
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cecilia Jiménez-López | + 5815 word(s) | 5815 | 2020-08-05 08:12:39 | | | |
| 2 | Nicole Yin | Meta information modification | 5815 | 2020-08-24 04:54:20 | | | | |
| 3 | Nicole Yin | Meta information modification | 5815 | 2020-11-05 08:33:05 | | |
Video Upload Options
Extra virgin olive oil (EVOO) is responsible for a large part of many health benefits associated with Mediterranean diet as it is a fundamental ingredient of this diet. The peculiarities of this golden highly valued product are in part due to the requirements that must be met to achieve this title, namely, it has to be obtained using exclusively mechanical procedures, its free acidity cannot be greater than 0.8%, it must not show sensory defects and it has to possess a fruity taste. All these characteristics are key factors to EVOO quality, thus the chemical composition of these many health-promoting compounds, such as unsaturated fatty acids (which are also the major compounds, especially oleic acid), as well as minor components such as tocopherols or phenolic compounds (which behave as natural antioxidants) must be preserved. Due to the presence of all these compounds, the daily consumption of EVOO entails health benefits such as cardioprotective, antioxidant, anti-inflammatory, anti-tumor properties or acting as regulators of the intestinal microbiota, among others. Taking all together, conserving EVOO chemical composition is essential to preserve its properties, so it is worth to control certain factors during storage like exposure to light, temperature, oxygen presence or the chosen packaging material, to maintain its quality and extend its shelf-life until its consumption.
1. Definition
Among virgin olive oil (VOO), the EU establishes three types of oils: extra virgin olive oil (EVOO), VOO, and lampante olive oil. Furthermore, IOC adds an extra subgroup called ordinary virgin oil. All VOOs are characterized for being obtained by mechanical processes (only washing, decantation, centrifugation and filtration) under specific thermal conditions which do not cause any alteration. Afterward, they are divided according to their acidity, which gives an idea of the content in free fatty acids based on the percentage of oleic acid. Lower acidity values guarantee a high-quality oil, showing it has been obtained from healthy olives and under ideal conditions. Moreover, they are submitted to sensory analysis to asses some requirements[1].
Particularly, EVOO is obtained directly from olives, that is, pure olive juice. It is considered the highest quality oil, and in general, it is characterized for having a low acidity, up to 0.8% and a sensory grade higher than 6.5 points, thus, having perfect aroma and flavor[2][3]. Regarding sensory aspects, EVOO must show a fruity note higher than 0 and, more importantly, a median of zero defects[4]. In addition, and to offer the most possible information to the consumer, these oils are usually labeled as intense, medium or light depending on their positive attributes[5].
2. Introduction
Regarding its composition, EVOO is mainly composed of triglycerides (97–99%) and minor compounds (1–3%), which are the principal responsible for its biological properties and sensory attributes. It has a high content of MUFA (65–83%), especially oleic acid, and some polyunsaturated fatty acids (PUFA) such as linoleic acid, which is considered a potent fatty acid on the reduction of low-density lipoprotein (LDL) cholesterol. This lipid profile and also high ω6/ω3 ratio have been linked to protective effects on coronary, autoimmune and inflammatory disorders but also as anti-thrombotic and regulators of blood pressure[6][7][8]. Concerning bioactive compounds, their main representatives are the same of oil in general, namely phenolic compounds such as hydroxytyrosol and derivatives (oleuropein and tyrosol), tocopherols but also other compounds as hydrocarbons (i.e., squalene) or pigments like provitamin A compounds[2][8]. However, it must be mentioned that some of these compounds such as squalene might be lost during refinery, so they can only be found on this type of oil[7].
As mentioned, all these bioactive compounds are known for their biological properties and positive effects on human health. EVOO inclusion in the diet and its bioactive molecules have been studied to identify its effects. EVOO is known for having a high content of antioxidant compounds with protective properties against free radicals. Therefore, it has been pointed out that its high consumption is related to a generally low risk of suffering colon, breast or skin cancer as well as beneficial effects on aging and coronary diseases[9]. It has also been proposed as a preventing tool of Alzheimer’s and other neurodegenerative diseases[2], as anti-inflammatory[10] and also as immune-stimulating[6]. Another study proved that rats fed with EVOO in substitution of lipids and complemented with physical exercise, could avert cartilage diseases as osteoarthritis[11]. Additionally, EVOO consumption has shown positive effects on gut microbiota[12]. Some studies have researched the bioavailability of phenolic compounds of EVOO and found that 55–60% of them can be absorbed, most of them at small intestine[13]. Moreover, their different compounds have shown other beneficial properties like antimicrobial, antitumor or protective agents against cellular damage[8][14]. More specific studies have also related EVOO treatment with positive gene regulation and with micro ribonucleic acid (miRNA) modulation of target genes associated with synaptic plasticity as well as to motor and cognitive behavior[15]. There are multiple bibliographic references directed towards proving all these promoting effects. Nevertheless, a specific study must be highlighted, the PREDIMED trial (prevention through MED, in Spanish). This is one of the largest nutritional studies ever conducted in Spain, which evaluated the effects on primary prevention of CVD when following a MED supplemented by EVOO or nuts mix[16]. This project groups together different studies, which have brought to light several positive consequences: reduction of CVD risk, reduction of C-reactive protein, reduction of atrial fibrillation, prevention of diabetes and metabolic syndrome, reduction of diastolic blood pressure, higher protection against breast cancer or lower prevalence of non-alcoholic fatty acid liver disease[7][17][18][19]. However, more epidemiologic studies and controlled trials are necessary to better validate and understand the beneficial effects of EVOO consumption. At last, it is worth mentioning that new disciplines (encompassed as nutrigenomics) are also working on new approaches for evaluating the health-promoting effects, characterizing new markers, and understanding their action mechanisms[15][20].
3. Main Components of EVOO
Virgin olive oils are oils obtained from the fruit of the olive tree (Olea europaea L.) solely by mechanical or other physical means under conditions, particularly thermal conditions, that do not lead to alterations in the oil, and which have not undergone any treatment other than washing, decantation, centrifugation and filtration[21]. The use of said physical techniques allows the preservation of many compounds that make EVOO one of a kind among plant oils. Its uniqueness is due to the abundance of fatty acids, PUFA and MUFA but also the occurrence of many bioactive molecules, like hydrophilic phenols, phytosterols, tocopherols and carotenes that provide several functional properties as well as a long storage time due to their high oxidative stability[22][23][24]. Other vegetable oils, like palm oil, are rich in saturated fats, which are more stable during the cooking or frying processes than the unsaturated ones, avoiding degradation to toxic compounds, but they do not have beneficial properties for the human health as the unsaturated one. On the other hand, sunflower oil is rich in unsaturated fats, especially in linoleic and oleic acids that enhance its healthy properties but decrease its thermal stability[25]. EVOO has a good PUFA:MUFA balance, which confers it stability properties against oxidative thermal degradation, particularly regarding the formation of volatile aldehydes, so EVOO is a proper and recommendable oil to use in food frying[26]. This relation between PUFA and MUFA and the low content of saturated fats also makes EVOO one of the healthiest vegetable oils to be consumed raw because it helps reduce LDL cholesterol levels in the human body[27].
The composition of EVOO is a result of several factors like genotypic potential, environmental factors, fruit ripening, harvest time, agricultural factors (irrigation, sunlight, orchard management) and also technological factors like the method applied for oil extraction or the storage conditions[28]. The concentration of the minor and major fruit components changes and depends on all those variables. Apart from that, the olives ripening process lasts a few months in which the atmospheric, environmental and agricultural conditions play a very important role despite the strict genetic control that can be applied[29][30]. During the maturation and ripening process, the photosynthetic activity decreases as the oil content in the fruits increases[31]. In the first stage of ripening, denominated green stage, the ripe fruits have already acquired their final size, so the maturation proceeds, and the chlorophylls in the skin are slowly swapped by anthocyanins, turning the olives from green to dark violet or purple until the end of the ripping process. These changes in color define the spotted, purple and black stages[29][32]. Olives have the highest phenolic compound content at the phase between green and darker skin, and therefore, the degree of maturation is an important factor to define the right harvest time that will originate the best quality olive oil[33]. Figure 1 shows a summary of representative chemical structures of some relevant compounds present in EVOO.
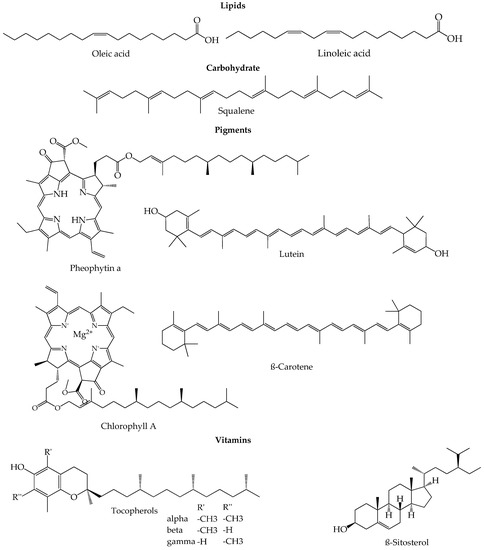
Figure 1. Representative chemical structures of some relevant compounds present in EVOO.
3.1. Primary Metabolites
3.1.1. Lipids
Lipids are one of the principal sources of energy for all living beings and additionally, they are also involved in many physiological functions, as their role as a structural component of cell membranes, nervous system, the production of hormones, brain development and also on the promotion of liposoluble vitamins absorption.
