
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vijayabhaskarreddy Junnuthula | + 1554 word(s) | 1554 | 2021-10-14 04:22:10 | | | |
| 2 | Nora Tang | + 408 word(s) | 1962 | 2021-11-17 10:32:29 | | |
Video Upload Options
Photodynamic therapy (PDT) involves activation of a photosensitizer by photon irradiation that generates reactive oxygen species (ROS) that selectively occlude CNV in the target ocular segment. It has found extensive applications in exudative AMD treatment before the rise of anti-VEGF therapy. Visudyne® is a liposomal formulation of a hydrophobic photosensitizer, Verteporfin that was approved for nAMD administration by the US FDA in 2000.
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of vision loss in developed countries with prevalence rates ranging from 5–40% based on ethnicity [1][2]. In 2020, around 200 million people were suffering from AMD, and projections are nearly 300 million in 2040 worldwide [1][2]. The pathophysiology of AMD includes inflammation mechanisms affecting the retina coupled with oxidative stress. The disease is broadly classified into dry AMD and wet AMD. Dry AMD could progress into wet AMD (neovascular AMD) after the progression of new blood vessels into the retina and subretinal space. Subsequently, these vessels cause bleeding, leakage of serum and fluid retention, distortion in vision, and central vision loss after progression [3]. Various treatment modalities are under consideration, intravitreal biologics (Bevacizumab, Ranibizumab, and Aflibercept) being the standard treatment of care given as intravitreal injections. The intravitreal injection (IVT) route is currently in use for anti-VEGF (Vascular Endothelial Growth Factor) agents. It is an invasive route and causes difficulty to patients, but alternative clinical outcomes are bleak at present [4][5]. Various administrative routes are explored, such as periocular, suprachoroidal, sub-retinal, systemic, and topical routes for the delivery of small molecules, biologics, and gene therapy [6]. Molecular therapies such as cell therapy and gene therapy are in focus due to their restoration ability [7][8][9][10]. AMD is a disease of the posterior eye segment which brings various challenges. There are two basic ways to improve the therapeutic outcome: prolonged delivery of intravitreal drugs and use of various other routes [5]. There were numerous attempts to deliver drugs and biologics from the topical routes due to its simple and non-invasive administration. However, the barriers in delivering biologics to the cornea (Absorption, Distribution, Metabolism, and Excretion (ADME); degradation of biologics; and their interactions with corneal layers) currently prevent the exploitation this route [11]. In general, biologics have longer half-lives in vitreous, and small molecules have shorter half-lives. If the patient misses the clinician’s appointment for any reason, the IVT therapy could decrease the efficacy, hence the need for long-acting injectables or implants. In recent years, nanoparticles (NPs) have been extensively investigated and used in drug delivery and biomedical application [12][13][14][15]. Several delivery vehicles are reported in the literature including, micelles, nanoparticles, polymersomes, liposomes, and reviews on the details of nanoparticle preparation and characterization, including their evaluation with respect to storage stability, toxicity potential, and in vivo fate [16][17][18][19][20][21]. Recently, we have reported a comprehensive review on “Nanodiagnostics and Nanotherapeutics for Age-related macular degeneration” [22] and hence this will not be discussed here. In the present review, we highlight preclinical and clinical studies of wet AMD treatment modalities such as anti-VEGF therapy, including antibodies, bispecific antibodies, small molecules, photodynamic therapy, radiation therapy, gene therapy, and cell therapy. We are also providing information on sustained-release strategies and pharmacokinetics aspects to reduce the need for frequent injections [23].
