type of extraction the chemical compositions of different essential oils obtained fromA. mexicanasubspecies. Plants introduced to other countries have essential oils with different chemical compositions. Extraction methods also influence the variability of the physical and chemical characteristics of the essential oils, but different distillation apparatus does not affect the quality ofA. mexicanaessential oil [22].
Chemical study of aqueous and organic extracts from aerial plant parts and whole plants led to the isolation of monoterpenes, diterpenes, triterpenes, flavones, and flavonoids. Table 1 shows the chemical compositions of non-polar and polar extracts fromA. mexicanasubspecies. The compounds in both subspecies are tilianin, acacetin, ursolic acid, salvigenine, 5-hydroxy-7,4′dimethoxyflavone, (2-acetyl)-7-O-glucosyl acacetin, diosmetin 7-O-β-D-(6″-O-malonyl)-glucoside, tilianin and acacetin are more abundant in the subspecies xolocotziana [23].
Table 1. Chemical composition of aqueous and organic extracts of Agastache mexicana.
| Taxa | Flavonoids | Flavones | Terpenes | Organic Acids | Esters | Alcohols, Aldehydes, and Ketones | Hydrocarbons |
|---|---|---|---|---|---|---|---|
| Agastache mexicana | Tilianin [24], hesperitin, quercetin [16]. | Limonene, linalool, menthone, α-terpineol, pulegone, eugenol [25]. |
|||||
| Agastache mexicana Linton & Epling spp. mexicana |
Tilianin [23][26][27][28], gardenin A, 5-hydroxy-7,4′ dimethoxy flavone [14]. | Acacetin [2][23][29], 7-O-glucosyl acacetin, (2-acetyl)-7-O-glucosyl acacetin [2], diosmetin 7-O-β-D-(6″-O-malonyl)-glucoside, acacetin 7-O-β-glucoside, acacetin 7-O-β-D-(6″-O-malonyl)-glucoside, acacetin-7-O-β-glucoside-D-(2 ″-acetyl-6″ malonyl), diosmetin, 5,6,7,8,3-pentahydroxy-4-methoxy flavone [14], luteolin 7-O-β-D-glucoside, luteolin 7-O-β-D-(6-O-malonyl)-glucoside [14]. | Ursolic acid [2][29], oleanolic acid [29], salvigenine, 8-hydroxy-salvigenin [14], estragole, oleanoic acid [2]. | Malic acid [14], hexadecanoic acid, 9-hexadecenoic acid [2]. |
Butanoic acid- hexane-dioctyl, hexanedioc-dioctyl ester, 6-octen-1-ol- 3,7-dimethyl propionate [2]. |
3-methoxy-cinnamaldehyde, 2,6-dimethoxy-4-(2-propenyl)-phenol [2] |
9-Eicosyne [2] |
| Agastache mexicana spp. xolocotziana Bye, E.L. Linares & Ramamoorthy |
Tilianin [23], pratol [12], gardenin A, pilosin [14]. | Acacetin [14][2][23][30], 5-hydroxy-7,4′ dimethoxy flavone, (2-acetyl)-7-O-glucosyl acacetin [2], acacetin 7-O-β-glucoside, acacetin 7-O-β-D-(6″-O-malonyl)-glucoside, acacetin-7-O-β-glucoside-D-(2 ″-acetyl-6″-malonyl), diosmetin 7-O-β-D-(6 ″ -O-malonyl)-glucoside, diosmetin, 5,6,7,8,3-pentahydroxy-4-methoxy flavone; diosmetin 7-β-O-glucoside, 8-hydroxy-flavone [2], chrysene [12]. | Salvigenine, corosolic acid, maslinic acid [2], ursolic acid [2][30], β-amirin, 8-hydroxy-salvigenin [14], breviflorine [12], nerol, pulegone, camphor, p-menth-6-ene-2,8-diol, α-terpineol, isopiperitenone, geraniol, α-terpineol-methyl ether, p-menthane-1,8-diol, neryl acetate, thymol acetate, piperitone, p-menth-2-ene-1,8-diol, isoeugenol, diosphenol, β-terpinyl acetate, ocimenol, 2,8-dihydroxy-p-menth-3-en-5-one, p-menth-1-en-7,8-diol, linalool 3,7-oxide, oleic acid [2]. | Butanoic acid [2]. | Hexadecanoic acid methyl ether, ethyl palmitate [2]. | 2-hydroxy-6-methoxyacetophenone, 2-pentadecanone [2]. | 9-octadecyne, 3,3,6-trimethyl 1,5-heptadiene [2]. |
The biological activity attributed toA. mexicanadiffers between subspecies because each one has a different chemical profile [2]. It also differs with respect to essential oils or extracts, as well as secondary metabolites present in them. Terpenes and flavonoids, such as ursolic acid, oleanolic acid, acacetin, apigenin, and tilianin, are the most active [24]. 3show the biological activities of the essential oils, extracts, and compounds for each subspecies.
