
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefan Radnev Stefanov | + 2238 word(s) | 2238 | 2021-11-03 10:06:57 | | | |
| 2 | Vicky Zhou | Meta information modification | 2238 | 2021-11-16 09:06:26 | | |
Video Upload Options
Lipid nanoparticles (LN) are recognized as promising drug delivery systems (DDS) in treating skin disorders. Solid lipid nanoparticles (SLN) together with nanostructured lipid carriers (NLC) exhibit excellent tolerability as these are produced from physiological and biodegradable lipids. Moreover, LN applied to the skin can improve stability, drug targeting, occlusion, penetration enhancement, and increased skin hydration compared with other drug nanocarriers. Furthermore, the features of LN can be enhanced by inclusion in suitable bases such as creams, ointments, gels (i.e., hydrogel, emulgel, bigel), lotions, etc.
1. Introduction
1.1. Skin Disorders
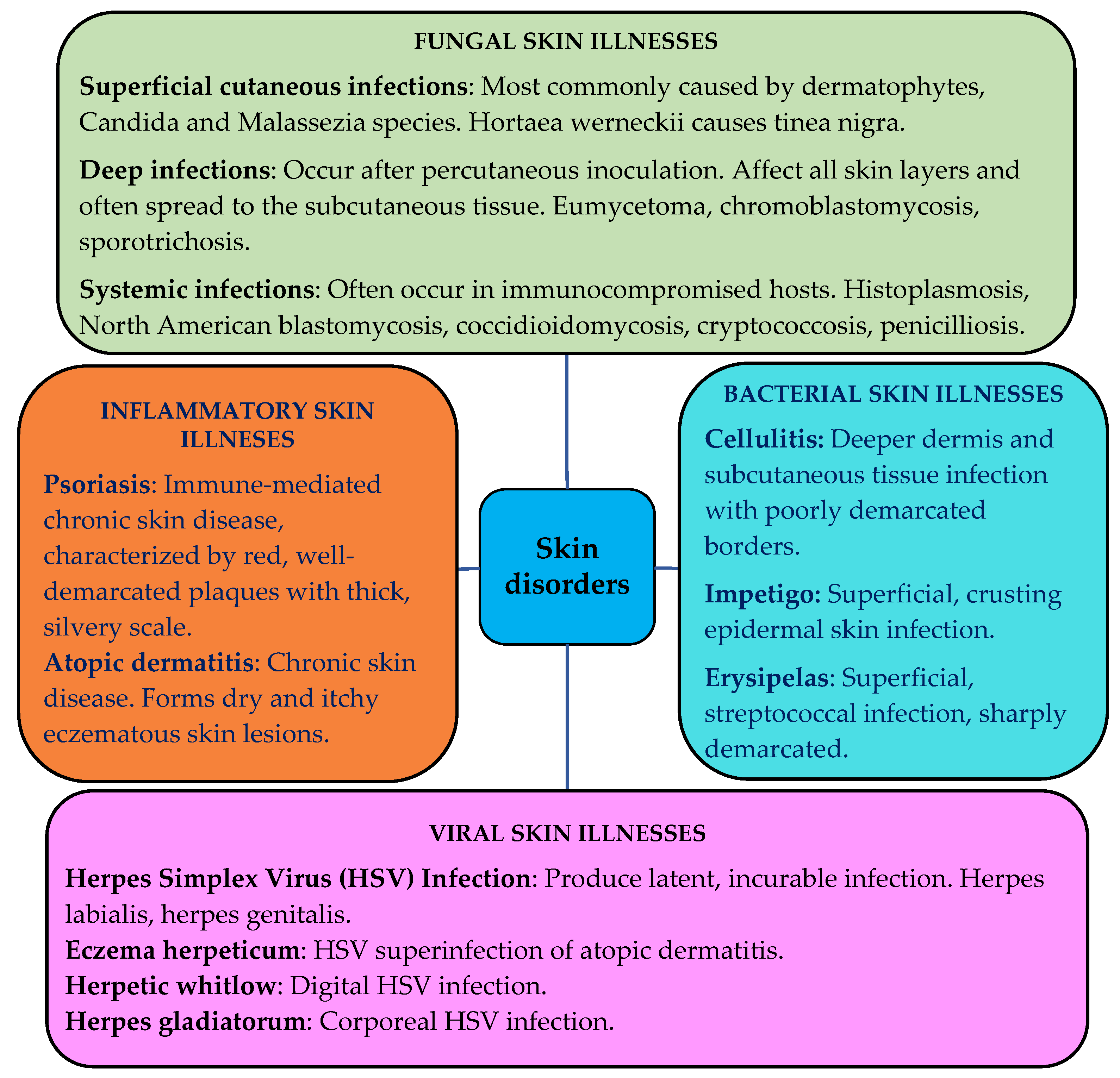
The skin can be affected by various pathological changes, i.e., inflammatory, neoplastic, traumatic, hormonal, degenerative, and even hereditarily determined [1]. Infectious skin diseases such as bacterial, fungal or viral affect people and cause various dermatological problems. Chronic inflammatory skin diseases such as atopic dermatitis, allergic contact dermatitis, psoriasis, etc., are a consequence of infiltration of inflammatory T cells [2]. The schematic representation of various skin disorders is shown in Figure 1 [3][4][5][6][7][8][9][10][11][12][13][14].
1.2. Lipid Nanoparticulate Drug Delivery Systems
Lipid-based drug delivery systems (LBDDS) are formulations containing a dissolved or suspended drug substance in lipidic excipients [17]. LBDDS are a progressive strategy to formulate pharmaceuticals for topical delivery [18]. Liposomes, which are “pioneers” among lipid DDS, have been used to improve drug solubility and traditionally for topical and transdermal drug delivery.
Table 1 presents a brief overview of lipid-based drug delivery systems.
Table 1. Presentation of some lipid-based delivery systems.
| Lipid-Based Delivery System |
Description | Advantages | Disadvantages |
|---|---|---|---|
| Nanovesicular carriers | |||
| Liposomes [19] | Conventional single or multilayer vesicles. Formed by contact of biodegradable lipids with an aqueous medium. Widely used as drug carriers for hydrophilic and lipophilic molecules. | Biocompatible and biodegradable lipids. Conventional production processes. Improved local delivery. Suitable for loading both hydrophobic and hydrophilic substances. | Insufficient chemical and physical stability. Short half-life. Inadequate penetration into the viable epidermis and dermis. High production costs. Difficulties in scalability. |
| Transfersomes [20][21][22] |
