Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sarana Rose Sommano | + 1776 word(s) | 1776 | 2021-11-15 04:29:39 | | | |
| 2 | Beatrix Zheng | + 261 word(s) | 2037 | 2021-11-16 03:50:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sommano, S. Mango Peel Pectin: Recovery, Functionality and Sustainable Uses. Encyclopedia. Available online: https://encyclopedia.pub/entry/16006 (accessed on 07 February 2026).
Sommano S. Mango Peel Pectin: Recovery, Functionality and Sustainable Uses. Encyclopedia. Available at: https://encyclopedia.pub/entry/16006. Accessed February 07, 2026.
Sommano, Sarana. "Mango Peel Pectin: Recovery, Functionality and Sustainable Uses" Encyclopedia, https://encyclopedia.pub/entry/16006 (accessed February 07, 2026).
Sommano, S. (2021, November 15). Mango Peel Pectin: Recovery, Functionality and Sustainable Uses. In Encyclopedia. https://encyclopedia.pub/entry/16006
Sommano, Sarana. "Mango Peel Pectin: Recovery, Functionality and Sustainable Uses." Encyclopedia. Web. 15 November, 2021.
Copy Citation
Mango peel is the byproduct of agro-processing and has been used for high value-added components such as polysaccharide biopolymers. Pectin derived from the peel is yet to be exploited to its greatest extent, particularly in terms of its separation and physiochemical properties, which limit its applicability to dietary fiber in culinary applications. The functionality of the mango peel pectin (MPP) strongly depends on the molecular size and degree of esterification which highlight the importance of isolation and characterisation of pectin from this novel resource.
extraction technique
fruit characteristic
mango peel biorefinery
pectic polysaccharide
pectin source
1. Mango Peel Pectin Recovery
General pectin recovery includes a raw material pre-treatment stage, an extraction operation and a post-extraction stage [1][2]. Nevertheless, the issue on the conventional process, particularly the extraction step, is whether or not it is worth the energy and economic demands that are currently required in the practice [3]. Therefore, several sustainable and quicker alternative approaches to extract pectin from biological materials have been developed. The innovative techniques for pectin extraction include enzyme-assisted extraction, ultrasounds, subcritical fluids and microwave heating. The benefits and drawbacks of the techniques are compared as shown in Table 1.
Table 1. Benefits and drawbacks of the novel techniques.
| Extraction Techniques | Benefits | Drawbacks |
|---|---|---|
| MAE |
|
|
| EAE |
|
|
| UAE |
|
|
| SWE |
|
|
1.1. Conventional Heating Extraction (CHE)
Pectin is traditionally extracted in water acidified with 0.05–2 M sulfuric, nitric, phosphoric, acetic or hydrochloric acid between 80–100 °C for 1 h with continuous stirring [16]. Conventional extraction (solid–liquid extraction) depends on a number of factors such as temperature, pH, solvent properties, solid to solvent ratio, dry solids, particle size and diffusion rate [17]. For pectin extraction, mango peel powder was initially treated with the acidified solution. Subsequently, the obtained solvent was treated with ethanol solution [18]. Through this method, an MPP yield as high as 30% can be achieved from the residue with the degree of esterification (DE) varying from approximately 60 to 90% [19][18].
1.2. Novel Extraction Techniques
-
Microwave-Assisted Extraction (MAE)
MAE involves dielectric heating of plant molecules through the exposure of microwaves. The microwave irradiation accelerates cell rupture by a sudden temperature rise and internal pressure increase inside the cells of plant sample, which promotes the destruction of sample surface and in turns the exudation of pectin within the plant cells into the surrounding solvents and increase [20][21][22]. The conventional “on-off” microwave operation, however, may lead to the overheating of the raw material, which may ultimately result in a low quality of MPP. Consequently, a phase controller (PCMAE), which regulates the electrical power input into the magnetron thereby generating smooth and adjustable microwave power was installed additionally for a better extraction performance [19]. The applications of the MAE for pectin extraction from mango peel were reported and the obtained pectin had higher content when compared with the CHE [19][23]. The microwave provides more efficient heat than the CHE approach due to the intense formation of vapour in polar substances generated by the electromagnetic field [24].
