Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joachim Mag. Dr. Greilberger | + 2593 word(s) | 2593 | 2021-11-18 05:04:05 | | | |
| 2 | Vivi Li | Meta information modification | 2593 | 2021-11-19 04:01:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mag. Dr. Greilberger, J. Alpha-Ketoglutarate and 5-HMF. Encyclopedia. Available online: https://encyclopedia.pub/entry/16138 (accessed on 07 February 2026).
Mag. Dr. Greilberger J. Alpha-Ketoglutarate and 5-HMF. Encyclopedia. Available at: https://encyclopedia.pub/entry/16138. Accessed February 07, 2026.
Mag. Dr. Greilberger, Joachim. "Alpha-Ketoglutarate and 5-HMF" Encyclopedia, https://encyclopedia.pub/entry/16138 (accessed February 07, 2026).
Mag. Dr. Greilberger, J. (2021, November 18). Alpha-Ketoglutarate and 5-HMF. In Encyclopedia. https://encyclopedia.pub/entry/16138
Mag. Dr. Greilberger, Joachim. "Alpha-Ketoglutarate and 5-HMF." Encyclopedia. Web. 18 November, 2021.
Copy Citation
Clinical and pre-clinical studies of an anti-tumoral solution containing aKG, 5-HMF, N-acetyl-selenomethionine, and N-acetylmethionine for treating tumors showed, on one hand, good therapeutic efficacy during infusion therapy in prostate cancer patients by increasing the PSA doubling time; on the other hand, a reduction of tumoral mass was shown in lung cancer patients.
alpha-ketoglutarate (aKG)
5-hydroxy-methyl-furfural (5-HMF)
reactive oxygen and nitrogen species (RONS)
leukemia
human fibroblasts (HF-SAR)
proliferation
caspase activity
carbonylated proteins (CP)
1. Introduction
The reduction of oxidatively modified proteins generated by cigarette smoke [1] demonstrated the impressive effects of aKG + 5-HMF as a better potential antioxidative solution compared to vitamin C or its single compounds. aKG itself is not only involved in the energy generation process in humans, but also in several metabolic processes for enzymatic regulation, such as those of hypoxia-inducing factor alpha [2] or 2-oxo-glutarate-dependent dioxygenases in cancer [3] and to suppress tumors in bladder cancer patients [3][4][5].
The compound 5-HMF occurs in honey and apple juice and in even higher rates in dried fruits, caramel products, and coffees [6]. Because there was speculation that 5-HMF is cancerogenic, the National Institute of Environmental Health Sciences demonstrated that no evidence of any carcinogenic activity was found when applying concentrations of 750 mg/kg over 2 years in rats and also provided some evidence in mice. Anti-proliferative and antioxidative activities were found in 5-HMF, suggesting its potential chemoprevention in cancer [7] as well as in melanoma cells [8]. aKG + 5-HMF was demonstrated to increase oxygen saturation during exercise in subjects with normobaric hypoxia [9] because of the antioxidative and anti-sickling effects of 5-HMF and its increased affinity for oxygen [10].
2. Estimation of Different AKG/5-HMF Ratios during Exposure of Cigarette Smoke on FCS Proteins
Figure 1 shows the oxidative modification of FCS protein after 2, 15, 30, and 60 min of exposure of cigarette smoke expressed with carbonylated proteins (nmol/mg protein) using different AKG+5-HMF combination solutions. The best significant reduction of carbonyl proteins was found using the 500 µg/mL + 125 µg/mL 5-HMF solution compared to control. After 2 min the carbonylated protein was significantly lower (3.06 ± 0.33 vs. 5.96 ± 0.70 nmol/mg; p < 0.01), also after 15 min (3.80 ± 0.99 vs. 7.96 ± 0.33 nmol/mg; p < 0.01), 30 min (4.49 ± 0.77 vs. 9.89 ± 1.20 nmol/mg; p < 0.01), and 60 min (11.51 ± 0.94 vs. 5.21 nmol/mg; p < 0.01). At time points 30 and 60 min, this solution (500 µg/mL + 125 µg/mL 5-HMF) also showed a significantly lower carbonylated protein content compared to 500 µg/mL aKG + 62.5 µg/mL 5-HMF (6.68 ± 0.90 nmol/mg, p < 0.05 and 7.56 ± 1.20 nmol/mg, p < 0.01). The highest used combination 500 µg/mL + 250 µg/mL 5-HMF showed no significant difference compared to 500 µg/mL + 125 µg/mL 5-HMF, but the carbonyl proteins were higher with the highest combination solution. For the following experiments we have used the 500 µg/mL aKG + 166.7 µg/mL 5-HMF solution and its dilutions.