Highly deformable, elastic or ultra-flexible liposomes. Vesicles, similar to conventional liposomes in terms of preparation and structure. Claimed to permeate as intact vesicles through the skin layers. Functionally deformed due to the presence of an edge activator. | Smaller vesicle size, higher elasticity. Compared with conventional liposomes—better penetration through the skin. High membrane hydrophilicity and elasticity allow them to avoid aggregation and fusion under osmotic stress, unlike the conventional liposomes. | Elasticity of these vesicles can be compromised by hydrophobic drug loading. Occlusive application and complete skin hydration limit transdermal delivery due to inhibition of transdermal hydration. Relatively high production costs. Absence of well-established regulatory guidance for skin delivery. |
| Ethosomes [23][24] | Lipid vesicles are composed of phospholipids, ethanol, and water. Similar to liposomes in terms of their preparation techniques and structure. Concentration of ethanol 20–45%. Their size decreases with an increase in the ethanol concentration. Exhibit high encapsulation efficiency. | Appropriate for both hydrophobic and hydrophilic drug loading. Enhanced skin delivery under both occlusive and nonocclusive conditions. Higher elasticity, smaller vesicle size, and higher entrapment efficiency than conventional liposomes. | High ethanol content can lead to skin irritation and toxicity. Possible structural and chemical instability during long-term storage. Need to optimize the concentration of lipids and ethanol for improved physicochemical properties and stability of ethosomes. |
| Lipid nanoparticulate carriers | |||
| Solid lipid nanoparticles [25][26] | Colloidal lipid nanoparticles are composed of a physiological biodegradable solid lipophilic matrix (solid at room temperature and body temperature), in which the drug molecules can be incorporated. | Increased drug stability. High drug payload. Incorporation of lipophilic and hydrophilic drugs. Avoidance of organic solvents. Lack of biotoxicity of the carrier. Relatively cost-effective. | SLN are incorporated into semisolid carriers such as ointments and gels due to the high water content. Potential expulsion of active compounds during storage. Cost-effective manufacturing process. |
| Nanostructure Lipid Carriers [27][28] |
Colloidal lipid nanoparticles composed of physiological mixing liquid lipid (oils) with the solid lipids, in which the liquid lipid is incorporated into the solid matrix or localized at the surface of solid particles | Improved drug loading compared with SLN. Lower water content compared with SLN. Firmly incorporates the drug substance during storage. Biodegradable and biocompatible. Large-scale production is easily possible. | Tendency to unpredictable gelation. Polymorphic transition. Low drug incorporation due to the crystalline structure of solid lipids. Lack of long-term stability data. |
| Lipospheres [29][30][31] |