EVOO is widely used in the human diet, especially in the MED and has been long renowned for its many health-promoting properties. Its consumption is associated with reduced risk of several chronic illnesses, like diabetes, hypertension, obesity and CVD[34][35]. These health properties are related to the presence of bioactive compounds like phenolic compounds but also with the high content in MUFA. Olive oil has a high content in oleic acid transforming it into a healthy fat, especially when compared with other vegetable oils[26]. This lipid can decrease the risk of CVD due to its effects on the lipids present in the blood vessels[36]. According to available data, there is 65.2–80.8% of MUFA in the lipidic fraction of olive oil[37]. Other fatty acids found in the total fatty acids composition of olive oils are palmitic acid, palmitoleic acid, stearic acid, linoleic acid, α-linolenic acid, and other minor ones that are listed in Table 1.
Triacylglycerols constitute a big part of the edible oil and a high percentage of the saponifiable fraction is constituted by MUFA[12]. The principal triacylglycerol detected in olive oil is oleic–oleic–oleic (OOO), representing about half of the total triacylglycerol portion found in EVOO. Other triacyclglycerols also present are palmitic–oleic–oleic (POO), oleic–oleic–linoleic (OOL), palmitic–oleic–linoleic (POL) and stearic–oleic–oleic (SOO)[29][38]. Diacylglycerols and monoacylglycerols have been identified in VOO at concentrations of 1%–2.8% and 0.25, respectively[39].
Table 1. Major EVOO components.
Component |
Concentration |
References |
|
|
Lipids |
|||
|
Fatty acids (%) |
|
||
|
Myristic acid |
C14:0 |
0.05 |
[39] |
|
Palmitic acid |
C16:0 |
9.4–19.5 |
|
|
Palmitoleic acid |
C16:1 |
0.6–3.2 |
|
|
Heptadecanoic acid |
C17:0 |
0.07–0.13 |
[37] |
|
Heptadecenoic acid |
C17:1 |
0.17–0.24 |
[37] |
|
Stearic acid |
C18:0 |
1.4–3.0 |
|
|
Oleic acid |
C18:1 |
63.1–79.7 |
|
|
Linoleic acid |
C18:2 |
6.6–14.8 |
|
|
α-Linolenic acid |
C18:3 |
0.46–0.69 |
|
|
Arachidic acid |
C20:0 |
0.3–0.4 |
|
|
Eicosenoic acid |
C20:1 |
0.2–0.3 |
|
|
Docosanoic acid |
C22:0 |
0.09–0.12 |
|
|
Lignoceric acid |
C24:0 |
0.04–0.05 |
[37] |
|
MUFA |
65.2–80.8 |
[37] |
|
|
PUFA |
7.0-15.5 |
[37] |
|
|
Other lipids |
|
|
|
|
Diacylglycerols (%) |
1–2.8 |
[39] |
|
|
Monoacylglycerols (%) |
0.25 |
[39][53] |
|
|
Total sterol content (mg/kg) |
1000–3040 |
||
|
Tocopherols (mg/kg) |
|||
|
α- Tocopherol |
10.2–208 |
||
|
β- Tocopherol |
0.75–1.05 |
[37] |
|
|
γ- Tocopherol |
0.7–2.1 |
[37] |
|
|
Carbohydrates (mg/kg) |
|||
|
Squalene |
200–8260 |
||
|
Pigments (mg/kg) |
|||
|
Total chlorophylls (mg/kg) |
0.15–61.96 |
||
|
Pheophytin-a (mg/kg) |
0.08–0.49 |
[42] |
|
|
Total carotenoids (mg/kg) |
0.53–31.51 |
||
|
β-carotene (mg/kg) |
0.15–0.67 |
[42] |
|
|
Lutein (mg/kg) |
0.65–3.60 |
[42] |
|
|
Other Compounds |
|||
|
Total phenolic compounds (mg/kg) |
213–450 |
[40] |
|
|
Triterpene dialcohols (% of total sterols) |
0.9–2.8 |
[41] |
|
|
β-sitosterol (mg/kg) |
530.2–2638.6 |
[42] |
|
Four classes of sterols also take place in olive oil and are commonly used to check its genuineness because their presence is linked to the quality of the oil. These four classes are common sterols (4-Desmethylsterols), 4α-Methylsterols, triterpene alcohols (4, 4-Dimethylsterols) and triterpene dialcohols[29]. Common sterols in EVOO are present in both free and esterified forms[45]. The leading components of this sterol fraction are campesterol, β-Sitosterol and Δ5-Avenasterol[46][47], and in smaller amounts, it is also possible to find stigmasterol, cholesterol, cholesterol, brassicasterol, sitostanol, ergosterol, campestanol, Δ7-Cholestenol, Δ7-Avenasterol, Δ7-Stigmasterol, Δ7-Campesterol, Δ5,24-Stigmastadienol, Δ5,23-Stigmastadienol, Δ7,24-Ergostadienol, Δ7,22-Ergostadienol, 22,23-Dihydrobrassicasterol and 24-Methylene-cholesterol[48][49]. The total sterol content of EVOO varies between 1000 and 2000 mg/kg, being the first value the inferior limit set by the EU Commission[29]. β-Sitosterol is the main compound in the sterol fraction with values between 75% and 90% of the total sterol fraction, while Δ5-Avenasterol has values between 5% and 20%[48]. Crop year, cultivar, ripeness of the fruit, storage time of the olives before oil extraction and geographic influences all contribute to sterol composition of the final EVOO obtained[50][51][52]. At the same time, storage time and conditions of the final product are also factors that can originate several important changes particularly in the concentrations of each individual sterol[29]. 4-Monomethylsterols are present in smaller amounts and signify part of sterol biosynthesis as intermediates. They can be found in their free and esterified forms[53]. The most common are gramisterol, obtusifoliol, cycloeucalenol and citrostadienol[46][48], and their concentrations vary between 50 and 360 mg/kg of oil[46][54]. Triterpene alcohols, also identified as 4,4-Dimethylsterol, are a very complex fraction that can be in free and esterified form, and whose main compounds are butyrospermol, β-Amyrin, cycloartenol and 24-Methylenecycloartanol. In smaller amounts or trace quantities, cyclosadol, cyclobranol, dammaradienol, germanicol, 24-Tirucalladienol, 24-Methylene-24-Dihydroparkeol, α-Amyrin, taraxerol, 7, parkeol and tirucallol can also be found[48]. Total triterpene alcohol levels range from values of 350 to 1500 mg/kg[54][55]. Lastly, among the triterpene dialcohols class, erythrodiol (5α-olean-12-ene-3β, 28-diol, homo-olestranol) in free and esterified form and uvaol (Δ12-Ursen-3β,28-diol) are the major triterpene dialcohols found in EVOO[56], and their presence is mainly affected by cultivation characteristics[54]. EVOO contains levels of total erythrodiol from 19 to 69 mg/kg of oil, and the free form is inferior to 50 mg/kg[45][54].
3.1.2. Tocopherols
Three isoforms of tocopherols are present in EVOO: α-, β- and γ-tocopherol. α-Tocopherol can be found in its free form and represents more than 90% of the identified section with ranges from 206.5 to 270.9 mg/kg of oil to 191.5 to 292.7 mg/kg of oil, values that fluctuate with variables as the year of harvest and spacing between olive trees[57]. Both, the distance between plants and the crop year influenced statistically tocopherols amount[29][57]. Besides, the high levels of this type of tocopherol may be linked to the high levels of chlorophyll pigments and the simultaneous necessity for singlet oxygen deactivation[58].
Lower quantities of β-Tocopherol (~10 mg/kg), γ-Tocopherol (~20 mg/kg) and δ-Tocopherol (~10 mg/kg) can also be found on EVOO. The total tocopherol concentration seems to decrease in the ripping of the fruits, and the refining or the hydrogenation process causes their degradation, so they are only found in the EVOO and VOO[59].
3.1.3. Carbohydrates
There are two hydrocarbons mainly present in olive oil, 2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-Tetracosahexaene also known as squalene and β-Carotene, which will be addressed in the pigments section of this review. Squalene is the last metabolite synthesized before the sterol ring formation. Some of the beneficial health effects of olive oil are partially linked with the presence of squalene, and it has also demonstrated antitumoral effects against certain types of cancer[60][61]. This compound constitutes more than 90% of the hydrocarbon fraction and is the most abundant compound in the unsaponifiable matter, with concentrations ranging from 200 to 7500 mg/kg oil[43]. In a different study, squalene was reported in even higher concentrations, up to 12,000 mg/kg oil. Squalene content depends on several variables like the type of olive cultivation and the oil extraction technique applied, and it decreases largely during the refining process so it is present in larger quantities in EVOO and VOO[62].
The remaining fraction of carbohydrates in EVOO is composed of triterpene and diterpene, isoprenoid polyolefins, hydrocarbons and n-paraffins[29][62].
3.2. Secondary Metabolites
3.2.1. Phenolic Compounds
The principal group of antioxidants in EVOO are hydrophilic phenols, and these compounds are extremely relevant when it comes to determining the quality of the oil regarding their sensory characteristics, like bitterness, pungency and stability[24][63], as well as determining the organoleptic characteristics of aroma and flavor of each EVOO[39]. The oxidative stability of EVOO depends not only on the olive variety and quality but also on the harvesting time; cultivation area; the degree of unsaturation and the levels of antioxidants present from tocopherols, hydrophilic phenols and carotenes. Besides, factors like oil extraction system and storage conditions also influence its conservation[64].