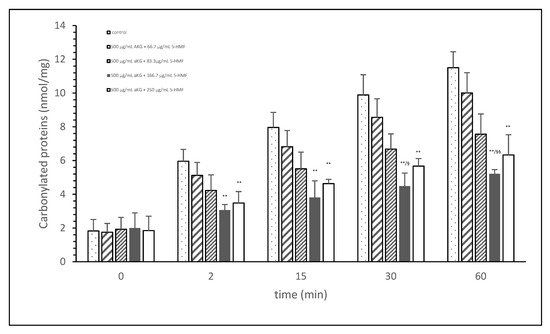
Figure 1. Cigarette smoke oxidatively modified proteins of FCS after cigarette smoke exposure in presence or absence of different AKG and 5-HMF combined solutions expressed with the content of carbonylated proteins (n = 3). ** p < 0.01: significance between the control (0 µg/mL aKG + 0 µg/mL 5-HMF) and different combinations of aKG + 5-HMF after 2, 15, 30, and 60 min exposure. § p < 0.05: significance between the 500 µg/mL aKG + 83.3 µg/mL 5-HMF and 500 µg/mL aKG + 125 µg/mL 5-HMF. §§ p < 0.01: significance between the 500 µg/mL aKG + 83.3 µg/mL 5-HMF and 500 µg/mL aKG + 125 µg/mL 5-HMF.
3. Cell Proliferation Experiments
Figure 2A describes the cell growth with different combinations of aKG + 5-HMF in Jurkat cells over 3 days. After 24 h, only the highest concentration (500 µg/mL aKG and 166.7 µg/mL 5-HMF) showed a significant reduction in cell growth compared to the control at 24 h (22,832 ± 2512 cells vs. 32,537 ± 5231 cells; p < 0.05). No significant changes were estimated between the control at 0 h and the Jurkat cells incubated in the presence of 500 µg/mL aKG + 166.7 µg/mL 5-HMF for 24 h. The cell growth at 48 h was significantly reduced compared to the control (52,123 ± 4232 cells, n = 5) by several different concentrations of the combination of aKG + 5-HMF: 125 µg/mL aKG + 41.7 µg/mL 5-HMF (43,511 ± 4209 cells; p < 0.05; n = 5), 200 µg/mL aKG + 66.7 µg/mL 5-HMF (36,823 ± 4845 cells; p < 0.001; n = 5), 250 µg/mL aKG + 83.3 µg 5-HMF (35,098 ± 2150 cells; p < 0.001; n = 5), 375 µg/mL aKG + 125 µg/mL 5-HMF (31,245 ± 4111 cells; p < 0.001; n = 5), and 500 µg/mL aKG + 166.7 µg/mL 5-HMF (21,243 ± 55,467 cells; p < 0.001; n = 5). After 72 h of incubation, the greatest combination, 500 µg/mL aKG + 166.7 µg/mL 5-HMF (23,224 ± 5445 cells; p < 0.001; n = 5), showed a significant reduction compared to the control after 72 h (82,131 ± 5197 cells; p < 0.001; n = 5), but did not show a reduction compared to the control cells after 0 or 24 h. A lesser reduction in the cell growth compared to the control after 72 h were obtained with 375 µg/mL aKG + 125 µg/mL 5-HMF (28,433 ± 5247 cells; p < 0.001; n = 5), 250 µg/mL aKG + 83.3 µg/mL 5-HMF (37,512 ± 5129 cells; p < 0.001; n = 5), 200 µg/mL aKG + 66.7 µg/mL 5-HMF (44,768 ± 3487 cells; p < 0.001; n = 5), and 125 µg/mL aKG + 41.7 µg/mL 5-HMF (54,227 ± 3655 cells; p <0.05; n = 5).