Pharmacological studies correlate with the ethnomedicinal uses ofA. mexicana. Additionally, the flavonoid tilianin, extracted from the plant, had antihypertensive and vasorelaxant effects on in vitro experiments, as observed on rat aortic rings and in vivo experiments in spontaneously hypertensive rats (SHR) [26]. mexicana and correlated the biological activity with tilianin content and extraction conditions. The methanolic extracts had higher concentrations of tilianin and were the more vasorelaxant on thoracic aorta rat rings compared to carbachol, while the methanol extracts from dried biomass at 100, 90, and 50 °C were potent vasorelaxants [31].
Furthermore, spasmogenic and spasmolytic activities differ between the two subspecies [23].A. mexicanassp. mexicana extracts were spasmogenic in guinea pig ileum, whileA. mexicanassp. xolocotziana contains a higher amount of acacetin and tilianin. xolocotziana should be used to treat gastrointestinal afflictions [23] (Table 2).
Table 2. Biological activity of extracts and compounds isolated from Agastache mexicana.
| Taxa | Antioxidant | Antimicrobial | Phytotoxic | Central Nervous System | Antihypertensive and Vasorelaxant | Spasmolytic and Antinociceptive |
|---|---|---|---|---|---|---|
| Agastache mexicana | Reduction percentage: Methanol extract: DPPH ~93%, ABTS ~99%, and TBARS ~94%. Eugenol: DPPH ~94%, ABTS ~98%, and TBARS ~98% [25]. Aqueous extract: DPPH (IC50 502.3 µg mL−1) and TEAC (926.9 µmol Trolox g extract−1) [16]. DPPH assay of herbal products containing A. mexicana: Hydroalcoholic extracts reduction percentage: A, 80.3%; B, 81.4%; C, 80.9%; D, 83.1% [32]. |
Aqueous extract for the synthesis of silver nanoparticles with activity against Escherichia coli [33]. | Phytotoxic activity at 1000 µg mL−1 (% of growth inhibition): hexane extract (60.5%) acetone extract (85.7%) and ethanolic extract (35.5%) on Amaranthus hypochondriacus L. Acetone extract (48.7%) on Echinochloa crus-galli (L.) P Beauv. [34]. | Aqueous extract: Anxiogenic-like effect in male Wistar rats at doses of 3–12 mg kg−1 in elevated plus-maze, forced swimming, and open field tests [35]. | Vasorelaxant effect on rat aortic rings: methanolic extract of wild plants (Emax = 31.96%, EC50 = 113.72 µg mL−1), in vitro plantlets (Emax = 37.0%, EC50 = 82.64 µg mL−1) and callus (Emax = 59.64%, EC50 = 105.43 µg mL−1) [24]. Aqueous extract: EC50 233.7 μg mL−1 and Emax 24.9% [16]. | |
| Agastachemexicana Linton & Epling ssp. mexicana | DPPH assay of hydroalcoholic extract: IC50 1.4 mg mL−1 [19]. | Anxiolytic effect in mice: Methanol extract and Tilianin at dosage of 30 mg kg−1 (ip.) or 300 mg kg−1 (po.) [28]. Aqueous extract: activity at low doses (0.1–10.0 mg kg−1). Reduced motor coordination and sedative-like actions at high doses (100–200 mg kg−1). Toxicity: LD50 > 5000 mg kg−1 [14]. | Vasorelaxant effect in rat aortic rings: Dichloromethane extract Emax 76.27%, IC50 189.06 µg mL−1 [26]. Methanolic extract: Emax 82.3% and EC50 291.25 µg mL−1 [31]. Acacetin: Emax 63.4% and EC50 210.84 µM. Ursolic acid: Emax 86% and EC50 39.56 µM and in vivo antihypertensive action on SHR [29]. Tilianin induced NO overproduction in rat aorta: 1.49–0.86 µM of nitrites g−1 of tissue and vasorelaxant effect at 0.002–933 µM, Emax 84.7% and EC50 104.4 µg mL−1. Antihypertensive action on SHR at 50 mg kg−1 [26][31]. LD50 of 6624 mg kg−1 in mice and antihypertensive effect (ED50 53.51 mg kg−1) in SHR [27]. | Methanolic extract: spasmogenic effect on guinea pig ileum. Maximal contractile response with 316 µg mL−1 (60%) [23]. | ||