Microspheres, composed of solid hydrophobic lipid core and stabilized by a monolayer of a phospholipid embedded on the surface. | Improved drug stability, especially for photo-labile drugs. Possibility for controlled drug release. Controlled particle size. High drug loading. Biodegradable and biocompatible. | Larger particle size and poor skin permeation compared with lipid-based vesicular carriers, SLN, and NLC. Poor drug loading for hydrophilic compounds. |
2. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
2.1. Solid Lipid Nanoparticles
2.2. Nanostructured Lipid Carriers
2.3. Preparation of SLN and NLC
-
Hot homogenization—the lipids are heated above their melting point;
2.4. SLN and NLC in the Treatment of Skin Disorders
2.4.1. Antioxidant Effect
2.4.2. Anti-Inflammatory Effect
2.4.3. Antifungal Effect
2.4.4. Anti-Acne Effect
| LNP Type | API/Drug | Application | Reference |
|---|---|---|---|
| SLN | Doxorubicin | Doxorubicin-loaded SLN for the treatment of skin cancer. | [63] |
| SLN | Adapalene | Adapalene-loaded SLN in the gel for anti-acne treatment. | [64] |
| SLN | Triamcinolone acetonide | Triamcinolone acetonide-loaded SLN for the topical treatment of psoriasis. | [65] |
| SLN | Resveratrol, vitamin E, and epigallocatechin gallate |
SLN containing resveratrol, vitamin E, and epigallocatechin gallate for antioxidant benefits. | [66] |
| SLN | Silybin | Silybin-loaded SLN enriched gel for irritant contact dermatitis. | [67] |
| SLN | Fluconazole | Fluconazole-loaded SLN topical gel for the treatment of pityriasis versicolor. | [68] |
| SLN | Tazarotene | Tazarotene-loaded SLN for the treatment of psoriasis. | [69] |
| SLN | Miconazole nitrate | Miconazole nitrate-loaded SLN for antifungal activity. | [70] |
| SLN | Adapalene | Adapalene-loaded SLN for anti-acne therapy. | [71] |
| SLN | Isotretinoin and α-tocopherol |
SLN loaded with retinoic acid and lauric acid for the topical treatment of acne vulgaris. | [72] |
| NLC | Spironolactone | Spironolactone-loaded NLC-based gel for the effective treatment of acne vulgaris. | [73] |
| NLC | Clobetasol propionate | NLC-based topical gel of clobetasol propionate for the treatment of eczema. | [74] |
| NLC | Tacrolimus and tumor necrosis factor α siRNA |
NLC co-delivering tacrolimus and tumor necrosis factor α siRNA for the treatment of psoriasis. | [75] |
| NLC | Itraconazole | Topical NLC containing itraconazole for the treatment of fungal infections. | [76] |
| NLC | Apremilast | NLC for topical delivery of apremilast for the treatment of psoriasis. | [77] |
| NLC | Dithranol | Dithranol-loaded NLC-based gel for the treatment of psoriasis. | [78] |
| NLC | Voriconazole | Voriconazole-loaded NLC for antifungal applications. | [79] |
| NLC | Mometasone furoate | NLC-based hydrogel of mometasone furoate for the treatment of psoriasis. | [80] |
| NLC | Antimicrobial peptide nisin Z |
Antimicrobial peptide nisin Z with conventional antibiotic-loaded NLC to enhance antimicrobial activity. | [81] |
| NLC | Adapalene and vitamin C | Adapalene- and vitamin C-loaded NLC for acne treatment. | [82] |
3. Conclusions
References
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60.