2. Pathogenesis of AMD
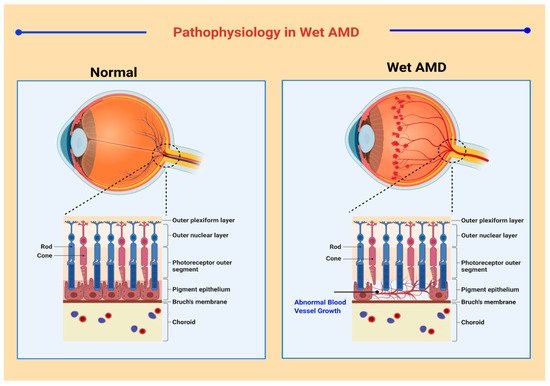
A broad spectrum of classification models has been developed for medical and research purposes to distinguish between the various manifestations of AMD. However, there exists no specific classification system that is accepted worldwide [24]. According to one convention, macular degeneration can be broadly classified as wet AMD and dry AMD [25]. Wet AMD, or neovascular AMD (nAMD), is an exudative degeneration that is caused by hyper-expression of VEGF along with rapid and progressive angiogenesis [26]. Pathophysiological features in neovascular AMD are shown in Figure 1 . Dry AMD is a non-exudative degeneration that is defined as the accumulation of “drusen” underneath the maculae [27]. Among the cases diagnosed, ~90% of the occurrences are associated to dry AMD whereas ~10% of cases are due to nAMD, mostly as all early forms of AMD are considered as dry AMD. Studies have depicted that 10–15% of non-neovascular AMD cases may progress into wet AMD [28][29].
The Wisconsin age-related maculopathy (ARM) [30] system, a convention based on the photographic retinal grading approach was adopted by The International ARM Epidemiological Study [31] to reinvent AMD diagnosis. The grading procedure was described by nominal or modest non-exudative age-related macular changes. According to the ARM criterion, the presence of retinal pigment epithelium (RPE) atrophy was requisite to signify the manifestation of wet AMD. Another rigorous classification protocol has been described in the Age-Related Eye Disease Study (AREDS). This study divided the occurrence of AMD into four classes based on protein drusen attributes. Drusen were categorized based on its average diameter into small (63 μm and less), intermediate (63–124 μm), and large (125 μm and more). According to the AREDS, class 1, or no AMD, specifies that there exist less than five small drusen; class 2, or early AMD, specifies that there exists many small drusen or few intermediate-sized drusen; class 3, or intermediate AMD, is characterized by multiple intermediate drusen or few large-sized drusen and non-foveal GA without pigmentary atrophy; and class 4, or advanced AMD, is denoted by foveal GA and pigmentary atrophy, vision impairment due to CNV occurrence, and visual acuity lower than 20/32 in either eye [32][33]. A non-clinical system has been developed by Klein et.al. It bases its categorization on variations in demography and involves phenotypic and genetic considerations [34].
3. Advanced Therapeutics and Delivery Strategies for nAMD
Another critical factor that has been evidenced in the pathogenesis of AMD is the intraocular inflammatory signals. This indicates that certain anti-inflammatory agents may play a role in the regression of the macular degeneration. The results from Blue Mountains Eye study [35] concluded that there was no apparent association between AMD prevalence and the administration of systemic non-steroidal anti-inflammatory drugs (NSAIDs).
A recent proof of concept clinical research study (NCT03022318, NCT03023059) concluded that levodopa oral formulation was safe and well-tolerated. In the case of patients taking anti-VEGF injections, the injection frequency was reduced. The application of levodopa as an adjuvant needs further evaluation due to the low sample size and limited racial diversity [36]. Levodopa acts as a ligand for G-protein coupled receptor (GPR143), upregulates the PEDF, and downregulates the production of VEGF [37][38][39].
Photodynamic therapy (PDT) involves activation of a photosensitizer by photon irradiation that generates reactive oxygen species (ROS) that selectively occlude CNV in the target ocular segment [40]. It has found extensive applications in exudative AMD treatment before the rise of anti-VEGF therapy. Visudyne ® is a liposomal formulation of a hydrophobic photosensitizer, Verteporfin that was approved for nAMD administration by the US FDA in 2000 [41]. Photodynamic therapy is generally used as alternative therapy or adjunct therapy along with anti-VEGF injections according to various country guidelines [42][43]. Table 1 summarizes the photodynamic therapy and delivery strategies for nAMD.