| Agastachemexicana ssp. xolocotziana Bye, E.L. Linares & Ramamoorthy | Anxiolytic effect in mice: Acacetin at dosage of 100–300 mg kg−1 in mice [30]. Aqueous extract activity at low doses (0.1–10.0 mg kg−1). Reduced motor coordination and sedative-like actions at high doses (100–200 mg kg−1). Toxicity: LD50 of 3807 mg kg−1 [14]. | Relaxant effect on rat tracheal rings. Hexane extract: Emax 100.16% and EC50 219 µg mL−1. Dichloromethane extract: Emax 97.78% and EC50 320.8 µg mL−1. Methanol extract: Emax 75.54% and EC50 644.44 µg mL−1 [36]. | Spasmolytic effect on guinea pig ileum: Methanolic extract maximal relaxant effects: 100 µg mL−1 (72.6%)–316.2 µg mL−1 (68.6%) [23]. Acacetin IC50 of 1.1 μM and antinociceptive activity in mice (ED50 2 mg kg−1). Ursolic acid: spasmolytic response and antinociceptive effect: ED50 3 mg kg−1 [30] and 2 mg kg−1 in mice [21]; ED50 44 mg kg−1 in rats [21]. Writhing test in mice: maximum latency at 300 mg kg−1 and antinociceptive response of extracts: hexane 73% (ED50: 56.68 mg kg−1), ethyl acetate 90% (ED50: 31.81 mg kg−1), and methanol 48% (ED50: 253.25 mg kg−1). Anti-inflammatory activity on the rat paw and formalin tests. Plantar test: antinociceptive responses of hexane extract from 30 to 300 mg kg−1 [30]. |
* DPPH: 1,1-Diphenyl-2-picrylhydrazyl; ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), TBARS: thiobarbituric acid reactive substance, TEAC: Trolox equivalent antioxidant capacity, NO: nitric oxide, LD50: median lethal dose, IC50: mean inhibitory concentration, EC50: mean effective concentration, Emax: maximum relaxant effect, ED50: median effective dose, SHR: spontaneously hypertensive rats, ip: intraperitoneal injection, po: oral administration.
Studies of the effect ofA. mexicanassp. xolocotziana hexanic, dichloromethanic and methanolic extracts on tracheal rat rings found a relaxant-like activity [36], while the essential oil ofA. mexicanassp. mexicana caused relaxation of guinea pig tracheal tissue. The essential oil contains primarily estragole and D-limonene, which act as relaxants and anti-asthmatic compounds [37]
Plant-tissue cultures ofA. mexicanassp. mexicana further confirmed the observed in vivo antihypertensive and vasorelaxant effects in SHR [24][26]. Tilianin isolated from methanolic extracts obtained from in vitro plantlets and calli confirmed the conservation of its vasorelaxant effects. The in vitro methanolic extracts contained a higher concentration of tilianin and produced a stronger vasorelaxant effect on aorta rat rings than extracts from wild plants [24]
The first pharmacological study on the effects of water-solubleA. mexicanaextract on the central nervous system showed an anxiogenic-like effect in behavioral experiments at the doses tested in male rats [35]. Chemical and pharmacological studies performed in 2014 to identify the effects aqueous extracts from both subspecies have on the central nervous system found similar chemical profiles but different compound abundances. In vivo experiments in different pain models in rodents confirmed the antinociceptive effect of organicA. mexicanassp. Taken together, results from pharmacological studies validate the traditional use of toronjil (lemon balm) to relieve gastrointestinal disorders, stomach pain, asthma, anxiety, insomnia, and hypertension.
Traditional Mexican medicine promotes lemon balm as an herbal product. However, herbal products lack strict quality control to guarantee their chemical composition or authenticity for manufacture. However, various herbal products containingA. mexicanafound significant antioxidant activity [32]. Additional reports detailed similar antioxidant activity ofA. mexicana[19][25] (Table 2).
A. mexicanavar. “Sangria” (ssp. mexicana) inflorescences are edible and have nutraceutical potential as they contain sugars and secondary metabolites. Compared to otherAgastachespecies and the Lamiaceae family members, lemon balm inflorescences have higher polyphenols and flavonoid content and higher antioxidant properties [19].