The correlation of the phenolic content of olive oil and oxidative stability was studied showing that these two are interconnected[65]. Furthermore, EVOO phenolic compounds provide benefits for human health in the prevention of several chronic diseases[66][67]. Various studies indicate that EVOO phenolic compounds have antioxidant, anti-inflammatory, antimicrobial and antitumoral activities, and they can also modulate gene expression to protect proteins that take part in the cellular mechanisms involved in the inflammation process, the oxidative stress resistance and in lipid metabolism[68][69]. Therefore, the major antioxidant substances found in EVOO are polar phenolic compounds that can be present in free, bound or esterified forms[29], and usually, its total phenolic content ranges between 50 and 1000 mg/kg[70], being more common in concentrations between 100 and 300 mg/kg[29]. Likewise, each EVOO has a different phenolic profile, content and composition due to the differences discussed above[64].
Phenolic compounds have been largely reported in EVOO composition, with more than 30 different compounds identified[33][71], being the major phenolic acids present in EVOO hydroxybenzoic, p-Coumaric, ferulic, gallic, syringic, vanillic, caffeic, o-coumaric and sinapic acids[39]. Other types of polyphenols that can also be found in EVOO are flavonoids, lignans, hydroxy-isocromans, secoiridoids and phenolic alcohols. The major flavonoids found in EVOO are luteolin, apigenin and many of their derivates[72][73], whereas the main lignans present are (+)-pinoresinol and (+)-1-Acetoxypinoresinol[74], being the usual lignan content in EVOO between 1 and 100 mg/kg[75].
Secoiridoids are rare phenolic compounds present in plant species, nevertheless, they are found in abundance in Oleaceae species, particularly in O. europaea leaves and fruits. However, they are insoluble in oil and therefore only a small percentage of these compounds ends up in the final EVOO after the mechanic extraction process. Nevertheless, they are one of the most important micronutrients on EVOO for their sensorial and heath properties[24][66]. The most common secoiridoids are demethyloleuropein, oleuropein, ligstroside and their aglycones, the last ones accounting for approximately 90% of the phenolic compounds in EVOO[76]. Secoiridoids are hydrolyzed through crushing and malaxation by enzymatic reactions catalyzed by endogenous b-glucosidases yielding secoiridoid aglycons[77]. The bitterness of olive oil is due to the secoiridoids present, especially the dialdehydic form of oleuropein aglycone[78].
Isochromans are only found at low concentration in EVOO, and the two mainly found are 1-Phenyl-6,7-dihydroxy-isochroman and 1-(3’Methoxy-4’-hydroxy)phenyl-6,7-dihydroxy-isochroman[79]. The concentration of these compounds increases during the extraction process because of the hydrolytic process that originates carbonyl compounds and hydroxytyrosol, which are isocromans derivatives[74]. Finally, the principal phenolic alcohols found in EVOO are tyrosol (p-Hydroxyphenyl ethanol) and hydroxytyrosol (2-[3,4-Dihydroxyphenyl] ethanol). These are present in small concentrations in fresh olive oil but tend to increase along the storage process because of the hydrolysis of olive oil secoiridoids[80].
3.2.2. Pigments
The lipophilic carotenoid and chlorophyll pigments occurring in olive oil are responsible for its characteristic color[81]. The coloration of EVOO is greener in the presence of green olives that have higher chlorophyll content whereas using mature olives with higher carotenoid content we obtain a more yellowish oil, so the final color is a result of the proportions of these pigments[82]. EVOO has a large variety of carotenoids and chlorophylls, from β-Carotene, violaxanthin, neoxanthin, lutein and other xanthophylls to chlorophyll a and b, pheophytin a and b and other minor derivatives[83][84]. These pigments can be found in amounts up to 100 ppm of total carotenoids and major pigments like pheophytin up to 25 ppm, β-carotene up to 15 ppm and lutein up to 10 ppm[82], although these values depend on various factors. The final concentration of each pigment in the final EVOO relies on the physicochemical characteristics of the fruit, the geographic origin, climate and irrigation conditions and the mechanic extraction process used. Storage conditions and final packaging also play a role in pigment concentration and type[82][85][86].
Quality and adulteration of EVOO are sometimes analyzed through the measuring of pigment compounds because they are correlated with EVOO nutritional value, freshness and antioxidant properties[85][87]. In addition, pigments can also be used for the authentication of EVOO, by measuring the chlorophyll and carotenoid pigments of EVOO and comparing them through a quality index, in which the total chlorophylls to total carotenoids ratio must be around 1 and the ratio of minor carotenoids to lutein must be around 0.5, to declare it an authentic olive oil[88]. These parameters are valid for any olive oil regardless of the studied variety. Furthermore, other pigments like violaxanthin, lutein and total pigment content can be useful as a tool to identify a monovarietal EVOO[88]. Chlorophylls, carotenoids and other minor pigments like lutein and violaxanthin can be stable for more than one year in storage regardless of the degree of ripeness and variety of the olives used to produce that oil[89].
The degradation of chlorophylls occurs as a consequence of a pheophytinization reaction that starts from the malaxation step during the extraction of the EVOO and increases throughout storage time[90]. During that process, the chlorophylls naturally present (a and b) are slowly but irreversibly converted into pheophytins a and b, where the central Mg+2 ion of the porphyrin ring is exchanged with two hydrogen atoms making the molecules more stable. These eventually turn to pyropheophytins by the removal of the carboxymethyl group, which are the ultimate products of chlorophyll degradation[91].
4. Biological Properties of EVOO
The Seven Country Study conducted in the middle of the 20th century first demonstrated the cardioprotective capacities and health benefits of MED[92], olive oil being the hallmark of this dietary pattern. Since then, plenty of observational and epidemiological studies have demonstrated the health-promoting effects of consuming olive oil.
Many health benefits of following a MED enriched in EVOO have been reported by the PREDIMED trial[93], such as protection against CVD[16] or oxidative damage[94][95] and prevention of breast cancer[96] and type 2 diabetes mellitus[97]. Many other randomized controlled trials, prospective study cohorts and meta-analysis, supported by in vitro experiments, indicate that EVOO possesses interesting biological activities and pharmaceutical-nutritional properties (Table 2) that exert a beneficial health impact that stands out over other fats and oils. Nevertheless, many attributes of EVOO are also related to the MED, the context in which its beneficial effects have been mainly evaluated.
Table 2. Main bioactivities associated with EVOO consumption.
|
Bioactivity |
Studies Description |
Main Results |
Ref |
|
Cardioprotection |
RCT, PREDIMED (n = 7447 participants at high CVD risk) |
Following a MED enriched with EVOO decreases CVD risk by 30% |
|
|
PREDIMED observational study (n = 7216 participants) |
For each 10g EVOO/day intake, CVD risk decreases by 10% |
[98] |
|
|
Systematic review of 15 RCTs |
10-50mL/day EVOO reduced diastolic blood pressure by 0.7 mm Hg |
[99] |
|
|
Meta-analysis of 9 studies (38,673 stroke and 101,460 CHD cases from RCT, case-control and prospective studies) |
For every increase of 25g of olive oil consumption the risk of CVD, stroke and CHD was reduced by 18%, 26% and 4% respectively |
[100] |
|
|
Meta-analysis of 26 RCTs |
High polyphenol olive oil intake significantly reduced CVD and inflammatory markers |
[101] |
|
|
Antioxidant properties |
European Food Safety Authority health claim. |
5 mg/day of olive oils polyphenols protects blood lipids from oxidation |
[102] |
|
RCTs evaluating the effect of olive oils consumption on blood lipids oxidative state. |
EVOO and high-phenolic olive oils consumption reduces LDL oxidation in a dose-dependent manner |
||
|
Controlled trials with sub-samples of PREDIMED cohort (n = 296) and (n = 210) |
Adherence to a MED enriched with EVOO improves HDL function and protects against LDL oxidation |
||
|
In vitro studies review. |
Lignans present in EVOO show antioxidant activity in vitro |
[107] |
|
|
Anti-inflammatory capacity |
Meta-analysis of 13 studies based on 9 RCTs |
Regular consumption of EVOO reduces IL-6, CRP and TNF-α levels |
[108] |
|
Meta-analysis of RCTs (3106 participants) |
Diet supplemented or enriched in olive oil reduces IL-6 and CRP plasmatic levels |
[109] |
|
|
Randomized crossover study (49 patients) |
High-phenolic virgin olive oil in breakfast reduces postprandial inflammatory response. |
[110] |
|
|
Antitumoral activity |
Meta-analysis of 19 case-control studies (comprising 13,800 cancer cases and 23,340 controls) |
Olive oil consumption is associated with lower odds of developing digestive and breast cancers |
[111] |
|
Cohort-study follow up (2321 breast cancer cases and 1665 controls) and meta-analysis |
Inverse association between adherence to MED and ERN breast cancer |
[112] |
|
|
RCT with a sub-sample of the PREDIMED cohort (n = 4152 women) |
Women following MED enriched in EVOO showed 62% relatively lower risk of breast cancer compared to control low-fat diet |
[96] |
|
|
Systematic review and meta-analysis of 83 studies, comprising a total of 2,130,753 subjects |
The adherence to MED is associated with lower risk of cancer mortality and lower risk of breast, colorectal, gastric and liver cancers, among others |
[113] |
|
|
In vitro experiments of antitumoral activity of phenolic compounds on cancer cell lines |
The phenolic fraction of EVOO, as well as isolated phenolic compounds, shows antitumoral and cytotoxic effect on different cancer cell lines |
||
|
Gut microbiota modulation |
RCT with 12 hypercholesterolemic participants |
Virgin olive oil enriched in phenolic compounds consumption favors gut bifidobacteria growth and decreases serum levels of oxidized LDL |
[117] |
|
Systematic review and meta-analysis of 17 RCTs |
Polyphenols exert a prebiotic action on gut microbiota, improving also CVD and CRC |
[118] |
EVOO: extra virgin olive oil; CVD: cardiovascular disease; CHD: coronary heart disease; RCT: randomized controlled trial; MED: Mediterranean diet; HDL: high-density lipoprotein; LDL: low-density lipoprotein; IL-6: interleukin-6; CRP: C-reactive protein; TNF-α: tumor necrosis factor alpha; IBD: inflammatory bowel disease; ERN: estrogen receptor negative; CRC: colorectal cancer.
