
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisabetta Aldieri | + 1662 word(s) | 1662 | 2021-11-16 07:18:56 | | | |
| 2 | Camila Xu | Meta information modification | 1662 | 2021-11-22 01:53:51 | | |
Video Upload Options
Malignant pleural mesothelioma (MPM) is an aggressive tumor mainly associated with asbestos exposure and is characterized by a very difficult pharmacological approach. Therefore, it is crucial to better understand the molecular mechanisms involved in MPM development and metastasis, in an attempt to open new scenarios that are useful in the identification of predictive markers and to improve the pharmacological approach against this aggressive cancer.
1. Introduction
2. Malignant Pleural Mesothelioma
3. Asbestos Effects and MPM Development
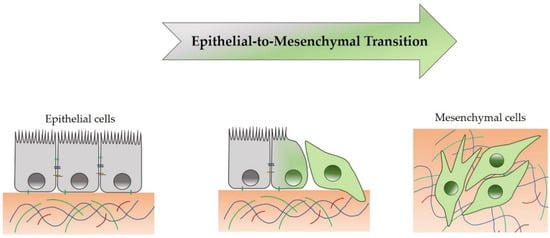
4. Epithelial-to-Mesenchymal Transition
5. EMT and TME Crosstalk in MPM: A Possible Therapeutic Approach
6. Conclusions
Malignant pleural mesothelioma (MPM) is such an aggressive tumor that it is currently very difficult to treat. Conventional therapies and current diagnostic biomarkers are not effective. Thus, the identification of new diagnostic markers to identify the early stages of tumor, and the individuation of novel drug targets, to have other available therapeutic approaches, are crucial goals. To reach this aim, it is necessary to understand MPM pathogenetic mechanisms induced by exposure to the main risk factor, particularly asbestos fibers. This entry shows how, among events that have been demonstrated to be involved in the MPM onset and progression, EMT is particularly crucial: this process mediates the effects of asbestos fibers on human mesothelial cells through a mechanism mediated by crosstalk between oxidative stress and TGFβ, which can cooperate in the MPM development and metastasis.
Taken as a whole, these mechanisms provide insights that can help improve the diagnosis and treatment of MPM, with the aim of achieving new ways to counteract this aggressive cancer.
References
- Gaudino, G.; Xue, J.; Yang, H. How asbestos and other fibers cause mesothelioma. Trans. Lung Cancer Res. 2020, 9 (Suppl. 1), S39–S46.
- Kamp, D.W. Asbestos-induced lung diseases: An update. Transl. Res. 2009, 153, 143–152.
- Cannito, S.; Novo, E.; Di Bonzo, L.V.; Busletta, C.; Colombatto, S.; Parola, M. Epithelial-mesenchymal transition: From molecular mechanisms, redox regulation to implications in human health and disease. Antioxid. Redox Signal. 2010, 12, 1383–1430.
- Giannoni, E.; Parri, M.; Chiarugi, P. EMT and oxidative stress: A bidirectional interplay affecting tumor malignancy. Antioxid. Redox Signal. 2012, 16, 1248–1263.
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression. Oxid. Med. Cell Longev. 2015, 2015, 654594.
- Reid, G. MicroRNAs in mesothelioma: From tumour suppressors and biomarkers to therapeutic targets. J. Thorac. Dis. 2015, 7, 1031–1040.
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.G.; Maskell, N.A.; Psallidas, I. Malignant pleural mesothelioma: An update on investigation, diagnosis and treatment. Eur. Respir. Rev. 2016, 25, 472–486.
- Qi, F.; Okimoto, G.; Jube, S.; Napolitano, A.; Pass, H.I.; Laczko, R.; Demay, R.M.; Khan, G.; Tiirikainen, M.; Rinaudo, C.; et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am. J. Pathol. 2013, 183, 1654–1666.s.
- Menis, J.; Pasello, G.; Remon, J. Immunotherapy in malignant pleural mesothelioma: A review of literature data. Transl. Lung. Cancer Res. 2021, 10, 2988–3000.
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386.
- Beasley, M.B.; Galateau-Salle, F.; Dacic, S. Pleural mesothelioma classification update. Virchows Arch. 2021, 478, 59–72.
- Klebe, S.; Brownlee, N.A.; Mahar, A.; Burchette, J.L.; Sporn, T.A.; Vollmer, R.T.; Roggli, V.L. Sarcomatoid mesothelioma: A clinical-pathologic correlation of 326 cases. Mod. Pathol. 2010, 23, 470–479.
- Quetel, L.; Meiller, C.; Assié, J.; Blum, Y.; Imbeaud, S.; Montagne, F.; Tranchant, R.; Wolf, J.; Caruso, S.; Copin, M.; et al. Genetic alterations of malignant pleural mesothelioma: Association with tumor heterogeneity and overall survival. Mol. Oncol. 2020, 14, 1207–1223.