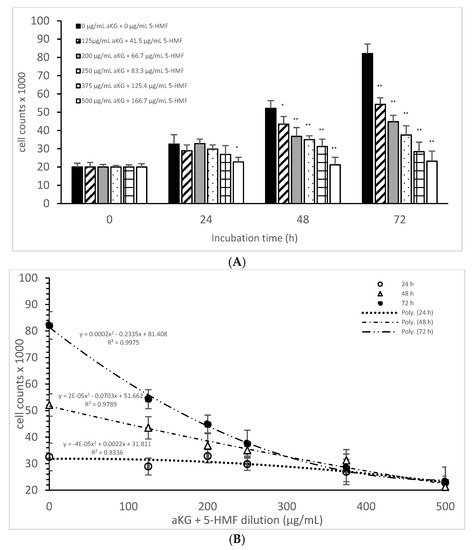
Figure 2. Cell growth of the Jurkat cell line in the absence or presence of different concentrations of the combined aKG + 5-HMF (A,B) correlation between cell growth and the combined solutions of aKG + 5-HMF after 24, 48, and 72 h of cultivation (n = 5). * p < 0.05: significance between the control (0 µg/mL aKG + 0 µg/mL 5-HMF) and different combinations of aKG + 5-HMF at the time points after 1, 2, and 3 days of cell culture. ** p < 0.001: significance between the control (0 µg/mL aKG + 0 µg/mL 5-HMF) and different combinations of aKG + 5-HMF at the time points after 1, 2, and 3 days of cell culture.
After correlating several concentrations of the combined AKG + 5-HMF solutions with cell growth (Figure 2B), all three curves showed a high polynomial correlation. The best correlation was calculated with the cell growth after 72 h of incubation (r = nearly 1; y = 0.0002x2 − 0.2335x + 81408) and the IC50% calculated for the 100 µg/mL AKG + 41.7 µg/mL 5-HMF solution, followed by 48 h of incubation (r = 0.99; y = 2 × 10−5x2 − 0.0703x + 51662) and the IC50% of the 200 µg/mL aKG + 66.7 µg/mL 5-HMF solution, and finally, the 24-h incubation (r = 0.91; y = y = −4 × 10−5x2 + 0.0022x + 31811) and the IC50% of around 375 µg/mL aKG + 125 µg/mL 5-HMF. These results showed also that the higher the incubation time with AKG + 5-HMF the lower concentrations are needed to reach the IC 50%. The decline of cell growth after 72 h incubation was higher compared to the 48 h and 24 h incubation.
Table 1 shows the cell proliferations (%) of Jurkat cells and HF-SAR cells after 0, 24, 48, and 72 h incubation in presence or absence of the combined solutions of aKG + 5-HMF. No significant difference was estimated between all used aKG + 5-HMF solutions during all incubations except with the highest concentration 500 µg/mL aKG + 166.7 µg/mL 5-HMF after 72 h incubation (p < 0.05) compared to 0, 24, and 48 h incubation.
Table 1. Cell proliferation (%) of the Jurkat cell line and of the HF-SAR after incubation for 0, 24, 48, and 72 h in the absence or presence of different combinations of aKG + 5-HMF solutions (n = 5).