| Therapeutic Modality | Active Ingredient | Delivery System | Clinical Progress | Highlights | Refs. |
|---|---|---|---|---|---|
| Port Delivery System | Ranibizumab | Ocular implant | Clinical development | Sustained and controlled release of Ranibizumab to neutralize VEGF | [44] |
| Photodynamic Therapy | Verteporfin | Liposomes | Approved for clinical use (Visudyne®) | Cessation of bleeding and selective occlusion of the newly formed blood vessels along with decreased exudate formation | [41] |
| Verteporfin | Cationic liposomes | Exploratory studies | CNV occlusion and reduced retinal deterioration compared to Visudyne® | [45] | |
| Paclitaxel/Succinyl-paclitaxel | Cationic liposomes | Exploratory studies | Inhibition of angiogenesis in rat models | [45] | |
| Hypocrellin B | Liposomes | Exploratory studies | Significant reduction in CNV area with reduced tissue damage | [46] | |
| Edaravone | Liposomes | Exploratory studies | Inhibition of ROS generation, reduced thickness of outer nuclear layer and no cytotoxicity | [47] | |
| Calcein | Gold nanoparticles | Exploratory studies | Light activated sustained and controlled drug release | [48] | |
| Calcein | ICG loaded liposomes | Exploratory studies | Light induced improved permeability of liposomes to control drug release | [49] | |
| Photocyanine and Sorafenib | RGD modified liposomes | Exploratory studies | Reduced CNV area and improved safety profile | [50] | |
| Verteporfin | Liposomes | Exploratory studies | Combination with anti-VEGF agents is more efficacious than anti-VEGF monotherapy | [51] | |
| Gene therapy | PEDF gene | AAV | Clinical trials | Inhibition of angiogenesis | [52] |
| PEDF gene | E1-, partial E3-, E4-deleted AAV | Clinical trials | No deleterious adverse effects were observed, however no concrete evidence is available to show the improved therapeutic efficacy and the relation between response and dose escalation | [53] | |
| sFLT-1 | Recombinant AAV | Clinical trials | Antiangiogenic activity and reduced progression of CNV but no promising results | [54][55][56] | |
| ADVM-022 and ADVM-032 | Vector capsid, AAV.7m8 | Clinical trials | Antiangiogenic activity in murine models with laser induced CNV | [57] | |
| sFLT1 | AAV2 with CBA promoter | Clinical trials | Higher expression with promoter introduction, however disappointing results | [58] | |
| Anti-VEGF mAb | AAV8 vector, RGX-314 | Clinical trials | High protein expression therefore tolerability is being studied | [59] | |
| Angiostatin and Endostatin | EIAV-lentiviral particle | Clinical trials | No specific complications observed and high levels of active maintained. | [60] | |
| mLP-CRISPR | Lentiviral particle | Clinical trials | Prevented progression of nAMD and no specific immune responses | [61] | |
| Stem cell therapy | Autologous iPSC derived RPE cells | Cell sheet | Clinical trials | Retained stability of transplanted sheet, corrected vision and no rejection of graft observed after 5 years | [7] |
| Allogeneic iPSC derived RPE cells | Cell suspension | Clinical trials | No specific cases of graft rejection were observed and mild cases of immune rejection that can be managed | [62] | |
| Allogeneic ESC derived RPE cells | Cell sheet | Clinical trials | Few cases of immediate adverse effect observed, no immune rejection responses. | [63] |
Studies suggest that, though VEGF inhibitors exhibit rapid therapeutic activity, it is restricted by several limitations. However, this drawback can be potentially overcome by the concurrent administration of anti-VEGF drugs and radiotherapy. As radiotherapy involves a major time lag and a prolonged duration of action, it can potentially augment the therapy of AMD in theory [64]. However, clinical trials and comparative studies negate such predictions and provide ambiguous conclusions. Recent clinical trials suggest that monotherapy of VEGF inhibitors is still superior to the combination therapy of anti-VEGF drugs and radiation therapy. Such results necessitate deeper understanding and large-scale trials to obtain accurate therapeutic results [65]. Table 1 summarizes the combination therapy of anti-VEGF agents with radiation for nAMD. In a 12 month follow-up study, comparing radiotherapy combined with anti-VEGF and anti-VEGF alone concluded that effectiveness for treating wet AMD was uncertain as to whether radiotherapy on its own, or with eye injections of anti-VEGF, was more beneficial [66].