- Attanoos, R.L.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; Roggli, V.L. Malignant Mesothelioma and Its Non-Asbestos Causes. Arch. Pathol. Lab. Med. 2018, 142, 753–760.
- Barlow, C.A.; Grespin, M.; Best, E.A. Asbestos fiber length and its relation to disease risk. Inhal. Toxicol. 2017, 9, 541–554.
- Manning, C.B.; Vallyathan, V.; Mossman, B.T. Diseases caused by asbestos: Mechanisms of injury and disease development. Int. Immunopharmacol. 2002, 2, 91–200.
- MacCorkle, R.A.; Sluttery, S.D.; Nash, D.R.; Brinkley, B.R. Intracellular protein binding to asbestos induces aneuploidy in human lung fibroblasts. Cell Motil. Cytoskel. 2006, 63, 646–657.
- Broaddus, V.C.; Everitt, J.I.; Black, B.; Kane, A.B. Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 153–178.
- Hiltbrunner, S.; Mannarino, L.; Kirschner, M.B.; Opitz, I.; Rigutto, A.; Laure, A.; Lia, M.; Nozza, P.; Maconi, A.; Marchini, S.; et al. Tumor Immune Microenvironment and Genetic Alterations in Mesothelioma. Front. Oncol. 2021, 11, 2223.
- Tamminen, J.A.; Myllärniemi, M.; Hyytiäinen, M.; Keski-Oja, J.; Koli, K. Asbestos exposure induces alveolar epithelial cell plasticity through MAPK/Erk signaling. J. Cell. Biochem. 2012, 113, 2234–2247.
- Katsuno, Y.; Derynck, R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-β family. Dev. Cell 2021, 56, 726–746.
- Schramm, A.; Opitz, I.; Thies, S.; Seifert, B.; Moch, H.; Weder, W.; Soltermann, A. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2010, 37, 566–572.
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84.
- Shah, P.P.; Dupre, T.V.; Siskind, L.J.; Beverly, L.J. Common cytotoxic chemotherapeutics induce epithelial-mesenchymal transition (EMT) downstream of ER stress. Oncotarget 2017, 8, 22625–22639.
- Song, W.; Mazzieri, R.; Yang, T.; Gobe, G.C. Translational significance for tumor metastasis of tumor-associated macrophages and epithelial-mesenchymal transition. Front. Immunol. 2017, 8, 1106.
- Taki, M.; Abiko, K.; Ukita, M.; Murakami, R.; Yamanoi, K.; Yamaguchi, K.; Hamanishi, J.; Baba, T.; Matsumura, N.; Mandai, M. Tumor Immune Microenvironment during Epithelial–Mesenchymal Transition. Clin. Cancer Res. 2021, 27, 4669–4679.
- Sakai, K.; Inoue, M.; Mikami, S.; Nishimura, H.; Kuwabara, Y.; Kojima, A.; Toda, M.; Ogawa-Kobayashi, Y.; Kikuchi, S.; Hirata, Y.; et al. Functional inhibition of heat shock protein 70 by VER-155008 suppresses pleural mesothelioma cell proliferation via an autophagy mechanism. Thorac. Cancer 2021, 12, 491–503.
- Birnie, K.A.; Prêle, C.M.; Thompson, P.J.; Badrian, B.; Mutsaers, S.E. Targeting microRNA to improve diagnostic and therapeutic approaches for malignant mesothelioma. Oncotarget 2017, 8, 78193–78207.
- Matsumoto, S.; Nabeshima, K.; Hamasaki, M.; Shibuta, T.; Umemura, T. Upregulation of microRNA-31 associates with a poor prognosis of malignant pleural mesothelioma with sarcomatoid component. Med. Oncol. 2014, 31, 303.
- Chintala, N.K.; Restle, D.; Quach, H.; Saini, J.; Bellis, R.; Offin, M.; Beattie, J.; Adusumilli, P.S. CAR T-cell therapy for pleural mesothelioma: Rationale, preclinical development, and clinical trials. Lung Cancer 2021, 157, 48–59.
- Wirawan, A.; Tajima, K.; Takahashi, F.; Mitsuishi, Y.; Winardi, W.; Hidayat, M.; Hayakawa, D.; Matsumoto, N.; Izumi, K.; Asao, T.; et al. A novel therapeutic strategy targeting the mesenchymal phenotype of malignant pleural mesothelioma by suppressing LSD1. Mol. Cancer Res. 2021, 21, 230.
- Bertoglio, P.; Aprile, V.; Ambrogi, M.; Mussi, A.; Lucchi, M. The role of intracavitary therapies in the treatment of malignant pleural mesothelioma. J. Thorac. Dis. 2017, 10, 2.