| Jurkat | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Cell growth | % | % | % | % |
| 0 µg/mL aKG + 0 µg/mL 5-HMF | 100 ± 7.5 | 190.5 ± 13.6 | 286.0 ± 2.1 | 376.5 ± 5.0 |
| 125 µg/mL aKG + 41.7 µg/mL HMF | 105 ± 3.5 | 150.5 ± 14.0 | 266.0 ± 6.0 | 331.0 ± 6.3 |
| 200 µg/mL aKG + 66.7 µg/mL 5-HMF | 101 ± 2.5 | 168.1 ± 20.2 | 216.7 ± 5.8 | 304.5 ± 5.9 |
| 250 µg/mL aKG + 83.3 µg/mL 5-HMF | 103 ± 9 | 165.5 ± 6.3 | 206.4 ± 7.8 | 290.5 ± 8.8 |
| 375 µg/mL aKG + 125 µg/mL 5-HMF | 98.7 ± 5.5 | 179.0 ± 8.7 | 195.5 ± 4.6 | 296.5 ± 7.1 |
| 500 µg/mL aKG + 166.7 µg/mL 5-HMF | 105 ± 3.5 | 165.5 ± 7.6 | 222.5 ± 3.4 | 256.0 ± 9.6 |
| HF-SAR | 0 h | 24 h | 48 h | 72 h |
| Cell growth | % | % | % | % |
| 0 µg/mL aKG + 0 µg/mL 5-HMF | 101.3 ± 7.5 | 88.7 ± 9.9 | 92.3 ± 12.1 | 126.6 ± 14.5 |
| 125 µg/mL aKG + 41.7 µg/mL HMF | 100.0 ± 3.1 | 85.0 ± 7.3 | 92.8 ± 9.2 | 121.7 ± 9.2 |
| 200 µg/mL aKG + 66.7 µg/mL 5-HMF | 104.1 ± 1.5 | 89.3 ± 8.9 | 100.6 ± 7.9 | 121.4 ± 13.1 |
| 250 µg/mL aKG + 83.3 µg/mL 5-HMF | 99.0 ± 6.1 | 82.7 ± 4.2 | 89.3 ± 4.1 | 137.6 ± 22.1 |
| 375 µg/mL aKG + 125 µg/mL 5-HMF | 99.9 ± 3.5 | 93.8 ± 11.0 | 104.3 ± 11.1 | 132.6 ± 29.1 |
| 500 µg/mL aKG + 166.7 µg/mL 5-HMF | 102.0 ± 3.1 | 86.2 ± 4.1 | 97.9 ± 8.2 | 140.5 * ± 23 |
4. Cytotoxic Assay
The mitochondrial activity of the Jurkat cells as it was expressed in the absorbance at 450 nm in the absence or presence of one of the dilutions of the aKG + 5-HMF solution is presented in Figure 3A. After 24 h of incubation, the highest concentration (500 µg/mL aKG and 166.7 µg/mL 5-HMF (0.112 ± 0.021)) showed a 41% reduction of the mitochondrial activity, which was significant compared to that of the control after 24 h (0.19 ± 0.02; n = 5; p < 0.001). Using the 375 µg/mL aKG + 125 µg/mL 5-HMF solution (0.136 ± 0.018; n = 5; p < 0.05) resulted in a 28% reduction compared to the control after 24 h.
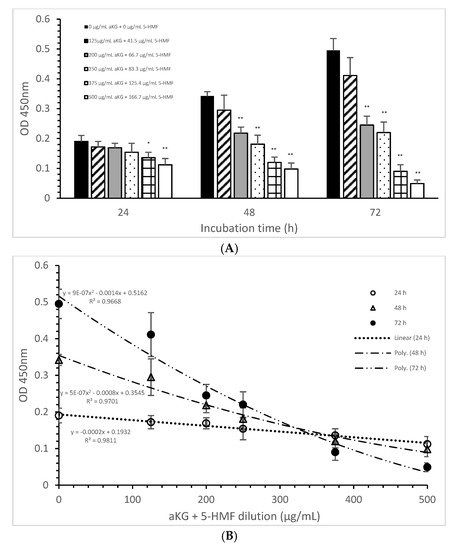
Figure 3. Mitochondrial activity of the Jurkat cell line in the absence or presence of different aKG + 5-HMF concentrations (A); (B) correlation between mitochondrial activity and the combined aKG + 5-HMF concentration after 24, 48, and 72 h of cultivation (n = 5). * p < 0.01: significance between the control (0 µg/mL aKG + 0 µg/mL 5-HMF) and different combinations of aKG + 5-HMF at time points after 24, 48, and 72 h of cell culture. ** p < 0.001: significance between the control (0 µg/mL aKG + 0 µg/mL 5-HMF) and different combinations of aKG + 5-HMF at time points after 1, 2, and 3 days of cell culture.