4.1. Cardioprotective Properties
The Seven Country Study started in the 1950s first demonstrated the cardioprotective abilities of MED[92] and has been supported by numerous further studies based both on MED and olive oil consumption.
More recently, the cardioprotective benefits of a MED enriched with EVOO have been proven by the PREDIMED study. This multicenter, randomized, controlled trial involved ~ 7500 subjects with potential cardiovascular risk, showing no CVD at enrolment. The PREDIMED trial resulted in a 30% decrease of a major CVD development, such as stroke or myocardial infarction, in comparison to a control group that followed a low-fat diet[16][93]. An observational study based on the PREDIMED cohort indicated that consumptions of 10g EVOO/day are related to CVD risk diminutions up to 10%[98]. A recent systematic review evaluating clinical trials reported that diets enriched with 10-50mL/day of EVOO (but not diets supplemented with EVOO capsules) significantly decreased diastolic blood pressure by 0.73mm Hg[99].
In another meta-analysis of randomized controlled trials, case-control and prospective cohort studies including ~40,000 cases of stroke and ~100,000 cases of coronary heart disease (CHD), it was reported that for each increase of 25 g of olive oil intake, stroke and CHD risk was reduced by 26% and 4%, respectively. When combined stroke and CHD, olive oil consumption also showed preventing effects, decreasing the risk of a CVD event by approximately 18%[100].
The preventive role of EVOO polyphenols against CVD was also documented in a meta-analysis of controlled trials that evaluated the effect of low versus high polyphenol olive oil on markers of CVD risk. Olive oil consumption ranged from 25–75 mL/day. High polyphenol olive oil significantly reduced the CVD-risk markers malondialdehyde, oxidized LDL, total cholesterol, high-density lipoprotein (HDL) cholesterol and also some inflammatory indicators like C-reactive protein (CRP) or interleukin-6 (IL-6)[101].
4.2. Antioxidant Activity
The antioxidant effects of EVOO have been deeply analyzed given the correlation between oxidative stress and CVD or atherosclerosis. Evidence from several meta-analyses and randomized controlled trials, such as the EUROLIVE study[103][105], demonstrated in their analyses the reduction of lipid oxidative damage, the LDL capacity to suffer oxidation and a decrease in oxidized LDL concentration after high-phenolic VOO and EVOO intake, in a dose-dependent way[104][106]. It is also worth noting the health claim allowed by the European Food Safety Authority (EFSA) concerning the protective effects of 5mg/day of olive oil phenolic compounds against LDL oxidation[102]. The PREDIMED cohort was also used to evaluate the antioxidant effects of EVOO. The intervention group with MED enriched with EVOO reported an improvement of HDL atheroprotective functions, oxidative status and composition and also increased resistance to LDL oxidation and low grade of LDL oxidative alterations in comparison to the control low-fat diet[94][95].
Pinoresinol and acetoxypinoresinol, phenolic compounds present in EVOO but not in olive fruits or refined oils, isolated from EVOO or other sources such as sesame seed, have reported in vitro antioxidant capacity[107]. The enzymatic hypoxanthine/xanthine oxidase assay reported a higher antioxidant potential of acetoxypinoresinol, compared to the classic antioxidants, vitamin E and dimethylsulfoxide (IC50 of 0.91, 12.4 and 2.30 nM, respectively). Pinoresinol possesses the ability to inhibit LDL oxidation but has shown inconsistent results (IC50 ranging from 24.6–558 µM, 2,2-Diphenyl-1-picryl-hydrazyl-hydrate free radical assay (DPPH ) colorimetric assay).
4.3. Anti-Inflammatory Activity
Recurrent or chronic inflammation is a main etiologic factor of several non-communicable pathologies, whose prevalence is promptly increasing. Thus, the anti-inflammatory effects of EVOO have gained attention and so have been widely evaluated.
A recent meta-analysis of randomized controlled trials evaluated regular olive oil intake effects on inflammation[108]. The authors reported a decrease in the levels of IL-6, tumor necrosis factor-α (TNF-α) and CRP, the three plasmatic inflammatory indicators considered. Such beneficial effects were shown in studies when EVOO was regularly consumed for more than 3 months. The overall health status of participants should also be taken into account, as the strongest positive effects were reported among unhealthy groups (with type 2 diabetes mellitus or at risk of CVD). Another meta-analysis comprising 3106 participants also showed a significant reduction of IL-6 and CRP levels, when olive oil was consumed as a supplementary or natural intake[109]. The adherence to a high-phenol VOO breakfast decreased the postprandial inflammatory response, reducing the levels of plasma lipopolysaccharides in patients with metabolic syndrome[110]. The higher polyphenol content of EVOO may mediate the mentioned favorable effect as it has demonstrated anti-inflammatory effects in vitro[119]. The anti-inflammatory effect of phenolic compounds-enriched EVOO has also been reported in the adipose tissue in mice, with anti-atherosclerotic effects[120].
Due to these mentioned capacities, EVOO has also been proposed as a potential therapeutic product, reducing inflammation in inflammatory bowel diseases, including ulcerative colitis and Crohn’s disease, being both related to chronic inflammation of the intestinal mucosa[121][122]. The benefits of EVOO consumption were evaluated in other autoimmune and chronic inflammatory diseases such as rheumatoid arthritis[123], systemic lupus erythematosus[124] or multiple sclerosis[125][126] with promising results in murine models. Besides, both in vitro and in vivo studies outline that the anti-inflammatory activity of EVOO provides a neuroprotective effects that could prevent cognitive decline and, therefore, the development of Alzheimer’s disease or elderly dementia[2][127].
4.4. Antitumoral Activity
Traditionally, a lower incidence of cancers such as breast, colorectal, endometrium and prostate cancer has been observed in Mediterranean countries linked to dietary factors, when compared to the USA or other European countries[128]. The antitumoral and anticancer activities of EVOO, as well as of specific fractions or isolated compounds, have been widely studied and evidenced both in vitro with cell cultures and in vivo with animal models, observational cohort studies and clinical trials[129].
Evidence from 19 case-control observational studies, including in total 13,800 cancer cases and 23,340 controls, suggests that olive oil intake is inversely associated with the risk of having any type of cancer (34% lower likelihood of cancer for high olive oil intake)[111]. More precisely, this meta-analysis associated lower odds for developing breast and digestive cancer with olive oil consumption (log odds ratio of −0.45 and −0.36, respectively).
The strongest beneficial effects of EVOO concerning cancer have been described in breast cancer prevention. A meta-analysis reported a statistically significant inverse association between estrogen receptor-negative postmenopausal breast cancer and the adherence to MED[112]. Breast cancer incidence was also included in the PREDIMED trial, which included ~ 4200 women. Those allocated to the MED enriched with EVOO showed a 62% relatively lower risk of breast cancer, compared to women who followed a low-fat diet[93][96]. To our knowledge, no recent large case-control or prospective cohort studies have been conducted about the relationship between colorectal cancer risk and EVOO consumption. Nevertheless, a meta-analysis has determined that MED consumption is related to a 14% lower risk or developing colorectal cancer[113]. Recently, it has been suggested that the antitumoral activity of EVOO, lowering colorectal tumor incidence in rats, could be mediated by epigenetic mechanisms, such as miRNA and deoxyribonucleic acid (DNA) methylation[130].
In vitro experiments have shown that both the phenolic fraction of EVOO and specific compounds such as hydroxytyrosol, caffeic acid, p-Coumaric acid, 1-acetoxypinoresinol and pinoresinol, among others, have antitumoral activity against breast cancer cell lines[114][115]. Other in vitro studies about the cytotoxic effect of the EVOO lignan pinoresinol have reported variable results, depending on the cancer cell line tested. Pinoresinol shows a cytotoxic effect against breast, lung and prostate cell lines, and it inhibits cell viability of colon cancer cells. A synergic effect with other EVOO phenolic compounds have been reported[107][116].
4.5. Positive Modulation of Gut Microbiota
Much of the health benefits of olive oil consumption are attributed to the metabolism of the phenolic compounds carried out by the gut microbiota[131]. It is estimated that 90–95% of total phenolic compounds intake is not absorbed in the small intestine; therefore, they remain in the large intestinal lumen where they are subjected to gut microbiota metabolic activities. As a consequence, polyphenols are converted to low-molecular-weight compounds that are absorbed and responsible for the health benefits derived from polyphenol-rich food, such as EVOO[118][132][133].