-
Enzyme-Assisted Extraction (EAE)
The enzymes are used to improve extraction process by hydrolyzing matrix of the plant cell wall. Cell wall degrading enzymes with minimum pectinolytic activity are used to hydrolyze non-pectin plant cell wall components in enzymatic extraction of pectin [25][10]. The EAE depends on reaction time, type and concentration of enzyme, temperature, pH value and particle size of plant material [26][27]. The EAE technique was applied to recover pectin from multiple bioresources such as lime [28], passion fruit [29] and apple pomace [30]. The yields of pectin were achieved with the enzymatic extraction which were greater than that obtained with the CHE method. However, the pectin extraction from mango peel using this technology has not yet been implemented.
-
Ultrasound-Assisted Extraction (UAE)
Sound waves consist of mechanical vibrations, which can be applied in treatments to the solid, liquid or gas with frequencies higher than 20 kHz [27][31]. Adapted for pectin extraction, the collapse of cavitation bubbles near cell walls induced by ultrasound produces cell disruption, thus causing stronger and enhanced solvent entrance into the cells and intensification of the mass transfer [32][33]. For pectin recovery, Guandalini et al. [34] found that the UAE provided an alternative choice for pectin extraction from mango peel because through this technique an MPP yield as high as 50% can be achieved without interfering the physicochemical properties (galacturonic acid content and degree of esterification).
-
Subcritical-Assisted Extraction (SWE)
Subcritical water is liquid water at elevated pressure which is able to attain temperatures higher than its normal boiling point without a change in phase. When such water is used as solvent in extraction, the process is known as subcritical water extraction (SWE) also known as pressurized hot water extraction (PHWE) and superheated water extraction (SHWE) [13]. The SWE is stated as a green route for the valorisation of mango peel in form of pectin product. Xiaa and Matharu [35] reported that the MPP extracted by the SWE with no mineral acid supplementation resulted in a great yield of 18.34%, while the DE of the pectin was more than 70%.
2. MPP Functionality
Pectin is mostly extracted from various plant sources and is of great variation in term of quality. Consequently, pectin is purified and restructured in order to achieve constant and reproducible gel strength, for example HMP is improved its quality by dilution with sucrose. MPP is typical of high methoxyl content which is unable to form gel by interaction with calcium ions due to an insufficient number of carboxylic groups [36][37]. Thus, to improve its functionality for a specific purpose, de-esterification using either acidic or basic chemicals is necessary. The characteristic compositions of the extracted MPP are illustrated in Table 2.
Table 2. Typical characteristics of mango peel pectin compared with commercial pectin.
| Characteristics | Commercial Pectin [38] |
Mango Peel Pectin | ||
|---|---|---|---|---|
| CHE [34] | UAE [39] |
MAE [40] |
||
| Galacturonic acid (%) | >65 (typically 75–80) | 76 | 52–53 | n/a |
| Degree of esterification (%) | 30–75 | 61 | 56–93 | 57–93 |
| Degree of acethylation | <5 (except for e.g., sugar beet pectin) | n/a | n/a | n/a |
| Neutral sugars (%) | <15% | n/a | n/a | n/a |
| Protein (N × 6.25) (%) | <5% | n/a | 4.7–5.9 | n/a |
| Molecular weight (g mol−1) | 100,000–200,000 | n/a | 378,400–2,858,000 | n/a |
n/a = not available; CHE = conventional heating extraction; UAE = ultrasound-assisted extraction; MAE = microwave-assisted extraction.