Aside from its use as a medicinal plant,A. mexicanaproduces bioactive compounds with antifungal activity. Research has shown the potential for its use as a non-toxic botanical fungicidal and as an alternative to synthetic fungicides [38]. A recent study tested the effect of addingA. mexicanaessential oil to wheat grains as a food preservative for flour and dough. These properties indicate the essential oil as a candidate non-toxic food preservative [39].
In addition, the phytotoxic potential of organic extracts obtained with hexane, acetone and ethanol was explored (Table 3). The acetone extract ofA. mexicana(subspecies not specified) leaves was the most active, with an IC50of 71 µg/mL on the radical growth ofAmaranthus hypochondriacusL. [34].
3. Potential and Perspectives
The holistic approach to studying theA. mexicanaspecies focuses on biology, ethnobotany, chemical composition, and biological activity. The species has potential pharmacological uses as a source of bioactive compounds, such as tilianin, acacetin, apigenin, ursolic acid, and oleanolic acid [24] in areas such as drug development, disease modeling, and other biological explorations [40].
Agro-industrial applications and the production of essential oils require greater knowledge and understanding of endemic and native species of cultivated aromatic plants, such asAgastache mexicana. It is also essential to develop appropriate technologies for industrial applications and products. The information provided in this review supports the cultivation of lemon balm to take advantage of the plant, extracts, and essential oils. Red lemon balm has high essential oil yields, averaging 2.26%, regardless of the type of distillation device [22], while white lemon balm yields about 1.2%.
Evidence from biotechnological studies show thatA. mexicanaplant tissue cultures have great potential as a source of tilianin and other bioactive compounds [24], but information is scarce in terms of a technological package of cultivation and standardization of its components. There is phenotypic variability between subspecies and populations concerning wild or cultivated plants [20]. These results suggest that there may be genetic variability and the potential for genetic improvement ofA. mexicanato increase plant biomass, improve resistance to climatic factors, resistance to pests and diseases. Furthermore, this variability could allow for the development of populations with specific chemotypes.
References
- Santillán, M.A.; López, M.E.; Aguilar, S.; Aguilar, A. Estudio etnobotánico, arquitectura foliar y anatomía vegetativa de Agastache mexicana ssp. mexicana y A. mexicana ssp. xolocotziana. Rev. Mex. Biodivers. 2008, 79, 513–524.
- Estrada-Reyes, R.; Aguirre Hernández, E.; García-Argáez, A.; Soto Hernández, M.; Linares, E.; Bye, R.; Heinze, G.; Martínez-Vázquez, M. Comparative chemical composition of Agastache mexicana subsp. mexicana and A. mexicana subsp. xolocotziana. Biochem. Syst. Ecol. 2004, 32, 685–694.
- Martínez-Gordillo, M.; Bedolla-García, B.; Cornejotenorio, G.; Fragoso-Martínez, I.; García-Peña, M.D.R.; González-Gallegos, J.G.; Lara-Cabrera, S.I.; Zamudio, S. Lamiaceae de México. Bot. Sci. 2017, 95, 780–806.
- Universitarios, D.G. de R. Dirección General de Repositorios Universitarios, Universidad Nacional Autónoma de México. Portal de Datos Abiertos UNAM, Colecciones Universitarias. Available online: https://datosabiertos.unam.mx/ (accessed on 21 March 2021).
- Verpoorte, R.; Crommelin, D.; Danhof, M.; Gilissen, L.J.W.J.; Schuitmaker, H.; van der Greef, J.; Witkamp, R.F. Commentary: “A systems view on the future of medicine: Inspiration from Chinese medicine”? J. Ethnopharmacol. 2009, 121, 479–481.
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative approaches for recovery of phytoconstituents from medicinal/aromatic plants and biotechnological production. Molecules 2020, 25, 309.
- Mushtaq, M.Y.; Verpoorte, R.; Kim, H.K. Zebrafish as a model for systems biology. Biotechnol. Genet. Eng. Rev. 2013, 29, 187–205.
- Zielińska, S.; Matkowski, A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416.
- Ayres, G.S.; Widrlechner, M.P. The Genus Agastache as Bee Forage: A Historical Perspective. Am. Bee J. 1994, 134, 341–348.
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial activity of Agastache honey and characterization of its bioactive compounds in comparison with important commercial honeys. Front. Microbiol. 2019, 10, 1–16.