A recent review and meta-analysis of randomized controlled trials supports the prebiotic action of polyphenols, capable of modulating and improving intestinal microbe populations, which affects to CVD and colorectal cancer markers[118]. Furthermore, another randomized controlled trial showed that the ingestion of VOO enriched with phenolic compounds decreases the serum levels of oxidized LDL in hypercholesterolemic participants as well as increases the presence of Bifidobacterium spp in feces. Slight changes in the profile of fecal microbial metabolites were also reported. These data suggest that the cardioprotective effect of phenolic compounds could be mediated by the populations of bifidobacteria present in the gut microbiota[117]. The possible modulation of gut microbiota by olive oil and its role in cancer prevention, especially colorectal cancer, has also been suggested[134].
However, the complexity of the human diet, the lack of accuracy in the measurement of dietary intake and the extensive variation on microbiota between individuals challenges the evaluation of how diet changes modulate gut microbiota and its metabolic activity[135].
4.6. Other Bioactivities
The detailed description above was limited to the main bioactivities attributed to EVOO and its specific components. However, some other activities of biological relevance are under study. A recent extensive review highlighted the anti-aging properties of the major phenolic compound in EVOO, hydroxytyrosol, suggesting that it can contribute to the correct regulation of mechanisms that maintain cell homeostasis, such as mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) pathways, whose imbalance is a hallmark of aging[136]. Moreover, this phenolic compound also modulates the metabolism of adipose tissue, stimulating mitochondrial biosynthesis and increasing the function of the mitochondrial respiratory chain in vitro[137].
EVOO consumption has also been associated with the enhancement of blood circulation and coagulation, by reducing platelet aggregation (mechanism related to CVD) and decreasing the levels of coagulation factor VII, effects attributed to minor components of EVOO[138]. Interestingly, a study carried out in murine models reported that polyphenols present in EVOO may improve learning and memory, by reversing the oxidative damage in the brain associated with aging and diseases related to the production of amyloid-β protein[139].
As a final remark, hydroxytyrosol, pinoresinol and oleuropein from EVOO have been reported to possess antimicrobial capacity. Pinoresinol has shown antifungal activity against several pathogenic fungi such as Fusarium verticillioides, Fusarium graminearum and Candida albicans [107]. Additionally, oleuropein and hydroxytyrosol were found to be effective against fungi and several strains of bacteria, viruses, including human immunodeficiency viruses (HIV) and parasites[137][140].
References
- M. Antonietta Baldo; Paolo Oliveri; Sabrina Fabris; Cristina Malegori; Salvatore Daniele; Fast determination of extra-virgin olive oil acidity by voltammetry and Partial Least Squares regression. Analytica Chimica Acta 2019, 1056, 7-15, 10.1016/j.aca.2018.12.050.
- Gustavo C. Román; R.E. Jackson; J. Reis; A.N. Román; J.B. Toledo; E. Toledo; Extra-virgin olive oil for potential prevention of Alzheimer disease. Revue Neurologique 2019, 175, 705-723, 10.1016/j.neurol.2019.07.017.
- Seçmeler, Ö.; Galanakis, C.M. Chapter 8-olive fruit and olive oil. In Innovations in Traditional Foods; Woodhead Publishing: Sawston, UK, 2019; pp. 193–220. ISBN 9780128148884.
- Mariotti, M. Virgin olive oil: Definition. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 11–19.
- Bertuccioli, M.; Monteleone, E. The sensory quality of extra-virgin olive oil. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 35–58.
- Mariotti, M.; Peri, C. The composition and nutritional properties of extra-virgin olive oil. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 21–34.
- Sánchez-Villegas, A.; Sánchez-Tainta, A. Virgin olive oil. In The Prevention of Cardiovascular Disease through the Mediterranean Diet; Academic Press: Cambridge, MA, USA, 2018; pp. 59–87. ISBN 9780128112595.
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2018; Volume 57, ISBN 9780444640574.
- Alexandra Foscolou; Elena Critselis; Demosthenes Panagiotakos; Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60-66, 10.1016/j.maturitas.2018.10.013.
- Mireia Urpi-Sarda; Rosa Casas; Gemma Chiva-Blanch; Edwin Saúl Romero-Mamani; Palmira Valderas-Martínez; Sara Arranz; Cristina Andres-Lacueva; Rafael Llorach; Alexander Medina-Remón; Rosa M Lamuela-Raventós; et al.Ramón Estruch Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacological Research 2012, 65, 577-583, 10.1016/j.phrs.2012.03.006.
- Giuseppe Musumeci; Guglielmo Trovato; Karin Pichler; Annelie Martina Weinberg; Carla Loreto; Paola Castrogiovanni; Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: an in vivo and in vitro study on lubricin expression. The Journal of Nutritional Biochemistry 2013, 24, 2064-2075, 10.1016/j.jnutbio.2013.07.007.
- Mohsen Gavahian; Amin Mousavi Khaneghah; José M. Lorenzo; Paulo E.S. Munekata; Izaskun Garcia-Mantrana; María Carmen Collado; Antonio J. Meléndez-Martínez; Francisco J. Barba; Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends in Food Science & Technology 2019, 88, 220-227, 10.1016/j.tifs.2019.03.008.
- Pérez-Rodrigo, C.; Aranceta, J. Olive oil: Its role in the diet. In The Encyclopedia of Healing Foods; Atria Books: New York, NY, USA, 2015; pp. 158–166.
- Sara Cicerale; Lj Lucas; Rsj Keast; Russell Keast; Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Current Opinion in Biotechnology 2012, 23, 129-135, 10.1016/j.copbio.2011.09.006.
- Marta Piroddi; Adriana Albini; Roberto Fabiani; Lisa Giovannelli; Cristina Luceri; Fausta Natella; Patrizia Rosignoli; Teresa Rossi; Agnese Taticchi; Maurizio Servili; et al.Francesco Galli Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2016, 43, 17-41, 10.1002/biof.1318.
- Ramón Estruch; Emilio Ros; Jordi Salas-Salvadó; María-Isabel Covas; Dolores Corella; Fernando Arós; Enrique Gomez-Gracia; Valentina Ruiz-Gutierrez; Miquel Fiol; Jose Lapetra; et al.Rosa M Lamuela-RaventósLluís Serra-MajemXavier PintóJosep BasoraMiguel-Angel MuñozJose V. SorliJosé Alfredo MartinezMontserrat FítoAlfredo GeaMiguel A. HernánMiguel Ángel Martínez-GonzálezPREDIMED Study Investigators Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts.. New England Journal of Medicine 2018, 378, e34, 10.1056/NEJMoa1800389.
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Martínez-González, M.A.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97.
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean diet rich in extra-virgin olive oil is associated with a reduced prevalence of nonalcoholic fatty liver disease in older individuals at high cardiovascular risk. J. Nutr. 2019, 149, 1920–1929.
- Mourouti, N.; Panagiotakos, D.B. The beneficial effect of a Mediterranean diet supplemented with extra virgin olive oil in the primary prevention of breast cancer among women at high cardiovascular risk in the PREDIMED Trial. Evid. Based Nurs. 2016, 19, 71.
- Edwin N. Frankel; Nutritional and Biological Properties of Extra Virgin Olive Oil. Journal of Agricultural and Food Chemistry 2011, 59, 785-792, 10.1021/jf103813t.
- International Olive Council. World Olive Encyclopaedia; International Olive Oil Council: Madrid, Spain, 1996; ISBN 9788401618819
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654.
- Sánchez, J.; Harwood, J.L. Biosynthesis of triacylglycerols and volatiles in olives. Eur. J. Lipid Sci. Technol. 2002, 104, 564–573.
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84.
- María Mercedes Echarte; Laila Alejandra Puntel; Luis A.N. Aguirrezábal; Assessment of the critical period for the effect of intercepted solar radiation on sunflower oil fatty acid composition. Field Crops Research 2013, 149, 213-222, 10.1016/j.fcr.2013.05.007.
- Lucía Molina-Garcia; Carla S.P. Santos; Sara C. Cunha; Susana Casal; José O. Fernandes; Comparative Fingerprint Changes of Toxic Volatiles in Low PUFA Vegetable Oils Under Deep-Frying. Journal of the American Oil Chemists' Society 2017, 94, 271-284, 10.1007/s11746-016-2943-1.
- Sun, Y.; Neelakantan, N.; Wu, Y.; Lote-oke, R.; Pan, A.; Dam, R.M.; Van palm oil consumption increases LDL cholesterol compared with vegetable oils low in saturated fat in a meta-analysis of clinical. J. Nutr. Nutr. Epidemiol 2015, 145, 1549–1558.
- M. Nieves Criado; J. Ramón Morelló; María-Jose Motilva; María P. Romero; Effect of growing area on pigment and phenolic fractions of virgin olive oils of the arbequina variety in Spain. Journal of the American Oil Chemists' Society 2004, 81, 633-640, 10.1007/s11746-004-954-z.
- Boskou, D.; Blekas, G.; Tsimidou, M. Olive Oil Composition, 2nd ed.; AOCS Press: Urbana, IL, USA, 2006; ISBN 9780128043547.
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562.
- Maria Desamparados Salvador; F. Aranda; Giuseppe Fregapane; Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality A study of four successive crop seasons. Food Chemistry 2001, 73, 45-53, 10.1016/s0308-8146(00)00276-4.
- María Roca; M». Isabel M Nguez-Mosquera; Change in the natural ratio between chlorophylls and carotenoids in olive fruit during processing for virgin olive oil. Journal of the American Oil Chemists' Society 2001, 78, 133-138, 10.1007/s11746-001-0233-z.
- Kalogeropoulos, N.; Kaliora, A.C. Effect of Fruit Maturity on Olive Oil Phenolic Composition and Antioxidant Capacity; AOCS Press: Urbana, IL, USA, 2015; ISBN 9781630670429
- Caramia, G.; Gori, A.; Valli, E.; Cerretani, L. Virgin olive oil in preventive medicine: From legend to epigenetics. Eur. J. Lipid Sci. Technol. 2012, 114, 375–388.