The residues of galacturonic acid (GA) (Figure 1a) are generally recognised as the backbone of the pectin structure. Its chemical structure composes of an aldehyde group at C1 and a carboxylic acid group at C6 [41]. The GA can be partially methyl-esterified at C6 with methanol and acetylated at the O2 or O3 positions with acetic acid (Figure 1b,c) [42]. The GA content can be determined by either the colorimetry [34] or high performance liquid chromatography [43]. The ratio of methyl-esterified galacturonic acid groups to the total galacturonic acid groups is defined as the degree of esterification (DE) [44][45][46]. The degree of esterification and acetylation of pectin affects the gelling properties of the pectin; a higher DE increases the capacity to form gels, whereas a higher degree of acetylation inhibits gelling [47]. The analytical quantification of DE include the titrimetric technique [34][48], gas liquid chromatography and colorimetric uronic acid analyses [49]. Furthermore, the content of GA in foods is very important because their presence can affect the chemical and sensorial characteristics of the matrix such as pH, total acidity, microbial stability, sweetness, consumer acceptability and therefore, provide precious information on the wholesome quality of the food or on the optimisation needed to impart select technical features [50]. Meanwhile, the molecular weight of pectin depends on the raw materials and the extraction techniques. Bagherian et al. [4] reported that continued heating of pectin extraction may lead to pectin networks disaggregation, thus decreasing the molecular weight.
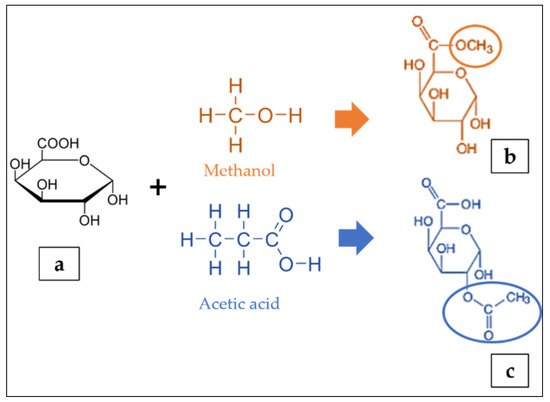
Figure 1. Structure of galacturonic acid (a) presenting methyl esterified (b) and acetylated (c) forms adapted from [42].
In case of pectin recovered from mango peel, the GalA contents varied depending on the extraction techniques. Process optimization of extraction methods to obtain the minimal GalA level of 65% in MPP has been highlighted in many research studies [39][51][52]. Geerkens et al. [53] claimed that the preparation processes of the peel (blanching, particle size reduction) and fruit ripening stage reduced the GalA content, however the highest content obtained was 48%. Regarding the DE content, the values were in a range between 56% and 93%, which categorized it as high methoxyl pectin [54]. Both GalA and DE of pectic polysaccharides are involved in the commercial uses of pectin as gelling and thickening agents [55][56].
3. MPP Applications
Pectins are widely used as additive in foods and beverages such as a gelling agent, thickener, texturiser, emulsifier and stabiliser [57]. In recent years, pectin has been applied as a fat or sugar alternative in low-calorie foods [23], dietetic food [58], food packaging [59] and drug carrier [37]. Selection of pectin for a particular food depends on many factors, including the texture required, pH, processing temperature, presence of ions, proteins and the expected shelf life of the product [60]. MPP was recovered from peel of ‘Nam dok mai’ variety (Mox > 8%) and was found suitable as fat replacement in a Chinese sausage formular in its original form and colour [23]. Additionally, MPP obtained from ‘Chok anan’ variety was utilised as a substrate for pectic oligosaccharide hydrolysate with pectinase. The digested monosaccharide compositions were mainly fructose and glucose while arabinose had prominent influence on prebiotic potentials of Bifidobacterium animalis [61]. Thin films have been used as food packaging polymer and many drug delivery systems of oral, buccal, and transdermal routes. In one study, thin film was fabricated from a mixture of LMP and MPP at 1:2 ratio with 40% (w/w) glycerol. The film attained the highest elongation at break (8.80%) and lowest Young’s modulus (83.19 MPa) with an increasing hydrophobicity when the content of MPP increased [36]. For a topical drug delivery, de-esterified MPP with NaOH was proposed for thin film development [37]. In this same study, the DE decreased when a higher volume (~3.0 mL) of 1 N NaOH at 25 °C was employed in the preparation.