The use of 48 h of incubation resulted in a higher reduction (of 36%) of the mitochondrial activity compared to that of the control after 48 h, with the 500 µg/mL aKG and 166.7 µg/mL 5-HMF solution (0.098 ± 0,02; n = 5; p < 0.001) showing the greatest reduction, followed by the 375 µg/mL aKG + 125 µg/mL 5-HMF solution (0.120 ± 0.018; n = 5; p < 0.001), the 250 µg/mL aKG + 83.3 µg 5-HMF solution (0.181 ± 0.02; n = 5; p < 0.001), and the 200 µg/mL aKG + 66.7 µg/mL 5-HMF solution (0.218 ± 0.05; n = 5; p < 0.001). The lowest concentration, 125 µg/mL aKG + 41.7 µg/mL 5-HMF (0.295 ± 0.05; n = 5), showed no effects. The same trend could be seen after 72 h of incubation. While no effects compared to the control (0.495 ± 0.04) after 72 h were obtained when using 125 µg/mL aKG + 41.7 µg/mL 5-HMF (0.411 ± 0.06; n = 5), all the other used concentrations showed significant reductions: 200 µg/mL aKG + 66.7 µg/mL 5-HMF (0.245 ± 0.03; n = 5; p < 0.001), 250 µg/mL aKG + 83.3 µg 5-HMF (0.222 ± 0.035; n = 5; p < 0.001), 375 µg/mL aKG + 125 µg/mL 5-HMF (0.090 ± 0.022; n = 5; p < 0.001), and 500 µg/mL aKG and 166.7 µg/mL 5-HMF (0.098 ± 0.02; n = 5; p < 0.001). The mitochondrial activity in the Jurkat cells incubated for 72 h in the presence of 500 µg/mL aKG and 166.7 µg/mL 5-HMF was significantly lower than that with 48 and 24 h of incubation when using the same combined concentration (p < 0.001). A significant difference was also estimated between incubation for 72 h (0.090 ± 0.022; n = 5; p < 0.001) and incubation for 24 h (0.136 ± 0.018) with the use of 375 µg/mL aKG + 125 µg/mL 5-HMF.
Figure 3B shows the correlations between the combined solutions of aKG + 5-HMF and the mitochondrial activity at different incubation times with nearly equal regression terms: r = 0.98 for 72 h with a polynomial function (y = 9 × 10−7 x2 − 0.0014x + 0.5162), r = 0.98 for 48 h with a polynomial function (y = 5 × 10−7 x2 − 0.0008x + 0.3545), and r = 0.99 for 24 h with a linear function (y = −0.0002x + 0.1932). The IC50% was calculated for all functions, with nearly the same result of 250 µg/mL + 83.3 µg/mL 5-HMF. The decline of the mitochondrial activity was higher during 72 h incubation followed by 48 h incubation compared to 24 h incubation because of its different functions. The usage of 500 µg/mL aKG and 166.7 µg/mL 5-HMF and 375 µg/mL aKG + 125 µg/mL 5-HMF solutions showed a lower mitochondrial activity in favor of 72 h incubation followed by 48 h incubation compared to 24 h incubation.
Table 2 shows the decrease in the mitochondrial activity in the presence of the combined solutions (aKG + 5-HMF). The longer the incubation time and the higher the concentration of aKG + 5-HMF, the lower the mitochondrial activity was. A reduction of nearly half was obtained by using 200 µg/mL aKG + 66.7 µg/mL 5-HMF after 72 h of incubation or by using 250 µg/mL aKG + 83.3 µg/mL 5-HMF after 48 and 72 h of incubation.