- Ferreira, K.C.B.; Valle, A.B.C.d.S.; Paes, C.Q.; Tavares, G.D.; Pittella, F. Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances. Pharmaceutics 2021, 13, 1454.
- Wolff, K.; Johnson, R.A.; Saavedra, A.P.; Roh, E.K. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2017; pp. 693–759. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2043§ionid=154893575 (accessed on 19 October 2021).
- Urban, K.; Chu, S.; Scheufele, C.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G.R. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int. 2020, 2, 22–27.
- Craddock, L.N.; Schieke, S.M. Superficial fungal infection. In Fitzpatrick’s Dermatology, 9th ed.; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw-Hill: New York, NY, USA, 2019; Chapter 160; pp. 1–48. Available online: https://accessmedicine.mhmedical.com/content.aspx?sectionid=210432218&bookid=2570&Resultclick=2 (accessed on 19 October 2021).
- Silverberg, B. A Structured Approach to Skin and Soft Tissue Infections (SSTIs) in an Ambulatory Setting. Clin. Pract. 2021, 11, 65–74.
- Pearson, D.R.; Margolis, D.J. Cellulitis and erysipelas. In Fitzpatrick’s Dermatology, 9th ed.; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw-Hill: New York, NY, USA, 2019; Chapter 151; pp. 1–19. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2570§ionid=210413306 (accessed on 19 October 2021).
- Sartelli, M.; Guirao, X.; Hardcastle, T.; Kluger, Y.; Boermeester, M.A.; Rasa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft tissue infections. World J. Emerg. Surg. 2018, 13, 58.
- Falcone, M.; Concia, E.; Giusti, M.; Mazzone, A.; Santini, C.; Stefani, S.; Violi, F. Acute bacterial skin and skin structure infections in internal medicine wards: Old and new drugs. Inter. Emerg. Med. 2016, 11, 637–648.
- Khadr, L.; Harfouche, M.; Omori, R.; Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J. The Epidemiology of Herpes Simplex Virus Type 1 in Asia: Systematic Review, Meta-analyses, and Meta-regressions. Clin. Infect. Dis. 2019, 68, 757–772.
- Poole, C.L.; Kimberlin, D.W. Antiviral Approaches for the Treatment of Herpes Simplex Virus Infections in Newborn Infants. Annu. Rev. Virol. 2018, 5, 407–425.
- Mustafa, M.; Illzam, E.M.; Muniandy, R.K.; Sharifah, A.M.; Nang, M.K.; Ramesh, B. Herpes simplex virus infections, Pathophysiology and Management. IOSR J. Dent. Med. Sci. 2016, 15, 85–91.
- Schwingen, J.; Kaplan, M.; Kurschus, F.C. Review-Current Concepts in Inflammatory Skin Diseases Evolved by Transcriptome Analysis: In-Depth Analysis of Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2020, 21, 699.
- Ho, A.W.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502.
- Golan, Y. Current Treatment Options for Acute Skin and Skin-structure Infections. Clin. Infect. Dis. 2019, 68, 206–212.