- Flora Melífera de la Ciudad de México. In Fortalecimiento de la Producción Apícola en Suelo de Conservación de la Ciudad de MéxicoN; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020.
- Bye, R.; Linares, E.; Ramamoorthy, T.P.; García, F.; Collera, O.; Palomino, G.; Corona, V. Agastache mexicana Subs. xolocotziana (Lamiaceae). A new taxon from mexican medicinal plants. Phytologia 1987, 62, 156–163.
- Gutiérrez, S.L.G.; Chilpa, R.R.; Jaime, H.B. Medicinal plants for the treatment of “nervios”, anxiety, and depression in Mexican Traditional Medicine. Rev. Bras. Farmacogn. 2014, 24, 591–608.
- Estrada-Reyes, R.; López-Rubalcava, C.; Ferreyra-Cruz, O.A.; Dorantes-Barrón, A.M.; Heinze, G.; Moreno Aguilar, J.; Martínez-Vázquez, M. Central nervous system effects and chemical composition of two subspecies of Agastache mexicana; An ethnomedicine of Mexico. J. Ethnopharmacol. 2014, 153, 98–110.
- Patwardhan, B.; Vaidya, A.; Chorghade, M.; Joshi, S. Reverse Pharmacology and Systems Approaches for Drug Discovery and Development. Curr. Bioact. Compd. 2008, 4, 201–212.
- Ibarra-Alvarado, C.; Rojas, A.; Mendoza, S.; Bah, M.; Gutiérrez, D.M.; Hernández-Sandoval, L.; Martínez, M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010, 48, 732–739.
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801.
- Linares, E.; Bye, R. Traditional Markets in Mesoamerica: A Mosaic of History and Traditions. Ethnobot. Mex. 2016, 151–177.
- Najar, B.; Marchioni, I.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Volatilomic analysis of four edible flowers from agastache genus. Molecules 2019, 24, 4480.
- Carrillo-Galván, G.; Bye, R.; Eguiarte, L.E.; Cristians, S.; Pérez-López, P.; Vergara-Silva, F.; Luna-Cavazos, M. Domestication of aromatic medicinal plants in Mexico: Agastache (Lamiaceae)- A n ethnobotanical, morpho-physiological, and phytochemical analysis. J. Ethnobiol. Ethnomed. 2020, 16, 1–16.
- Verano, J.; González-trujano, M.E.; Déciga-campos, M.; Ventura-martínez, R.; Pellicer, F. Pharmacology, Biochemistry and Behavior Ursolic acid from Agastache mexicana aerial parts produces antinociceptive activity involving TRPV1 receptors, cGMP and a serotonergic synergism. Pharmacol. Biochem. Behav. 2013, 110, 255–264.
- Jadczak, P.; Bojko, K.; Wesołowska, A. Chemical composition of essential oils isolated from Mexican giant hyssop via hydrodistillation in Deryng and Clevenger apparatuses. Ann. Hortic. 2017, 27, 11–17.
- Ventura-Martínez, R.; Rodríguez, R.; González-Trujano, M.E.; Ángeles-López, G.E.; Déciga-Campos, M.; Gómez, C. Spasmogenic and spasmolytic activities of Agastache mexicana ssp. mexicana and A. mexicana ssp. xolocotziana methanolic extracts on the guinea pig ileum. J. Ethnopharmacol. 2017, 196, 58–65.
- Carmona-Castro, G.; Estrada-Soto, S.; Arellano-García, J.; Arias-Duran, L.; Valencia-Díaz, S.; Perea-Arango, I. High accumulation of tilianin in in-vitro cultures of Agastache mexicana and its potential vasorelaxant action. Mol. Biol. Rep. 2019, 46, 1107–1115.
- Esquivel-Gutiérrez, E.R.; Coria-Orozco, E.; Torres-Martínez, R.; Hernández-García, A.; Ríos-Chávez, P.; Manzo-Ávalos, S.; Saavedra-Molina, A.; Salgado-Garciglia, R. Antioxidant effects of Agastache mexicana extracts: An in vitro approach. FASEB J. 2017, 31, lb117.
- Hernández-Abreu, O.; Castillo-España, P.; León-Rivera, I.; Ibarra-Barajas, M.; Villalobos-Molina, R.; González-Christen, J.; Vergara-Galicia, J.; Estrada-Soto, S. Antihypertensive and vasorelaxant effects of tilianin isolated from Agastache mexicana are mediated by NO/cGMP pathway and potassium channel opening. Biochem. Pharmacol. 2009, 78, 54–61.