Table 2. Mitochondrial activity (%) of the Jurkat cell line (A) and of the HF-SAR (B) after incubation for 24, 48, and 72 h in the absence or presence of different combinations of aKG + 5-HMF solutions (n = 5). * p < 0.01: significance between 24 h of incubation without aKG + 5-HMF and with the combined solutions of aKG + 5-HMF. ** p < 0.001: significance between 24 h of incubation without aKG + 5-HMF and with the combined solutions of aKG + 5-HMF. ++ p < 0.001: significance between 48 h of incubation without aKG + 5-HMF and with the combined solutions of aKG + 5-HMF. $$ p < 0.001: significance between 48 h of incubation without aKG + 5-HMF and with the combined solutions of aKG + 5-HMF.
| Jurkat | 24 h | 48 h | 72 h |
|---|---|---|---|
| Mitochondrial Activity | % | % | % |
| 0 µg/mL aKG + 0 µg/mL 5-HMF | 100 ± 10.5 | 100 ± 4.4 | 100 ± 8.1 |
| 125 µg/mL aKG + 41.7 µg/mL HMF | 90.5 ± 9.5 | 86.3 ± 14.6 | 83 ± 12.1 |
| 200 µg/mL aKG + 66.7 µg/mL 5-HMF | 88.9 ± 7.9 | 63.7 ± 5.8 ++ | 49.5 ± 6.1 $$ |
| 250 µg/mL aKG + 83.3 µg/mL 5-HMF | 81.1 ± 15.8 | 52.9 ± 8.8 ++ | 44.4 ± 7.1 $$ |
| 375 µg/mL aKG + 125 µg/mL 5-HMF | 71.6 ± 9.5 * | 35.1 ± 5.3 ++ | 18.2 ± 4.4 $$ |
| 500 µg/mL aKG + 166.7 µg/mL 5-HMF | 58.9 ± 11.1 ** | 28.7 ± 5.8 ++ | 9.9 ± 2.4 $$ |
| HF-SAR | 24 h | 48 h | 72 h |
| Mitochondrial Activity | % | % | % |
| 0 µg/mL aKG + 0 µg/mL 5-HMF | 100 ± 11.4 | 100 ± 4.1 | 100 ± 8.4 |
| 125 µg/mL aKG + 41.7 µg/mL HMF | 86.7 ± 5.3 | 90.7.3 ± 5.2 | 83.1 ± 6.2 |
| 200 µg/mL aKG + 66.7 µg/mL 5-HMF | 90.7 ± 6.9 | 88.2 ± 8.3 | 91.1 ± 4.2 |
| 250 µg/mL aKG + 83.3 µg/mL 5-HMF | 84.3 ± 5.1 | 85.3 ± 6.8 | 91.9 ± 5.1 |
| 375 µg/mL aKG + 125 µg/mL 5-HMF | 90.0 ± 5.5 | 89.6 ± 6.1 | 95.8 ± 5.9 |
| 500 µg/mL aKG + 166.7 µg/mL 5-HMF | 90.8 ± 4.6.1 | 83.7 ± 11.3 | 99.1 ± 9.2 |
Mitochondrial activity at a level of nearly 30% was obtained after 48 h of incubation by using 375 µg/mL + 125.4 µg/mL 5-HMF and 500 µg/mL aKG + 166.7 µg/mL 5-HMF. Mitochondrial activity at levels of 10% and 20% remained after 72 h of incubation with 500 µg/mL aKG + 166.7 µg/mL 5-HMF and after 72 h of incubation with 375 µg/mL aKG + 125.4 µg/mL 5-HMF, respectively.
The incubation of HF-SAR did not result in relevant differences between the different concentrations of aKG + 5-HMF and the incubation times compared to the control (Table 2).
5. Caspase-3 Activity Measurements
The loss of mitochondrial activity mostly induces caspase activity (Figure 4). Compared to the control (2.9 ± 2.3%), 250 µg/mL aKG + 83.3 µg/mL 5-HMF significantly increased the caspase activity in Jurkat cells after 72 h of incubation (13.5 ± 3.2; n = 3; p < 0.001), but 500 µg/mL aKG + 166.7 µg/mL 5-HMF did so even more (51.6 ± 5.2%; n = 3; p < 0.001). While the activity with 500 µg/mL + 166.7 µg/mL 5-HMF was significantly higher than that of the positive control with 4 µM CPT (43.8 ± 1.8; n = 3; p < 0.01), that of 250 µg/mL aKG + 83.3 µg/mL 5-HMF was lower (13.5 ± 3.2, n = 3; p < 0.001).