- Aktas, E.; Esin, M.N. Skin disease symptoms and related risk factors among young workers in high-risk jobs. Contact Dermat. 2016, 75, 96–105.
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Khan, M.M.; Parveen, F.; Torchilin, V. Lipid-Based Drug Delivery Systems in Regenerative Medicine. Materials 2021, 14, 5371.
- LePree, J.M. Lipid based delivery—Are lipid-based drug delivery systems in your formulation toolbox? Drug Dev. Deliv. 2017, 17, 20–25. Available online: https://drug-dev.com/lipid-based-delivery-are-lipid-based-drug-delivery-systems-in-your-formulation-toolbox/ (accessed on 19 October 2021).
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018, 25, 606–635.
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug. Deliv. 2017, 14, 613–633.
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855.
- Chaurasiya, P.; Ganju, E.; Upmanyu, N.; Ray, S.K.; Jain, P. Transfersomes: A novel technique for transdermal drug delivery. J. Drug Deliv. Ther. 2019, 9, 279–285.
- Nainwal, N.; Jawla, S.; Singh, R.; Saharan, V.A. Transdermal applications of ethosomes—A detailed review. J. Liposome Res. 2019, 29, 103–113.
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019, 25, 2338–2348.
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194.
- Liu, M.; Wen, J.; Sharma, M. Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status. Curr. Pharm. Des. 2020, 26, 3203–3217.
- Geng, Q.; Zhao, Y.; Wang, L.; Xu, L.; Chen, X.; Han, J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. AAPS Pharm. Sci. Tech. 2020, 21, 318.
- Araujo, V.H.S.; Delello Di Filippo, L.; Duarte, J.L.; Spósito, L.; de Camargo, B.A.F.; da Silva, P.B.; Chorilli, M. Exploiting solid lipid nanoparticles and nanostructured lipid carriers for drug delivery against cutaneous fungal infections. Crit. Rev. Microbiol. 2020, 47, 79–90.
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomedicine 2017, 13, 2251–2262.
- Esposito, E.; Sguizzato, M.; Bories, C.; Nastruzzi, C.; Cortesi, R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers 2018, 10, 160.
- Waghule, T.; Gorantla, S.; Rapalli, V.K.; Shah, P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Emerging Trends in Topical Delivery of Curcumin Through Lipid Nanocarriers: Effectiveness in Skin Disorders. AAPS Pharm. Sci. Tech. 2020, 21, 284.
- Silva, A.C.; Amaral, M.H.; Lobo, J.M.; Lopes, C.M. Lipid nanoparticles for the delivery of biopharmaceuticals. Curr. Pharm. Biotechnol. 2015, 16, 291–302.
- Chella, N.; Shastri, N.R. Lipid Carriers: Role and Applications in Nano Drug Delivery. In Particulate Technology for Delivery of Therapeutics; Jana, S., Jana, S., Eds.; Springer: Singapore, 2017; pp. 253–289.
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016, 7, 639–647.
- Xing, H.; Wang, H.; Wu, B.; Zhang, X. Lipid nanoparticles for the delivery of active natural medicines. Curr. Pharm. Des. 2017, 23, 6705–6713.
- De Souza, M.L.; Dos Santos, W.M.; de Sousa, A.L.M.D.; de Albuquerque Wanderley Sales, V.; Nóbrega, F.P.; de Oliveira, M.V.G.; Rolim-Neto, P.J. Lipid Nanoparticles as a Skin Wound Healing Drug Delivery System: Discoveries and Advances. Curr. Pharm. Des. 2020, 26, 4536–4550.
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313.
- Gratieri, T.; Krawczyk-Santos, A.P.; da Rocha, P.B.; Gelfuso, G.M.; Marreto, R.N.; Taveira, S.F. SLN- and NLC-Encapsulating Antifungal Agents: Skin Drug Delivery and their Unexplored Potential for Treating Onychomycosis. Curr. Pharm. Des. 2017, 23, 6684–6695.