4. Concluding Remarks and Future Perspectives
Over the past few decades, the irremediable blindness due to the fast progression of nAMD in the geriatric population has led researchers to shift their focus from intravitreal anti-VEGF biologics (Bevacizumab, Ranibizumab, Aflibercept, and Brolucizumab) to advanced therapeutic strategies that possess an improved safety and efficacy profile accompanied with minimal adverse effects. One step in this direction involves the exploration of therapy requiring administration of small molecules (Tyrosine Kinase Inhibitors and anti-inflammatory molecules), biologics, and gene products. In 2017, Luxturna® is approved for Leber’s congenital amaurosis (LCA) genetic disease, may boost further interest in gene therapy. Several research groups are focused on the progression of non-invasive delivery and sustained release strategies for the posterior segment. The Port Delivery System seems to be the front-runner in the long-acting strategy. Several particulate systems are under clinical and preclinical development. However, pre hand information of potency and dosage requirements are crucial in the success of such technologies. Most studies reported in this space are rather observational; quantitative estimations and mass balance of molecules kinetics is often missing. The therapeutic interest in radiation therapy is declining rapidly due to the emerging biologics. At present, bispecific antibodies are being continuously explored, and the first step towards their commercialization requires the reduction in the ocular cytotoxicity, followed by thorough investigation in clinical trials to expand the scope of therapy. BYOOVIZ™ (SB11), a biosimilar referencing LUCENTIS ® (Ranibizumab), recently approved by the U.S. Food and Drug Administration (FDA), may reduce the cost of treatment. Though anti-VEGF therapy has many challenges, it is going to remain the primary treatment until one of the described therapies effectively replaces it and subsequently improves patient compliance.
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116.
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia-Pac. J. Ophthalmol. 2017, 6, 493–497.
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021, 7, 1–25.
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12.
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185.
- Wan, C.-R.; Muya, L.; Kansara, V.; Ciulla, T. Suprachoroidal Delivery of Small Molecules, Nanoparticles, Gene and Cell Therapies for Ocular Diseases. Pharmaceutics 2021, 13, 288.
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779.
- Wang, Y.; Tang, Z.; Gu, P. Stem/progenitor cell-based transplantation for retinal degeneration: A review of clinical trials. Cell Death Dis. 2020, 11, 1–14.
- Scholl, H.P.N.; Strauss, R.W.; Singh, M.S.; Dalkara, D.; Roska, B.; Picaud, S.; Sahel, J.-A. Emerging therapies for inherited retinal degeneration. Sci. Transl. Med. 2016, 8, 368rv6.
- Baradaran-Rafii, A.; Sarvari, M.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Aghayan, H.R.; Larijani, B.; Rezaei-Tavirani, M.; Biglar, M.; Arjmand, B. Cell-based approaches towards treating age-related macular degeneration. Cell Tissue Bank. 2020, 21, 339–347.
- Wels, M.; Roels, D.; Raemdonck, K.; De Smedt, S.; Sauvage, F. Challenges and strategies for the delivery of biologics to the cornea. J. Control. Release 2021, 333, 560–578.
- Patil, A.; Dyawanapelly, S.; Dandekar, P.; Jain, R. Fabrication and Characterization of Non-spherical Polymeric Particles. J. Pharm. Innov. 2020, 1–12.
- Atale, S.S.; Dyawanapelly, S.; Jagtap, D.D.; Jain, R.; Dandekar, P. Understanding the nano-bio interactions using real-time surface plasmon resonance tool. Int. J. Biol. Macromol. 2019, 123, 97–107.
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29.
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246.