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294.
- Lukas Schwingshackl; Georg Hoffmann; Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 2012, 4, 1989-2007, 10.3390/nu4121989.
- Thays H. Borges; José Alberto Pereira; Carmen Cabrera-Vique; Luis Lara; Adelson F. Oliveira; Isabel Seiquer; Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chemistry 2017, 215, 454-462, 10.1016/j.foodchem.2016.07.162.
- F Aranda; Sergio Gómez-Alonso; R.M Rivera Del Álamo; Maria Desamparados Salvador; Giuseppe Fregapane; Triglyceride, total and 2-position fatty acid composition of Cornicabra virgin olive oil: Comparison with other Spanish cultivars. Food Chemistry 2004, 86, 485-492, 10.1016/j.foodchem.2003.09.021.
- Alu, M.H.; Rababah, T.; Alhamad, M.N. Application of Olive Oil as Nutraceutical and Pharmaceutical Food: Composition and Biofunctional Constituents and Their Roles in Functionality, Therapeutic, and Nutraceutical Properties; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128114124
- Roberto Ambra; Fausta Natella; Sabrina Lucchetti; Valentina Forte; Gianni Pastore; α-Tocopherol, β-carotene, lutein, squalene and secoiridoids in seven monocultivar Italian extra-virgin olive oils. International Journal of Food Sciences and Nutrition 2016, 68, 538-545, 10.1080/09637486.2016.1265099.
- Cynthia T. Srigley; Carolyn J. Oles; Ali Reza Fardin Kia; Magdi M. Mossoba; Authenticity Assessment of Extra Virgin Olive Oil: Evaluation of Desmethylsterols and Triterpene Dialcohols. Journal of the American Oil Chemists' Society 2015, 93, 171-181, 10.1007/s11746-015-2759-4.
- Paula Mapelli-Brahm; Dolores Hernanz; Carla M. Stinco; Francisco J. Heredia; Antonio J. Meléndez-Martínez; Isoprenoids composition and colour to differentiate virgin olive oils from a specific mill. LWT 2018, 89, 18-23, 10.1016/j.lwt.2017.10.021.
- Perrin, J.L.; Minor components and natural antioxidants in olives and olive oil. Rev. Fr. Des Corps Gras 1992, 39, 25–32.
- Moyano, M.J.; Meléndez-Martínez, A.J.; Alba, J.; Heredia, F.J.; A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (I): CIEXYZ non-uniform colour space. Food Res. Int. 2008, 41, 505–512.
- Konrad Grob; Mauro Lanfranchi; Cario Mariani; Evaluation of olive oils through the fatty alcohols, the sterols and their esters by coupled LC-GC. Journal of the American Oil Chemists' Society 1990, 67, 626-634, 10.1007/bf02540412.
- Itoh, T.; Tamura, T.; Matsumoto, T. Methylsterol compositions of 19 vegetable oils. J. Am. Oil Chem. Soc. 1973, 50, 300–303.
- Boskou, D.; Morton, I.D. Changes in the sterol composition of olive oil on heating. J. Sci. Food Agric. 1975, 26, 1149–1153.
- Itoh, T.; Yoshida, K.; Yatsu, T.; Tamura, T.; Matsumoto, T.; Spencer, G.F. Triterpene alcohols and sterols of Spanish olive oil. J. Am. Oil Chem. Soc. 1981, 58, 545–550.
- Boarelli, M.C.; Biedermann, M.; Peier, M.; Fiorini, D.; Grob, K. Ergosterol as a marker for the use of degraded olives in the production of olive oil. Food Control 2020, 112, 107136.
- Gutiérrez, F.; Varona, I.; Albi, M.A. Relation of acidity and sensory quality with sterol content of olive oil from stored fruit. J. Agric. Food Chem. 2000, 48, 1106–1110.
- Ranalli, A.; Modesti, G.; Patumi, M.; Fontanazza, G. The compositional quality and sensory properties of virgin olive oil from a new olive cultivar-I-77. Food Chem. 2000, 69, 37–46.
- Rivera Del Álamo, R.M.; Fregapane, G.; Aranda, F.; Gómez-Alonso, S.; Salvador, M.D. Sterol and alcohol composition of Cornicabra virgin olive oil: The campesterol content exceeds the upper limit of 4% established by EU regulations. Food Chem. 2004, 84, 533–537.
- Dimitrios Chryssafidis; Pantelis Maggos; Vassilis Kiosseoglou; Dimitrios Boskou; Composition of total and esterified 4α-monomethylsterols and triterpene alcohols in virgin olive oil. Journal of the Science of Food and Agriculture 1992, 58, 581-583, 10.1002/jsfa.2740580419.
- Ramón Aparicio; Guadalupe Luna; Characterisation of monovarietal virgin olive oils. null 2002, 104, 614-627, 10.1002/1438-9312(200210)104:9/10<614::AID-EJLT614>3.0.CO;2-L.
- A. Ranalli; M. L. Ferrante; G. De Mattia; N. Costantini; Analytical evaluation of virgin olive oil of first and second extraction.. Journal of Agricultural and Food Chemistry 1999, 47, 417-424, 10.1021/jf9800256.
- Mariani, C.; On the complexity of sterol fraction in olive oil|Sulla complessità della frazione sterolica nell’olio di oliva. Riv. Ital. Delle Sostanze Grasse 2016, 93, 147.
- Nuno Rodrigues; Susana Casal; António M. Peres; Paula Baptista; Albino Bento; Hugo Martin; M. Carmen Asensio-S.-Manzanera; José Alberto Pereira; Effect of olive trees density on the quality and composition of olive oil from cv. Arbequina. Scientia Horticulturae 2018, 238, 222-233, 10.1016/j.scienta.2018.04.059.
- G. W. Grams; K. Eskins; Dye-sensitized photooxidation of tocopherols. Correlation between singlet oxygen reactivity and vitamin E activity. Biochemistry 1972, 11, 606-608, 10.1021/bi00754a020.
- Nikolaos K Andrikopoulos; Maria N Hassapidou; Athanasios G Manoukas; The tocopherol content of greek olive oils. Journal of the Science of Food and Agriculture 1989, 46, 503-509, 10.1002/jsfa.2740460412.
- Rao, C.V.; Newmark, H.L.; Reddy, B.S. Chemopreventive effect of squalene on colon cancer. Carcinogenesis 1998, 19, 287–290.
- Smith, T.J.; Yang, G.Y.; Seril, D.N.; Liao, J.; Kim, S. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis by dietary olive oil and squalene. Carcinogenesis 1998, 19, 703–706.
- Augusto Lanzón; Tomas Albi; Arturo Cert; Jaime Gracián; The hydrocarbon fraction of virgin olive oil and changes resulting from refining. Journal of the American Oil Chemists' Society 1994, 71, 285-291, 10.1007/bf02638054.
- Verónica Sánchez De Medina; Milad El Riachy; F. Priego Capote; M.D. Luque De Castro; Mass spectrometry to evaluate the effect of the ripening process on phenols of virgin olive oils. European Journal of Lipid Science and Technology 2013, 115, 1053-1061, 10.1002/ejlt.201300059.
- E. Gimeno; Ana I. Castellote; Rosa M Lamuela-Raventós; M.C. De La Torre; Maria Carmen López-Sabater; The effects of harvest and extraction methods on the antioxidant content (phenolics, α-tocopherol, and β-carotene) in virgin olive oil. Food Chemistry 2002, 78, 207-211, 10.1016/s0308-8146(01)00399-5.
- Fito Colomer, M. Efectos Antioxidantes del Aceite de Oliva y de Sus Compuestos Fenólicos. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2003
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S.J. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236.
- Boskou, D. Olive Oil: Minor Constituents and Health; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9780367387143.
- Covas, M.I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor components of olive oil: Evidence to date of health benefits in humans. Nutr. Rev. 2006, 64, S20–S30.
- Konstantinidou, V.; Covas, M.; Muñoz-Aguayo, D.; Khymenets, O.; Torre, R.; Saez, G.; Carmen Tormos, M.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557
- Tsimidou, M.; Polyphenols and quality of virgin olive oil in retrospect. Ital. J. Food Sci. 1998, 10, 99–116.
- Segura-Carretero, A.; Menéndez-Menéndez, J.; Fernández-Gutiérrez, A. Polyphenols in olive oil: The importance of phenolic compounds in the chemical composition of olive oil. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2010; ISBN 9780123744203.
- Morelló, J.R.; Vuorela, S.; Romero, M.P.; Motilva, M.J.; Heinonen, M. Antioxidant activity of olive pulp and olive oil phenolic compounds of the arbequina cultivar. J. Agric. Food Chem. 2005, 53, 2002–2008.
- Murkovic, M.; Lechner, S.; Pietzka, A.; Bratacos, M.; Katzogiannos, E. Analysis of minor components in olive oil. J. Biochem. Biophys. Methods 2004, 61, 155–160.
- Alessandra Bendini; L. Cerretani; Alegria Carrasco-Pancorbo; Ana Maria Gómez-Caravaca; Antonio Segura-Carretero; Alberto Fernández-Gutiérrez; Giovanni Lercker; Phenolic Molecules in Virgin Olive Oils: a Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679-1719, 10.3390/12081679.