Wongkaew et al. [40] explained the industrial value chain process of MPP as illustrated in Figure 2. First, the biomass was dried and pectin extraction can be achieved with MAE techniques. The dried peel powder was suspended in diluted acidic solution (distilled H2O adjusted to pH 1.5 with 2 M HCl) and heated in a microwave oven followed by separating the residue from the solution using filtration technique. The liquid is combined with a 1:1 ethanol-water mixture to precipitate the pectin, and then it is separated by filtration. The pectin was dried at 40 °C until a consistent weight was attained. The final product can be applied to food additives or sources of prebiotic or in pharmaceutical application.
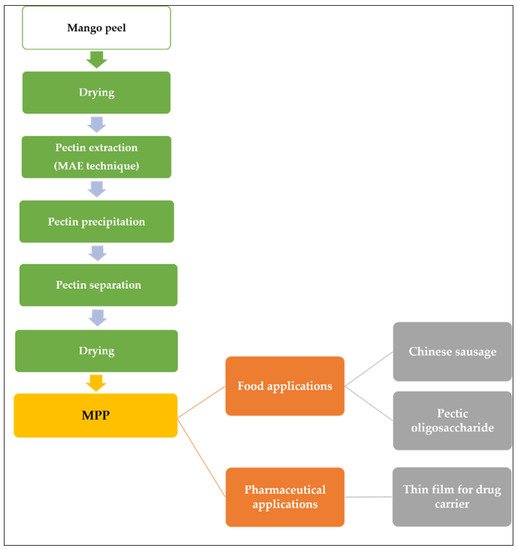
Figure 2. MPP value chain and applications.
4. Future Direction of MPP Utilisation
Plant polysaccharides are vital for the modulation of human gut microbiota which can impact on health generally recognised as prebiotics [62]. Among the most common prebiotic candidates, pectin oligosaccharide (POS) is receiving attention in the functional food industry [63]. MPP can possibly be hydrolysed into small molecules of pectic oligosaccharide or MPOS, as shown in Figure 3 [64]. The MPOS obtained highly stimulated the probiotic growth as well as the total short-chain fatty acids (SCFAs) production of Bifidobacterium animalis TISTR 2195 and Lactobacillus reuteri DSM 17938. It is also confirmed in our previous study that the MPOS illustrates a high potential as a prebiotic property [61]. The subsequently obtained SCFAs provide a great variety of health effects, including inhibition of pathogenic bacteria, constipation relief, reduction in blood glucose levels, improvement in mineral absorption, reduction of colonic cancer and modulation of the immune system [65].
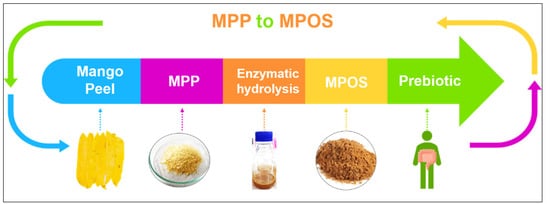
Figure 3. Future direction of MPP utilisation to MPOS production.
References
- Gentilini, R.; Bozzini, S.; Munarin, F.; Petrini, P.; Visai, L.; Tanzi, M.C. Pectins from Aloe Vera: Extraction and production of gels for regenerative medicine. J. Appl. Polym. Sci. 2014, 131, 1–9.
- Peng, K.; Zhang, Y.; Wang, S.; Liao, X.; Hu, X. Effect of microwave drying pretreatment on extraction of pectin from apple pomace. Int. Food Res. J. 2008, 24, 222–226.
- Casas-Orozco, D.; Villa, A.L.; Bustamante, F.; Gonzalez-Rodriguez, L.-M. Process development and simulation of pectin extraction from orange peels. Food Bioprod. Process. 2015, 96, 86–98.
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process. Intensif. 2011, 50, 1237–1243.
- Fishman, M.L.; Chau, H.K.; Hoagland, P.; Ayyad, K. Characterization of pectin, flash-extracted from orange albedo by microwave heating, under pressure. Carbohydr. Res. 1999, 323, 126–138.
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250.
- Ptichkina, N.; Markina, O.; Rumyantseva, G. Pectin extraction from pumpkin with the aid of microbial enzymes. Food Hydrocoll. 2008, 22, 192–195.