- Hernández-Abreu, O.; Torres-Piedra, M.; García-Jiménez, S.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Montes, S.; Rembao, D.; Estrada-Soto, S. Dose-dependent antihypertensive determination and toxicological studies of tilianin isolated from Agastache mexicana. J. Ethnopharmacol. 2013, 146, 187–191.
- González-Trujano, M.E.; Ponce-mu, H.; Hidalgo-figueroa, S.; Navarrete-Vaquez, G.; Estrada-Soto, S. Depressant effects of Agastache mexicana methanol extract and one of major metabolites tilianin. Asian Pac. J. Trop. Med. 2015, 185–190.
- Flores-Flores, A.; Hernández-Abreu, O.; Rios, M.Y.; León-Rivera, I.; Aguilar-Guadarrama, B.; Castillo-España, P.; Perea-Arango, I.; Estrada-Soto, S. Vasorelaxant mode of action of dichloromethane-soluble extract from Agastache mexicana and its main bioactive compounds. Pharm. Biol. 2016, 54, 2807–2813.
- González-Trujano, M.E.; Ventura-Martínez, R.; Chávez, M.; Díaz-Reval, I.; Pellicer, F. Spasmolytic and antinociceptive activities of ursolic acid and acacetin identified in Agastache mexicana. Planta Med. 2012, 78, 793–799.
- Hernández-Abreu, O.; Durán-Gómez, L.; Best-Brown, R.; Villalobos-Molina, R.; Rivera-Leyva, J.; Estrada-Soto, S. Validated liquid chromatographic method and analysis of content of tilianin on several extracts obtained from Agastache mexicana and its correlation with vasorelaxant effect. J. Ethnopharmacol. 2011, 138, 487–491.
- Salazar-Aranda, R.; de la Torre-Rodríguez, Y.C.; Alanís-Garza, B.A.; Pérez-López, L.A.; Waksman-de-Torres, N. Evaluación de la actividad biológica de productos herbolarios comerciales Ricardo. Med. Univ. 2009, 11, 156–164.
- López, J.L.; Baltazar, C.; Torres, M.; Ruíz-Baltazar, A.; Esparza, R.; Rosas, G. Biosynthesis of Silver Nanoparticles Using Extracts of Mexican Medicinal Plants. In Characterization of Metals and Alloys; Campos Pérez, R., Cuevas Contreras, A., Muñoz Esparza, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; p. 255. ISBN 9783319316949.
- Santiago, R.; Rojas, I.; Arvizu, G.; Muñoz, D.; Pérez, D.; Sucilla, M. Caracterización del potencial fitotóxico de Agastache mexicana (kunth.) Lint et Epling. Investig. Univ. Multidiscip. 2005, 4, 14–20.
- Molina-Hernández, M.; Téllez-Alcántara, P.; Martínez, E. Agastache mexicana may produce anxiogenic-like actions in the male rat. Phytomedicine 2000, 7, 199–203.
- Sánchez-Recillas, A.; Mantecón-Reyes, P.; Castillo-España, P.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Estrada-Soto, S. Tracheal relaxation of five medicinal plants used in Mexico for the treatment of several diseases. Asian Pac. J. Trop. Med. 2014, 7, 179–183.
- Navarrete, A.; Ávila-Rosas, N.; Majín-León, M.; Balderas-López, J.L.; Alfaro-Romero, A.; Tavares-Carvalho, J.C. Mechanism of action of relaxant effect of agastache mexicana ssp. Mexicana essential oil in guinea-pig trachea smooth muscle. Pharm. Biol. 2017, 55, 96–100.
- Juárez, Z.N.; Hernández, L.R.; Bach, H.; Sánchez-arreola, E.; Bach, H. Antifungal activity of essential oils extracted from Agastache mexicana ssp. xolocotziana and Porophyllum linaria against post-harvest pathogens. Ind. Crop. Prod. 2015, 74, 178–182.
- Juárez, Z.N.; Bach, H.; Bárcenas-Pozos, M.E.; Hernández, L.R. Impact of the Persistence of Three Essential Oils with Antifungal Activities on Stored Wheat Grains, Flour, and Baked Products. Foods 2021, 10, 213.
- Calvo-Irabien, L.M. Native Mexican aromatic flora and essential oils: Current research status, gaps in knowledge and agro-industrial potential. Ind. Crops Prod. 2018, 111, 807–822.