- Junnuthula, V.; Boroujeni, A.S.; Cao, S.; Tavakoli, S.; Ridolfo, R.; Toropainen, E.; Ruponen, M.; van Hest, J.; Urtti, A. Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region. Pharmaceutics 2021, 13, 445.
- Ridolfo, R.; Tavakoli, S.; Junnuthula, V.; Williams, D.S.; Urtti, A.; Van Hest, J.C.M. Exploring the Impact of Morphology on the Properties of Biodegradable Nanoparticles and Their Diffusion in Complex Biological Medium. Biomacromolecules 2021, 22, 126–133.
- Sharma, P.; Mittal, S. Nanotechnology: Revolutionizing the delivery of drugs to treat age-related macular degeneration. Expert Opin. Drug Deliv. 2021, 18, 1131–1149.
- Iglicki, M.; González, D.P.; Loewenstein, A.; Zur, D. Longer-acting treatments for neovascular age-related macular degeneration—Present and future. Eye 2021, 35, 1111–1116.
- Formica, M.L.; Alfonso, H.G.A.; Palma, S.D. Biological drug therapy for ocular angiogenesis: Anti-VEGF agents and novel strategies based on nanotechnology. Pharmacol. Res. Perspect. 2021, 9, e00723.
- Iyer, S.; Radwan, A.E.; Hafezi-Moghadam, A.; Malyala, P.; Amiji, M. Long-acting intraocular Delivery strategies for biological therapy of age-related macular degeneration. J. Control. Release 2019, 296, 140–149.
- Sarkar, A.; Dyawanapelly, S. Nanodiagnostics and Nanotherapeutics for age-related macular degeneration. J. Control. Release 2021, 329, 1262–1282.
- Skelly, A.; Bezlyak, V.; Liew, G.; Kap, E.; Sagkriotis, A.; Liew, K. Treat and Extend Treatment Interval Patterns with Anti-VEGF Therapy in nAMD Patients. Vision 2019, 3, 41.
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330.
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159.
- Chong, V. Ranibizumab for the treatment of wet AMD: A summary of real-world studies. Eye 2016, 30, 270–286.
- Holz, F.G.; Schmitz-valckenberg, S.; Invest, J.C.; Holz, F.G.; Schmitz-valckenberg, S.; Fleckenstein, M. Recent developments in the treatment of age- related macular degeneration Find the latest version: Recent developments in the treatment of age-related macular degeneration. J. Clin. Investig. 2014, 124, 1430–1438.
- Chou, R.; Dana, T.; Bougatsos, C.; Grusing, S.; Blazina, I. Screening for Impaired Visual Acuity in Older Adults: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force. JAMA 2016, 315, 915–933.
- Tielsch, J.; Javitt, J.C.; Coleman, A.; Katz, J.; Sommer, A. The Prevalence of Blindness and Visual Impairment among Nursing Home Residents in Baltimore. N. Engl. J. Med. 1995, 332, 1205–1209.
- Klein, R.; Davis, M.D.; Magli, Y.L.; Segal, P.; Klein, B.E.; Hubbard, L. The Wisconsin Age-related Maculopathy Grading System. Ophthalmology 1991, 98, 1128–1134.
- Bird, A.; Bressler, N.; Bressler, S.; Chisholm, I.; Coscas, G.; Davis, M.; de Jong, P.; Klaver, C.; Klein, B.; Klein, R.; et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv. Ophthalmol. 1995, 39, 367–374.
- Davis, M.D.; Gangnon, R.E.; Lee, L.-Y.; Hubbard, L.D. The Age-Related Eye Disease Study Severity Scale for Age-Related Macular Degeneration. AREDS Rep. 2005, 123, 1484–1498.
- Ferris, F.; Wilkinson, C.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical Classification of Age-related Macular Degeneration. Am. Acad. Ophthalmol. 2013, 120, 844–851.