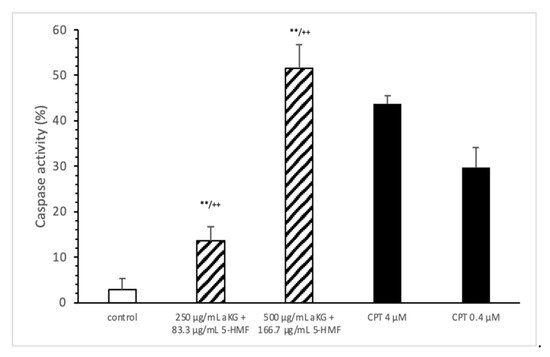
Figure 4. Caspase activity of the Jurkat cell lines after 72 h of cell growth using combined solutions of 250 µg/mL aKG + 83.3 µg/mL 5-HMF solution, 500 µg/mL aKG + 166.7 µg/mL 5-HMF, and 4 or 0.4 µM CPT as positive controls (n = 3). ** p < 0.01: significance between the control and combined solutions of aKG + 5-HMF. ++ p < 0.001: significance between the positive control (4 µM CPT) and combined solutions of aKG + 5-HMF.
6. Detection of the Mitochondrial Membrane Potential through Flow Cytometry
The estimation of apoptotic cells (Figure 5) was significantly increased with 250 µg/mL aKG + 83.3 µg/mL 5-HMF after 72 h of incubation (31.4 ± 3.2%) compared to the control (14.9 ± 2.2%; n=3; p < 0.001), but was significantly decreased compared to 4 µM CPT (50.7 ± 5.6%). The 500 µg/mL aKG + 166.7 µM 5-HMF combination showed a higher significance (63.2 ± 5.6%; n = 3; p < 0.001) compared to the control and to 4 µM CPT (n = 3; p < 0.05).
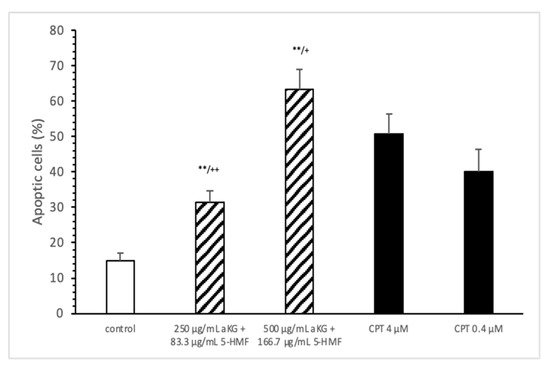
Figure 5. Estimation of apoptotic cells (JC-1) of the Jurkat cell lines after 72 h of cell growth using a combined solutions of 250 µg/mL aKG + 83.3 µg/mL 5-HMF, 500 µg/mL aKG + 166.7 µg/mL 5-HMF, and 4 µM or 0.4 µM CPT as positive controls (n = 3). ** p < 0.001: significance between the control and combined solutions of aKG + 5-HMF. + p < 0.01: significance between the positive control (4 µM CPT) and combined solutions of aKG + 5-HMF. ++ p < 0.001: significance between the positive control (4 µM CPT) and combined solutions of aKG + 5-HMF.