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161.
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocoll. 2015, 44, 156–161.
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44.
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436.
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312.
- Zakaria, S.M.; Kamal, S.M.M. Subcritical water extraction of bioactive compounds from plants and algae: Applications in pharmaceutical and food ingredients. Food Eng. Rev. 2016, 8, 23–34.
- Ueno, H.; Tanaka, M.; Hosino, M.; Sasaki, M.; Goto, M. Extraction of valuable compounds from the flavedo of Citrus junos using subcritical water. Sep. Purif. Technol. 2008, 62, 513–516.
- Khajavi, S.H.; Kimura, Y.; Oomori, T.; Matsuno, R.; Adachi, S. Degradation kinetics of monosaccharides in subcritical water. J. Food Eng. 2005, 68, 309–313.
- Georgiev, Y.; Ognyanov, M.; Yanakieva, I.; Kussovski, V.; Kratchanova, M. Isolation, characterization and modification of citrus pectins. J. Biosci. Biotechnol. 2013, 2012, 223–233.
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37.
- Oliveira, A.D.N.; Paula, D.D.A.; de Oliveira, E.B.; Saraiva, S.H.; Stringheta, P.C.; Ramos, A.M. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int. J. Biol. Macromol. 2018, 113, 395–402.
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S.; Page, P.; Colelli, G. Characterisation and physiochemical properties of mango peel pectin extracted by conventional and phase control microwave-assisted extractions. Int. Food Res. J. 2018, 25, 2657–2665.
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013, 97, 703–709.
- Maran, J.P.; Prakash, K.A. Process variables influence on microwave assisted extraction of pectin from waste Carcia papaya L. peel. Int. J. Biol. Macromol. 2015, 73, 202–206.
- Pandit, S.G.; Vijayanand, P.; Kulkarni, S. Pectic principles of mango peel from mango processing waste as influenced by microwave energy. LWT 2015, 64, 1010–1014.
- Wongkaew, M.; Sommano, S.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango peel pectin by microwave-assisted extraction and its use as fat replacement in dried Chinese sausage. Foods 2020, 9, 450.
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185.
- Fissore, E.N.; Ponce, N.M.; Wider, E.A.; Stortz, C.A.; Gerschenson, L.N.; Rojas, A.M. Commercial cell wall hydrolytic enzymes for producing pectin-enriched products from butternut (Cucurbita moschata, Duchesne ex Poiret). J. Food Eng. 2009, 93, 293–301.
- Poojary, M.M.; Orlien, V.; Passamonti, P.; Olsen, K. Enzyme-assisted extraction enhancing the umami taste amino acids recovery from several cultivated mushrooms. Food Chem. 2017, 234, 236–244.
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N. Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Eng. Rev. 2016, 8, 214–234.
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.; Meyer, A.S.; Mikkelsen, J.D. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocoll. 2014, 40, 273–282.
- Liew, S.; Chin, N.; Yusof, Y.; Sowndhararajan, K. Comparison of acidic and enzymatic pectin extraction from passion fruit peels and its gel properties. J. Food Process. Eng. 2016, 39, 501–511.
- Dranca, F.; Oroian, M. Optimization of pectin enzymatic extraction from malus domestica ‘fălticeni’ apple pomace with Celluclast 1.5L. Molecules 2019, 24, 2158.
- Zinoviadou, K.G.; Galanakis, C.; Brnčić, M.; Grimi, N.; Boussetta, N.; Mota, M.; Saraiva, J.A.; Patras, A.; Tiwari, B.K.; Barba, F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015, 77, 743–752.
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess Technol. 2015, 8, 1139–1148.
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47.
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461.
- Xia, H.; Matharu, A.S. Unavoidable food supply chain waste: Acid-free pectin extraction from mango peel via subcritical water. Faraday Discuss. 2017, 202, 31–42.
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.-H.; Chambin, O.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611.
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of clindamycin hydrochloride loaded de-esterified low-methoxyl mango peel pectin film used as a topical drug delivery system. Polymers 2020, 12, 1006.