- Klein, R.; Meuer, S.M.; Myers, C.E.; Buitendijk, G.H.S.; Rochtchina, E.; Choudhury, F.; De Jong, P.T.V.M.; McKean-Cowdin, R.; Iyengar, S.; Gao, X.; et al. Harmonizing the Classification of Age-related Macular Degeneration in the Three-Continent AMD Consortium. Ophthalmic Epidemiol. 2014, 21, 14–23.
- Wang, J.J.; Mitchell, P.; Smith, W.; Gillies, M.; Billson, F. Systemic use of anti-inflammatory medications and age-related maculopathy: The Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003, 10, 37–48.
- Figueroa, A.G.; Boyd, B.M.; Christensen, C.A.; Javid, C.G.; McKay, B.S.; Fagan, T.C.; Snyder, R.W. Levodopa Positively Affects Neovascular Age-Related Macular Degeneration. Am. J. Med. 2021, 134, 122–128.e3.
- Lopez, V.M.; Decatur, C.L.; Stamer, W.D.; Lynch, R.M.; McKay, B.S. L-DOPA Is an Endogenous Ligand for OA1. PLoS Biol. 2008, 6, e236.
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144.
- Falk, T.; Congrove, N.R.; Zhang, S.; McCourt, A.D.; Sherman, S.J.; McKay, B. PEDF and VEGF-A Output from Human Retinal Pigment Epithelial Cells Grown on Novel Microcarriers. J. Biomed. Biotechnol. 2012, 2012, 278932.
- Nishie, H.; Kataoka, H.; Yano, S.; Kikuchi, J.-I.; Hayashi, N.; Narumi, A.; Nomoto, A.; Kubota, E.; Joh, T. A next-generation bifunctional photosensitizer with improved water-solubility for photodynamic therapy and diagnosis. Oncotarget 2016, 7, 74259–74268.
- Christie, J.G.; Kompella, U.B. Ophthalmic light sensitive nanocarrier systems. Drug Discov. Today 2008, 13, 124–134.
- Recommendations|Age-Related Macular Degeneration|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng82/chapter/Recommendations#pharmacological-management-of-amd (accessed on 21 September 2021).
- AMD Treatment Guidelines|Harvard Medical School Department of Ophthalmology. Available online: https://eye.hms.harvard.edu/eyeinsights/2015-january/age-related-macular-degeneration-amd (accessed on 21 September 2021).
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2019, 126, 1141–1154.
- Gross, N.; Ranjbar, M.; Evers, C.; Hua, J.; Martin, G.; Schulze, B.; Michaelis, U.; Hansen, L.L.; Agostini, H.T. Choroidal neovascularization reduced by targeted drug delivery with cationic liposome-encapsulated paclitaxel or targeted photodynamic therapy with verteporfin encapsulated in cationic liposomes. Mol. Vis. 2013, 19, 54–61.
- Li, T.; Hou, X.; Deng, H.; Zhao, J.; Huang, N.; Zeng, J.; Chen, H.; Gu, Y. Liposomal hypocrellin B as a potential photosensitizer for age-related macular degeneration: Pharmacokinetics, photodynamic efficacy, and skin phototoxicity in vivo. Photochem. Photobiol. Sci. 2015, 14, 972–981.
- Shimazaki, H.; Hironaka, K.; Fujisawa, T.; Tsuruma, K.; Tozuka, Y.; Shimazawa, M.; Takeuchi, H.; Hara, H. Edaravone-Loaded Liposome Eyedrops Protect against Light-Induced Retinal Damage in Mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7289–7297.
- Lajunen, T.; Viitala, L.; Kontturi, L.-S.; Laaksonen, T.; Liang, H.; Vuorimaa-Laukkanen, E.; Viitala, T.; Le Guével, X.; Yliperttula, M.; Murtomäki, L.; et al. Light induced cytosolic drug delivery from liposomes with gold nanoparticles. J. Control. Release 2015, 203, 85–98.
- Lajunen, T.; Nurmi, R.; Wilbie, D.; Ruoslahti, T.; Johansson, N.; Korhonen, O.; Rog, T.; Bunker, A.; Ruponen, M.; Urtti, A. The effect of light sensitizer localization on the stability of indocyanine green liposomes. J. Control. Release 2018, 284, 213–223.