7. Estimation of Carbonylated Proteins in Jurkat and HF-Sar Cells
After 72 h incubation with 250 µg/mL aKG + 83.3 µg/mL 5-HMF the carbonylated protein content of isolated membrane proteins of Jurkat cell line was significantly lower compared to 0 h (11.6 ± 0.67 vs. 7.44 ± 0.93 nmol/mg; p < 0.01), but not of HF-SAR as presented in Table 3. Using 500 µg/mL aKG + 166.7 µg/mL 5-HMF the carbonylated protein level showed a significant reduction (10.6 ± 0.37 vs. 5.55 ± 1.22; p < 0.01) in Jurkat cells and also in HF-SAR (2.5 ± 0.6 vs. 1.73 ± 0.52 nmol/mg; p < 0.05). Furthermore, the carbonylated protein content of Jurkat lysates showed a significantly higher content (11.1 ± 0.70 nmol/mg) compared to HF-SAR lysate (2.30 ± 0.66 nmol/mg; p < 0.01) before incubation with 250 µg/mL aKG + 83.3 µg/mL 5-HMF or 500 µg/mL aKG + 166.7 µg/mL 5-HMF.
Table 3. Carbonylated proteins of Jurkat and HF-SAR lysates after 0 and 72 h incubation in absence or presence of 500 µg/mL aKG + 125 µg/mL 5-HMF and 500 µg/mL aKG + 62.5 µg/mL 5-HMF (n = 5). * p < 0.05: significance between 0 h and 72 h incubated aKG + 5-HMF solutions. ** p < 0.01: significance between 0 h and 72 h incubated aKG + 5-HMF solutions. §§ p < 0.01 = significance between Jurkat cell and HF-SAR lysate.
| Carbonylated Proteins (nmol/mg Protein) | ||||
|---|---|---|---|---|
| Jurkat Lysate | HF-SAR Lysate | |||
| 0 h | 72 h | 0 h | 72 h | |
| 250 µg/mL aKG + 83.3 µg/mL 5-HMF | 11.6 §§ ± 0.67 | 7.44 ± 0.93 ** | 2.10 ± 0.72 | 1.91 ± 0.82 |
| 500 µg/mL aKG + 166.7 µg/mL 5-HMF | 10.6 §§ ± 0.37 | 5.55 ± 1.22 ** | 2.5 ± 0.6 | 1.73 ± 0.52 * |
References
- Greilberger, J.F.; Wintersteiger, R.; Astrid, O.; Greilberger, M.; Herwig, R. Combination of 2-oxoglutarate/ascorbic acid/5-hydroxy-methyl-furfur-aldehyde/carnosine inhibits protein oxidation during radical exposure of cigarette smoke. Proteins 2018, 5, 10–12.
- Kaelin, W.G. Cancer and altered metabolism: Potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 335–345.
- Losman, J.A.; Koivunen, P.; Kaelin, W.G. 2020. 2-Oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer 2020, 20, 710–726.
- Yi, W.; Yu, Y.; Li, Y.; Yang, J.; Gao, S.; Xu, L. The tumor-suppressive effects of alpha-ketoglutarate-dependent dioxygenase FTO via N6-methyladenosine RNA methylation on bladder cancer patients. Bioengineered 2021, 12, 5323–5333.
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234.
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35.
- Zhao, L.; Su, J.; Li, L.; Chen, J.; Hu, S.; Zhang, X. Mechanistic elucidation of apoptosis and cell cycle arrest induced by 5-hydroxymethylfurfural, the important role of ROS-mediated signaling pathways. Food Res. 2014, 66, 186–196.
- Bito, T.; Koseki, K.; Asano, R.; Ueda, N. 5-hydroxymethyl-2-furaldehyde purified from Japanese pear (Pyrus pyrifolia Nakai cv. Nijisseiki) juice concentrate inhibits melanogenesis in B16 mouse melanoma. Bioscience 2020, 4, 2374–2384.
- Kössler, F.; Mair, L.; Burtscher, M. 5-Hydroxymethylfurfural and α-ketoglutaric acid supplementation increases oxygen saturation during prolonged exercise in normobaric hypoxia. Int. J. 2019, 91, 63–68.
- Kassa, T.; Wood, F.; Strader, M.B.; Alayash, A.I. Antisickling Drugs Targeting βCys93 Reduce Iron Oxidation and Oxidative Changes in Sickle Cell Hemoglobin. Front. Physiol. 2019, 10, 931.
More
Information
Subjects:
Oncology; Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
19 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