- Pham, D.T.T.; Tran, P.H.L.; Tran, T.T.D. Development of solid dispersion lipid nanoparticles for improving skin delivery. Saudi Pharm. J. 2019, 27, 1019–1024.
- Waghule, T.; Rapalli, V.K.; Gorantla, S.; Saha, R.N.; Dubey, S.K.; Puri, A.; Singhvi, G. Nanostructured Lipid Carriers as Potential Drug Delivery Systems for Skin Disorders. Curr. Pharm. Des. 2020, 26, 4569–4579.
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166.
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308.
- Rajpoot, K. Solid Lipid Nanoparticles: A Promising Nanomaterial in Drug Delivery. Curr. Pharm. Des. 2019, 25, 3943–3959.
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56.
- Trivino, A.; Gumireddy, A.; Chauhan, H. Drug-Lipid-Surfactant Miscibility for the Development of Solid Lipid Nanoparticles. AAPS Pharm. Sci. Tech. 2019, 20, 46.
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Sahafi, S.M. Formulation and characterization of novel nanostructured lipid carriers made from beeswax, propolis wax and pomegranate seed oil. Food Chem. 2018, 244, 83–92.
- Sebaaly, C.; Charcosset, C.; Stainmesse, S.; Fessi, H.; Greige-Gerges, H. Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous and lyophilized states: From laboratory to large scale using a membrane contactor. Carbohydr. Polym. 2016, 138, 75–85.
- Shah, M.K.; Khatri, P.; Vora, N.; Patel, N.K.; Jain, S.; Lin, S. Lipid nanocarriers: Preparation, characterization and absorption mechanism and applications to improve oral bioavailability of poorly water-soluble drugs. In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 117–147.
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781.
- Chaudhary, S.A.; Patel, D.M.; Patel, J.K.; Patel, D.H. Solvent Emulsification Evaporation and Solvent Emulsification Diffusion Techniques for Nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer: Cham, Switzerland, 2021; pp. 287–300.
- Singh, D.; Tiwary, A.K.; Bedi, N. Self-microemulsifying Drug Delivery System for Problematic Molecules: An Update. Recent Pat. Nanotech. 2019, 13, 92–113.
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259.
- Jensen, L.B.; Petersson, K.; Nielsen, H.M. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J. Pharm. Biopharm. 2011, 79, 68–75.
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formula-tions for topical administration. Int. J. Pharm. 2015, 478, 726–735.
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl. Res. 2017, 7, 37–52.
- Montenegro, L.; Messina, C.M.; Manuguerra, S.; Santagati, L.M.; Pasquinucci, L.; Turnaturi, R.; Parenti, C.; Arena, R.; Santulli, A. In Vitro Antioxidant Activity and In Vivo Topical Efficacy of Lipid Nanoparticles Co-Loading Idebenone and Tocopheryl Acetate. Appl. Sci. 2019, 9, 845.
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290.
- Gad, H.A.; El-Rahman, F.A.A.; Hamdy, G.M. Chamomile oil loaded solid lipid nanoparticles: A naturally formulated remedy to enhance the wound healing. J. Drug Deliv. Sci. Technol. 2019, 50, 329–338.
- Butani, D.; Yewale, C.; Misra, A. Topical Amphotericin B solid lipid nanoparticles: Design and development. Colloids Surf. B Biointerfaces 2016, 139, 17–24.
- Carbone, C.; do Céu Teixeira, M.; do Céu Sousa, M.; Martins-Gomes, C.; Silva, A.M.; Souto, E.M.B.; Musumeci, T. Clotrima- zole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics 2019, 11, 231.
- Ghate, V.M.; Lewis, S.A.; Prabhu, P.; Dubey, A.; Patel, N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016, 108, 253–261.
- Malik, D.S.; Kaur, G. Exploring therapeutic potential of azelaic acid loaded NLCs for the treatment of acne vulgaris. J. Drug Deliv. Sci. Technol. 2020, 55, 101418.
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016, 33, 372–380.