- Thibault, J.F.; Ralet, M.-C. Physico-Chemical Properties of Pectins in the Cell Walls and After Extraction; Springer: Berlin/Heidelberg, Germany, 2003; pp. 91–105.
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803.
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R. Fruit characteristics, peel nutritional compositions, and their relationships with mango peel pectin quality. Plants 2021, 10, 1148.
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277.
- Melton, L.D.; Smith, B.G. Determining the degree of methylation and acetylation of pectin. Curr. Protoc. Food Anal. Chem. 2001, 1, E3.4.1–E3.4.6.
- Moreira, M.M.; Guido, L.F.; Cruz, J.M.; Barros, A.A. Determination of galacturonic acid content in pectin from fruit juices by liquid chromatographydiode array detection-electrospray ionization tandem mass spectrometry. Open Chem. 2010, 8, 1236–1243.
- Gnanasambandam, R.; Proctor, A. Preparation of soy hull pectin. Food Chem. 1999, 65, 461–467.
- Salminen, H.; Weiss, J. Effect of pectin type on association and ph stability of whey protein—Pectin complexes. Food Biophys. 2013, 9, 29–38.
- Yavuz-Düzgün, M.; Zeeb, B.; Dreher, J.; Özçelik, B.; Weiss, J. The impact of esterification degree and source of pectins on complex coacervation as a tool to mask the bitterness of potato protein isolates. Food Biophys. 2020, 15, 376–385.
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.G.; Berger, A.P.A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599.
- Pinheiro, E.s.R.; Silva, I.M.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.; Amboni, R.D. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566.
- Maness, N.O.; Ryan, J.D.; Mort, A.J. Determination of the degree of methyl esterification of pectins in small samples by selective reduction of esterified galacturonic acid to galactose. Anal. Biochem. 1990, 185, 346–352.
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–130.
- Kermani, Z.J.; Shpigelman, A.; Pham, T.T.H.; Van Loey, A.; Hendrickx, M.E. Functional properties of citric acid extracted mango peel pectin as related to its chemical structure. Food Hydrocoll. 2015, 44, 424–434.
- Nagel, A.; Neidhart, S.; Anders, T.; Elstner, P.; Korhummel, S.; Sulzer, T.; Wulfkühler, S.; Winkler, C.; Qadri, S.; Rentschler, C. Improved processes for the conversion of mango peel into storable starting material for the recovery of functional co-products. Ind. Crop. Prod. 2014, 61, 92–105.
- Geerkens, C.H.; Nagel, A.; Just, K.M.; Miller-Rostek, P.; Kammerer, D.R.; Schweiggert, R.M.; Carle, R. Mango pectin quality as influenced by cultivar, ripeness, peel particle size, blanching, drying, and irradiation. Food Hydrocoll. 2015, 51, 241–251.
- Sharma, B.R.; Dhuldhoya, N.; Merchant, S.U.; Merchant, U. An overview on pectins. Times Food Proc. J. 2006, 23, 44–51.
- Koubala, B.; Kansci, G.; Mbome, L.; Crépeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Améliorée” and “Mango” mango peels. Food Hydrocoll. 2008, 22, 1345–1351.
- Willats, W.G.; Knox, P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104.
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73.
- Ciurzyńska, A.; Szerszeń, J.; Lenart, A. Pectin—A functional component of diet. Int. J. Res. Stud. Sci. Eng. Technol. 2016, 3, 20–27.
- Zhang, Y.; Rempel, C.; McLaren, D. Chapter 12-Edible coating and film materials: Carbohydrates. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2013; pp. 305–323.
- Speiser, R.; Copley, M.J.; Nutting, G.C. Effect of molecular association and charge distribution on the gelation of pectin. J. Phys. Chem. 1947, 51, 117–133.
- Wongkaew, M.; Tinpovong, B.; Sringarm, K.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Hanmoungjai, P.; Sommano, S. Crude pectic oligosaccharide recovery from Thai Chok Anan mango peel using pectinolytic enzyme hydrolysis. Foods 2021, 10, 627.
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota-introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412.
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 244, 232–237.
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127.
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