- Wang, J.-L.; Xi, Y.; Liu, Y.-L.; Wang, Z.; Zhang, Q. Combination of Targeted PDT and Anti-VEGF Therapy for Rat CNV by RGD-Modified Liposomal Photocyanine and Sorafenib. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7983–7989.
- Gao, Y.; Yu, T.; Zhang, Y.; Dang, G. Anti-VEGF Monotherapy Versus Photodynamic Therapy and Anti-VEGF Combination Treatment for Neovascular Age-Related Macular Degeneration: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4307–4317.
- Campochiaro, P.A. Gene transfer for ocular neovascularization and macular edema. Gene Ther. 2011, 19, 121–126.
- Campochiaro, P.A.; Nguyen, Q.D.; Shah, S.M.; Klein, M.L.; Holz, E.; Frank, R.N.; Saperstein, D.A.; Gupta, A.; Stout, J.T.; Macko, J.; et al. Adenoviral Vector-Delivered Pigment Epithelium-Derived Factor for Neovascular Age-Related Macular Degeneration: Results of a Phase I Clinical Trial. Hum. Gene Ther. 2006, 17, 167–176.
- Lai, C.-M.; Estcourt, M.J.; Himbeck, R.P.; Lee, S.-Y.; Yeo, I.Y.-S.; Luu, C.; Loh, B.K.; Lee, M.W.; Barathi, A.; Villano, J.; et al. Preclinical safety evaluation of subretinal AAV2.sFlt-1 in non-human primates. Gene Ther. 2011, 19, 999–1009.
- Lai, C.-M.; Brankov, M.; Zaknich, T.; Lai, Y.K.-Y.; Shen, W.-Y.; Constable, I.J.; Kovesdi, I.; Rakoczy, P.E. Inhibition of Angiogenesis by Adenovirus-Mediated sFlt-1 Expression in a Rat Model of Corneal Neovascularization. Hum. Gene Ther. 2001, 12, 1299–1310.
- Lai, Y.K.Y.; Sharma, S.; Lai, C.-M.; Brankov, M.; Constable, I.J.; Rakoczy, P.E. Virus-mediated secretion gene therapy--A potential treatment for ocular neovascularization. Single Mol. Single Cell Seq. 2003, 533, 447–453.
- Moore, N.A.; Bracha, P.; Hussain, R.M.; Morral, N.; Ciulla, T.A. Gene therapy for age-related macular degeneration. Expert Opin. Biol. Ther. 2017, 17, 1235–1244.
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 2017, 390, 50–61.
- de Guimaraes, T.A.C.; Georgiou, M.; Bainbridge, J.W.B.; Michaelides, M. Gene therapy for neovascular age-related macular degeneration: Rationale, clinical trials and future directions. Br. J. Ophthalmol. 2021, 105, 151–157.
- Campochiaro, P.A.; Lauer, A.K.; Sohn, E.H.; Mir, T.A.; Naylor, S.; Anderton, M.C.; Kelleher, M.; Harrop, R.; Ellis, S.; Mitrophanous, K.A. Lentiviral Vector Gene Transfer of Endostatin/Angiostatin for Macular Degeneration (GEM) Study. Hum. Gene Ther. 2017, 28, 99–111.
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156.
- Maeda, T.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Trends of Stem Cell Therapies in Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 1785.
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097.
- Woodward, B.W. Comparison of Efficacy of Proton Beam and 90Sr/90Y Beta Radiation in Treatment of Exudative Age-Related Macular Degeneration; NeoVista Inc.: Fremont, CA, USA, 2008.
- Kishan, A.U.; Modjtahedi, B.S.; Morse, L.S.; Lee, P. Radiation Therapy for Neovascular Age-related Macular Degeneration. Int. J. Radiat. Oncol. 2013, 85, 583–597.
- Evans, J.R.; Igwe, C.; Jackson, T.; Chong, V. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2020, 2020.