- Manuel Brenes; Aranzazu García; Pedro García; Antonio Garrido; Rapid and complete extraction of phenols from olive oil and determination by means of a coulometric electrode array system.. Journal of Agricultural and Food Chemistry 2000, 48, 5178-5183, 10.1021/jf000686e.
- Karina De La Torre-Carbot; Olga Jáuregui; Eva Gimeno; Ana I. Castellote; Rosa M Lamuela-Raventós; Maria Carmen López-Sabater; Characterization and Quantification of Phenolic Compounds in Olive Oils by Solid-Phase Extraction, HPLC-DAD, and HPLC-MS/MS. Journal of Agricultural and Food Chemistry 2005, 53, 4331-4340, 10.1021/jf0501948.
- Rovellini, P.; Cortesi, N.; Liquid chromatography-mass spectrometry in the study of oleuropein and ligstroside aglycons in virgin olive oil: Aldehydic, dialdehydic forms and their oxidized products. Riv. Ital. Sostanze Grasse 2002, 79, 1–14.
- Raquel Mateos; Arturo Cert; M. Carmen Pérez-Camino; José M. García; Evaluation of virgin olive oil bitterness by quantification of secoiridoid derivatives. Journal of the American Oil Chemists' Society 2004, 81, 71-75, 10.1007/s11746-004-0859-x.
- A. Bianco; F. Coccioli; M. Guiso; C. Marra; The occurrence in olive oil of a new class of phenolic compounds: hydroxy-isochromans. Food Chemistry 2002, 77, 405-411, 10.1016/s0308-8146(01)00366-1.
- Sergio Gómez-Alonso; Maria Desamparados Salvador; Giuseppe Fregapane; Phenolic Compounds Profile of Cornicabra Virgin Olive Oil. Journal of Agricultural and Food Chemistry 2002, 50, 6812-6817, 10.1021/jf0205211.
- Cristina Montealegre; María Luisa Marina; Carmen Garcı́a-Ruiz; Carmen García-Ruiz; Traceability Markers to the Botanical Origin in Olive Oils. Journal of Agricultural and Food Chemistry 2010, 58, 28-38, 10.1021/jf902619z.
- Lazzerini, C.; Cifelli, M.; Domenici, V. Pigments in extra-virgin olive oil: Authenticity and quality. In Products from Olive Tree; Books on Demand: McFarland, WI, USA, 2016.
- Lazzerini, C.; Domenici, V. Pigments in extra-virgin olive oils produced in Tuscany (Italy) in different years. Foods 2017, 6, 25.
- Uncu, O.; Ozen, B. Importance of some minor compounds in olive oil authenticity and quality. Trends Food Sci. Technol. 2020, 100, 164–176.
- Lazzerini, C.; Cifelli, M.; Domenici, V. Pigments in extra virgin olive oils produced in different mediterranean countries in 2014: Near UV-vis spectroscopy versus HPLC-DAD. LWT Food Sci. Technol. 2017, 84, 586–594.
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and carotenoids in food products from olive tree. In Products from Olive Tree; Books on Demand: McFarland, WI, USA, 2016
- Noelia Tena Pajuelo; Selina C Wang; Ramón Aparicio‐Ruiz; Diego L. García-González; Ramón Aparicio; In-Depth Assessment of Analytical Methods for Olive Oil Purity, Safety, and Quality Characterization. Journal of Agricultural and Food Chemistry 2015, 63, 4509-4526, 10.1021/jf5062265.
- Beatriz Gandul-Rojas; Maria Roca-L. Cepero; M. Isabel Mínguez-Mosquera; Use of chlorophyll and carotenoid pigment composition to determine authenticity of virgin olive oil. Journal of the American Oil Chemists' Society 2000, 77, 853-858, 10.1007/s11746-000-0136-z.
- María Roca; Beatriz Gandul-Rojas; Lourdes Gallardo-Guerrero; Ma Isabel Mínguez-Mosquera; Pigment parameters determining spanish virgin olive oil authenticity: Stability during storage. Journal of the American Oil Chemists' Society 2003, 80, 1237-1240, 10.1007/s11746-003-0848-0.
- Ramón Aparicio‐Ruiz; Beatriz Gandul-Rojas; Decoloration kinetics of chlorophylls and carotenoids in virgin olive oil by autoxidation. Food Research International 2014, 65, 199-206, 10.1016/j.foodres.2014.05.046.
- Angela Giuliani; L. Cerretani; Angelo Cichelli; Chlorophylls in Olive and in Olive Oil: Chemistry and Occurrences. Critical Reviews in Food Science and Nutrition 2011, 51, 678-690, 10.1080/10408391003768199.
- Alessandro Menotti; Paolo Emilio Puddu; How the Seven Countries Study contributed to the definition and development of the Mediterranean diet concept: A 50-year journey. Nutrition, Metabolism and Cardiovascular Diseases 2015, 25, 245-252, 10.1016/j.numecd.2014.12.001.
- Miguel Ángel Martínez-González; Dolores Corella; Jordi Salas-Salvadó; Emilio Ros; María Isabel Covas; Miquel Fiol; Julia Wärnberg; Fernando Arós; Valentina Ruíz-Gutiérrez; Rosa M Lamuela-Raventós; et al.José LapetraMiguel-Angel MuñozJosé Alfredo MartínezGuillermo SáezLluís Serra-MajemXavier PintóMaria Teresa MitjavilaJosep Antoni TurMaría Del Puy PortilloRamón Estruchfor the PREDIMED Study Investigators Cohort Profile: Design and methods of the PREDIMED study. International Journal Of Epidemiology 2010, 41, 377-385, 10.1093/ije/dyq250.
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals. Circulation 2017, 135, 633–643.
- Hernáez, Á.; Castañer, O.; Goday, A.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; et al. The Mediterranean diet decreases LDL atherogenicity in high cardiovascular risk individuals: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1601015.
- Estefanía Toledo; Jordi Salas-Salvadó; Carolina Donat-Vargas; Pilar Buil Cosiales; Ramón Estruch; Emilio Ros; Dolores Corella; Montserrat Fitó; Frank B. Hu; Fernando Arós; et al.Enrique Gómez-GraciaDora RomagueraManuel Ortega-CalvoLluís Serra-MajemXavier PintóHelmut SchröderJosep BasoraJose V. SorliMònica BullóMercè Serra-MirMiguel Ángel Martínez-González Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial. JAMA Internal Medicine 2015, 175, 1752-1760, 10.1001/jamainternmed.2015.4838.
- Jordi Salas-Salvadó; Mònica Bulló; Ramón Estruch; Emilio Ros; M. I. Covas; Núria Ibarrola-Jurado; Dolores Corella; Fernando Arós; Enrique Gómez-Gracia; Valentina Ruíz-Gutierrez; et al.Dora RomagueraJosé LapetraRosa M Lamuela-RaventósLluís Serra-MajemXavier PintóJosep BasoraMiguel-Angel MuñozJose V. SorliMiguel Ángel Martínez-González Prevention of Diabetes With Mediterranean Diets. Annals of Internal Medicine 2014, 160, 1-10, 10.7326/m13-1725.
- Marta Guasch-Ferre; Frank B. Hu; Miguel Ángel Martínez-González; Montserrat Fitó; Mònica Bulló; Ramón Estruch; Emilio Ros; Dolores Corella; Javier Recondo; Enrique Gómez-Gracia; et al.Miquel FiolJosé LapetraLluís Serra-MajemMiguel-Angel MuñozXavier PintóRosa M Lamuela-RaventósJosep BasoraPilar Buil-CosialesJose V. SorliValentina Ruíz-GutierrezAlfredo MartínezJordi Salas-Salvadó Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Medicine 2014, 12, 78-78, 10.1186/1741-7015-12-78.
- Francisca Zamora Zamora; Juan Miguel Martínez-Galiano; José J. Gaforio; M. Delgado-Rodríguez; Effects of olive oil on blood pressure: A systematic review and meta-analysis. Grasas y Aceites 2018, 69, 272, 10.3989/gya.0105181.
- Miguel Ángel Martínez-González; Ligia J. Dominguez; Miguel Delgado-Rodríguez; Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case–control, cohort and intervention studies. British Journal of Nutrition 2014, 112, 248-259, 10.1017/s0007114514000713.
- Elena S. George; Skye Marshall; Hannah L. Mayr; Gina L. Trakman; O.A. Tatucu-Babet; Annie-Claude M. Lassemillante; Andrea Bramley; Anjana J. Reddy; Adrienne Forsyth; Audrey C. Tierney; et al.Colleen J. ThomasCatherine ItsiopoulosWolfgang Marx The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Critical Reviews in Food Science and Nutrition 2018, 59, 2772-2795, 10.1080/10408398.2018.1470491.
- EFSA.; Panel on dietetic products nutrition and allergies (NDA) scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte. EFSA J. 2011, 9, 1–25.
- Covas, M.-I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616.
- Fitó, M.; Cladellas, M.; de la Torre, R.; Martí, J.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; Vila, J.; et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158.
- Hernáez, Á.; Remaley, A.T.; Farràs, M.; Fernández-Castillejo, S.; Subirana, I.; Schröder, H.; Fernández-Mampel, M.; Muñoz-Aguayo, D.; Sampson, M.; Solà, R.; et al. Olive oil polyphenols decrease LDL concentrations and LDL atherogenicity in men in a randomized controlled trial. J. Nutr. 2015, 145, 1692.
- Hohmann, C.D.; Cramer, H.; Michalsen, A.; Kessler, C.; Steckhan, N.; Choi, K.; Dobos, G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine 2015, 22, 631–640.