- Harde, H.; Agrawal, A.K.; Katariya, M.; Kale, D.; Jain, S. Development of a topical adapalene-solid lipid nanoparticle load-ed gel with enhanced efficacy and improved skin tolerability. RSC Adv. 2015, 5, 43917–43929.
- Pradhan, M.; Singh, D.; Singh, M.R. Influence of selected variables on fabrication of Triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif. Cells Nanomed. Biotechnol. 2016, 44, 392–400.
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Car-riers. AAPS Pharm. Sci. Tech. 2017, 18, 509–516.
- Shrotriya, S.N.; Vidhate, B.V.; Shukla, M.S. Formulation and development of Silybin loaded solid lipid nanoparticle enriched gel for irritant contact dermatitis. J. Drug Deliv. Sci. Technol. 2017, 41, 164–173.
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Flucona-zole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2018, 25, 78–90.
- Aland, R.; Ganesan, M.; Rajeswara, R.P. Development and optimization of tazarotene loaded solid lipid nanoparticles for topical delivery. Asian J. Pharm. Clin. Res. 2019, 12, 63–77.
- Al-Maghrabi, P.M.; Khafagy, E.-S.; Ghorab, M.M.; Gad, S. Influence of formulation variables on miconazole nitrate-loaded lipid based nanocarrier for topical delivery. Colloids Surf. B Biointerfaces 2020, 193, 111046.
- Bhalekar, M.; Upadhaya, P.; Madgulkar, A. Formulation and evaluation of Adapalene-loaded nanoparticulates for epidermal localization. Drug Deliv. Transl. Res. 2015, 5, 585–595.
- Gupta, S.; Wairkar, S.; Bhatt, L.K. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J. Microencapsul. 2020, 37, 1–9.
- Kelidari, H.R.; Saeedi, M.; Hajheydari, Z.; Akbari, J.; Morteza-Semnani, K.; Akhtari, J.; Valizadeh, H.; Asare-Addo, K.; Nokhodchi, A. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate ac-ne vulgaris: A randomized, double-blind, prospective trial. Colloids Surf. B Biointerfaces 2016, 146, 47–53.
- Nagaich, U.; Gulati, N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: Design and in vivo characterization. Drug Deliv. Transl. Res. 2016, 6, 289–298.
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Ciampo, J.O.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660.
- Passos, J.S.; De Martino, L.C.; Dartora, V.F.C.; De Araujo, G.L.B.; Ishida, K.; Lopes, L.B. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur. J. Pharm. Sci. 2020, 149, 105296.
- Madan, J.R.; Khobaragade, S.; Dua, K.; Awasthi, R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol. Ther. 2020, 33, e13370.
- Sathe, P.; Saka, R.; Kommineni, N.; Raza, K.; Khan, W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev. Ind. Pharm. 2019, 45, 826–838.
- Waghule, T.; Rapalli, V.K.; Singhvi, G.; Manchanda, P.; Hans, N.; Dubey, S.K.; Hasnain, M.S.; Nayak, A.K. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 303–315.
- Kaur, N.; Sharma, K.; Bedi, N. Topical Nanostructured Lipid Carrier Based Hydrogel of Mometasone Furoate for the Treatment of Psoriasis. Pharm. Nanotechnol. 2018, 6, 133–143.
- Lewies, A.; Wentzel, J.F.; Jordaan, A.; Bezuidenhout, C.; Du Plessis, L.H. Interactions of the antimicrobial peptide nisin Z with conventional antibiotics and the use of nanostructured lipid carriers to enhance antimicrobial activity. Int. J. Pharm. 2017, 526, 244–253.
- Jain, A.; Garg, N.K.; Jain, A.; Kesharwani, P.; Jain, A.K.; Nirbhavane, P.; Tyagi, R.K. A synergistic approach of adapalene-loaded nanostructured lipid carriers, and vitamin C co-administration for treating acne. Drug Dev. Ind. Pharm. 2016, 42, 897–905.