- Alicia López-Biedma; Cristina Sánchez-Quesada; Miguel Delgado-Rodríguez; José J. Gaforio; The biological activities of natural lignans from olives and virgin olive oils: A review. Journal of Functional Foods 2016, 26, 36-47, 10.1016/j.jff.2016.07.005.
- João Fernandes; Mónica Fialho; Rodrigo Feteira-Santos; Catarina Peixoto-Plácido; Teresa Madeira; Nuno Sousa-Santos; Ana Virgolino; Osvaldo Santos; António Vaz Carneiro; Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition 2020, 69, 110559, 10.1016/j.nut.2019.110559.
- Lukas Schwingshackl; Marina Christoph; Georg Hoffmann; Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651-7675, 10.3390/nu7095356.
- Antonio Camargo; Oriol Alberto Rangel-Zuñiga; Carmen Haro; Eliana Romina Meza-Miranda; Patricia Peña-Orihuela; Maria Eugenia Meneses; Carmen Marín; Elena M. Yubero-Serrano; Pablo Pérez-Martínez; Javier Delgado-Lista; et al.Jose Manuel Fernández-RealM.D. Luque De CastroFrancisco TinahonesJosé López-MirandaFrancisco Pérez-Jiménez Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chemistry 2014, 162, 161-171, 10.1016/j.foodchem.2014.04.047.
- Theodora Psaltopoulou; Rena I. Kosti; Dimitrios A Haidopoulos; Meletios Dimopoulos; Demosthenes Panagiotakos; Olive oil intake is inversely related to cancer prevalence: a systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids in Health and Disease 2011, 10, 127-127, 10.1186/1476-511X-10-127.
- Piet A. Van Den Brandt; Maya Schulpen; Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. International Journal of Cancer 2017, 140, 2220-2231, 10.1002/ijc.30654.
- Lukas Schwingshackl; Carolina Schwedhelm; Cecilia Galbete; Georg Hoffmann; Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063, 10.3390/nu9101063.
- Menendez, J.A.; Vazquez-Martin, A.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Fernandez-Gutierrez, A.; Segura-Carretero, A. tabAnti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial extra-virgin olive oil (EVOO). BMC Cancer 2008, 8, 377.
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Analyzing effects of extra-virgin olive polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008, 22, 433–439.
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM–p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146.
- Sandra Martín-Peláez; Juana Ines Mosele; Neus Pizarro; Marta Farràs; Rafael De La Torre; Isaac Subirana; Francisco José Pérez-Cano; Olga Castañer; Rosa Solà; Sara Fernández-Castillejo; et al.Saray HerediaMagi FarréMaría-Jose MotilvaMontserrat Fitó Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: implications of human gut microbiota. European Journal of Nutrition 2015, 56, 119-131, 10.1007/s00394-015-1063-2.
- Mohanambal Moorthy; Nathorn Chaiyakunapruk; Sabrina Anne Jacob; Uma Devi Palanisamy; Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends in Food Science & Technology 2020, 99, 634-649, 10.1016/j.tifs.2020.03.036.
- Patrizia Rosignoli; Raffaela Fuccelli; R. Fabiani; Maurizio Servili; Guido Morozzi; Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. The Journal of Nutritional Biochemistry 2013, 24, 1513-1519, 10.1016/j.jnutbio.2012.12.011.
- Amparo Luque Sierra; Leticia Alvarez-Amor; Robert Kleemann; Franz Martin; Lourdes M. Varela; Extra-Virgin Olive Oil with Natural Phenolic Content Exerts an Anti-Inflammatory Effect in Adipose Tissue and Attenuates the Severity of Atherosclerotic Lesions inLdlr−/−.Leiden Mice. Molecular Nutrition & Food Research 2018, 62, 1800295, 10.1002/mnfr.201800295.
- Limketkai, B.N.; Wolf, A.; Parian, A.M. Nutritional Interventions in the patient with inflammatory bowel disease. Gastroenterol. Clin. North Am. 2018, 47, 155–177.
- Cabré, E.; Domènech, E. Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J. Gastroenterol. 2012, 18, 3814.
- Ma Ángeles Rosillo; Marina Sanchez-Hidalgo; Susana Sánchez-Fidalgo; Marina Aparicio-Soto; Isabel Villegas; Catalina Alarcón; Dietary extra-virgin olive oil prevents inflammatory response and cartilage matrix degradation in murine collagen-induced arthritis. European Journal of Nutrition 2015, 55, 315-325, 10.1007/s00394-015-0850-0.
- Marina Aparicio-Soto; Marina Sanchez-Hidalgo; Ana Cárdeno; Alejandro González-Benjumea; José G. Fernández-Bolaños; Catalina Alarcón; Dietary hydroxytyrosol and hydroxytyrosyl acetate supplementation prevent pristane-induced systemic lupus erythematous in mice. Journal of Functional Foods 2017, 29, 84-92, 10.1016/j.jff.2016.12.001.
- Martín, R.; Carvalho-Tavares, J.; Hernández, M.; Arnés, M.; Ruiz-Gutiérrez, V.; Nieto, M.L. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: A potential therapeutic role. Biochem. Pharm. 2010, 79, 198–208.
- Martín, R.; Hernández, M.; Córdova, C.; Nieto, M. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: Therapeutic implications for multiple sclerosis. Br. J. Pharm. 2012, 166, 1708–1723.
- Blanka Klimova; Michal Novotný; Teodorico De Castro Ramalho; Martin Valis; Effect Of An Extra-Virgin Olive Oil Intake On The Delay Of Cognitive Decline: Role Of Secoiridoid Oleuropein?. Neuropsychiatric Disease and Treatment 2019, 15, 3033-3040, 10.2147/NDT.S218238.
- A Trichopoulou; P Lagiou; H Kuper; D Trichopoulos; Cancer and Mediterranean dietary traditions.. Cancer Epidemiology Biomarkers & Prevention 2000, 9, 869–873.
- Patricia Reboredo Rodríguez; Carmen González-Barreiro; B. Cancho-Grande; Tamara Y. Forbes‐Hernández; Massimiliano Gasparrini; Sadia Afrin; Danila Cianciosi; Alegría Carrasco-Pancorbo; Jesús Simal-Gandara; Francesca Giampieri; et al.Maurizio Battino Characterization of phenolic extracts from Brava extra virgin olive oils and their cytotoxic effects on MCF-7 breast cancer cells. Food and Chemical Toxicology 2018, 119, 73-85, 10.1016/j.fct.2018.05.026.
- Neha Nanda; Safrun Mahmood; Alka Bhatia; Akhtar Mahmood; D. K. Dhawan; Chemopreventive role of olive oil in colon carcinogenesis by targeting noncoding RNAs and methylation machinery. International Journal of Cancer 2018, 144, 1180-1194, 10.1002/ijc.31837.
- Annalisa Romani; Francesca Ieri; Silvia Urciuoli; Annalisa Noce; Giulia Marrone; C. Nediani; Roberta Bernini; Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L.. Nutrients 2019, 11, 1776, 10.3390/nu11081776.
- Lin, S.; Wang, Z.; Lam, K.L.; Zeng, S.; Tan, B.K.; Hu, J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 2019, 63, 1518.
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78
- Antonio Maria Borzì; Antonio Biondi; Francesco Basile; Salvatore Luca; Enzo Vicari; Marco Vacante; Olive Oil Effects on Colorectal Cancer. Nutrients 2018, 11, 32, 10.3390/nu11010032.
- Kati Mokkala; Noora Houttu; Tugce Cansev; Kirsi Laitinen; Interactions of dietary fat with the gut microbiota: Evaluation of mechanisms and metabolic consequences. Clinical Nutrition 2020, 39, 994-1018, 10.1016/j.clnu.2019.05.003.
- Rocío M. De Pablos; Ana María Espinosa-Oliva; Ruth Hornedo-Ortega; Mercedes Cano; Sandro Arguelles; Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacological Research 2019, 143, 58-72, 10.1016/j.phrs.2019.03.005.
- Monika Gorzynik-Debicka; Paulina Przychodzeń; Francesco Cappello; Alicja Kuban-Jankowska; Antonella Marino Gammazza; Narcyz Knap; Michal Wozniak; Magdalena Gorska-Ponikowska; Potential Health Benefits of Olive Oil and Plant Polyphenols. International Journal of Molecular Sciences 2018, 19, 686, 10.3390/ijms19030686.
- Elena M. Yubero-Serrano; Javier Lopez-Moreno; Javier Delgado-Lista; José López-Miranda; Extra virgin olive oil: More than a healthy fat. European Journal of Clinical Nutrition 2018, 72, 8-17, 10.1038/s41430-018-0304-x.
- Susan A. Farr; Tulin O. Price; Ligia J. Dominguez; Antonio Motisi; Filippo Saiano; Michael L. Niehoff; John E. Morley; William A. Banks; Nuran Ercal; Mario Barbagallo; et al. Extra Virgin Olive Oil Improves Learning and Memory in SAMP8 Mice. Journal of Alzheimer's Disease 2012, 28, 81-92, 10.3233/jad-2011-110662.
- Matteo Bertelli; Aysha Karim Kiani; Stefano Paolacci; Elena Manara; Danjela Kurti; Kristjana Dhuli; Vilma Bushati; Jan Miertus; Domenico Pangallo; Mirko Baglivo; et al.Tommaso BeccariSandro Michelini Hydroxytyrosol: A natural compound with promising pharmacological activities. Journal of Biotechnology 2020, 309, 29-33, 10.1016/j.jbiotec.2019.12.016.




