| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leonardo Mancini | + 3291 word(s) | 3291 | 2020-08-05 08:18:52 | | | |
| 2 | Leonardo Mancini | -434 word(s) | 2857 | 2020-08-12 12:47:57 | | | | |
| 3 | Peter Tang | -881 word(s) | 1976 | 2020-08-13 07:53:34 | | | | |
| 4 | Peter Tang | Meta information modification | 1976 | 2020-10-29 06:48:20 | | |
Video Upload Options
Biomaterials derive either from nature or synthesized in the laboratory using chemical approaches utilizing metallic components, polymers, ceramics, or composite materials. They are often used and adapted for medical applications. A biomaterial is also defined as autograft, allograft, or xenograft used as a transplant material. In the last decade also tissue engineering and stem cells were deeply studied trying to make better autologous biomaterials ready to be used in regenerative procedures. Micrografts are a new concept of biomaterial, they are enriched of progenitor cells that are a particular type of stem cell excellent in enhancing the regenerative potential. Moreover, another advantage is the easy handling and tissue availability.
1. Definition
Micrografts are tissue particles enriched with progenitor cells (PCs), which are defined as descendants of stem cells that can differentiate into specialized cells belonging to the same tissue. Rigenera® is a new type of autologous graft (micrograft) that involves a chair-side mechanical disaggregation device and utilizes PCs derived from various sites such as the periosteum or dental pulp.
2. Introduction or History
The use of grafting materials in periodontology, implantology, and oral surgery has become very common over the last two decades. New products are brought to market every year with various protocols and uses [1]. Autologous biomaterials still remain the gold standard in oral regeneration due to their osteoinductive, osteoconductive, and osteogenic properties [2]. The use of these materials is limited owing to rapid resorption, collection of inadequate amounts of tissue, high biological cost, and donor site morbidity [3][4]. During recent years, different types of allografts and xenografts have also been proposed due to their biocompatibility and their potential as scaffolds for tissue regeneration [5]. These bone substitutes are limited by the fact that they are not formed from osteogenic cells and osteoinductive molecules, which are important for better tissue regeneration [6][7]. In the literature, several studies have proposed the use of mesenchymal stem cells (MSCs) or progenitor cells (PCs) isolated from various tissues and combined with various biomaterials in oral regeneration [8][9][10]. Specifically, PCs are defined as descendants of stem cells that are able to differentiate into specialized cells belonging to the same tissue. The process from isolation through culture is still at a crucial point. The most commonly used protocol for isolation of cells is the enzymatic process which utilizes the application of different chemical solutions to extract stem cells and requires an incubator and cell storage. Obviously, this process is not suitable in clinical practice where time and handling are crucial for clinical outcomes . A recent protocol proposed by Trovato et al. and later by Monti et al. [12][13], using the Rigenera® micrografting technology, suggests the use of a new type of autologous graft (micrograft) that involves a chair-side mechanical disaggregation device and utilizes PCs derived from various sites such as the periosteum or dental pulp. Such cells can be obtained from a tissue fragment a few millimeters in length that is harvested directly during the intervention phase, even from the same surgical site, and can be used without any manipulation or cell culture. A micrograft might be compared to an autologous graft due to the presence of MSCs that are able to enhance the healing process and regenerate the damaged tissue [14][15]. Considering the limitations of autologous biomaterials, such as their availability and quantity, and furthermore considering the osteoconductivity of xenografts, the use of micrografts leads to a greater regenerative potential.
3.Data, Model, Applications and Influences
3.1. Search Methods
A search was done on various databases such as PubMed and Scopus, and a manual search was done on relevant journals with the following words: micrografts AND Rigenera AND regeneration. Randomized clinical trials, retrospective studies, case series, and case reports were included in this review. The inclusion criteria were human studies (randomized clinical trials, case series, and case reports) using micrografts during oral regenerative procedures with clinical, histological, and radiographic evaluation. Exclusion criteria were not assessed due to the novel technique and scarce literature regarding this novel protocol.
3.2. Population
Males and females between 25 and 69 years of age were included in these studies. Healthy subjects needing implant rehabilitation of the upper jaw with a sinus lift procedure and periodontal patients with periodontitis stage III (probing depth of ≥ 6 mm) were involved. All patients with systemic diseases were excluded. In each study, patients were subjected to a presurgical phase of oral hygiene, and they were instructed to perform domiciliary oral hygiene in the correct modalities using chlorhexidine associated with a brushing technique until the day of surgery.
3.3. Autologous Micrograft Generation—Rigenera® Micrografting Technology
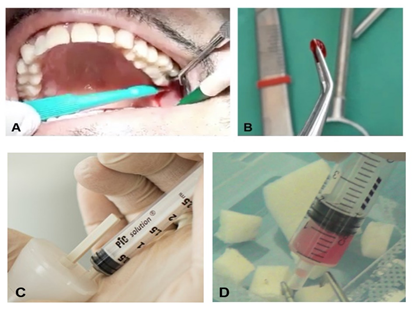
Depending on the type of intervention, tissue samples (1–2 mm up to 10 mm) were collected from the periosteum of an access flap or from the dental pulp of a third molar extracted simultaneously for mobility or malposition. The samples were all disaggregated using a particular chair-side medical device, the Rigeneracons® (Human Brain Wave LLC, Turin, Italy), which mechanically disaggregates the tissue with a particular micro-blade grid and a filter for cells with a diameter of 80 μm. This device leads to the generation of a micrograft suspension that is ready for use and rich in PCs, thanks to the particular 80 μm filter that enables selective collection of this cellular subtype, extracellular matrix fragments, and growth factors, and facilitates and enhances the regenerative potential of the isolated tissue fragments. Briefly, the tissue sample is inserted into the Rigeneracons® in addition to 1 mL of physiological solution. Following that, the sample is disaggregated at a rotation of 75 R/min and 15 N cm, activating the disruption. After 2 min, the micrografts, suspended in 1 mL of physiological solution, are collected with a syringe through a dedicated hole on the upper part of the Rigeneracons®. The micrograft suspension is stored inside the syringe and used to embed a collagen sponge or hydroxyapatite for 10 min in order to form a biocomplex used in periodontal defects, socket preservation, or sinus floor augmentation (Figure 1).
Figure 1. Schematic representation of Rigenera® technology use. (A) Collection of an autologous sample. (B) Average size of the tissue sample. (C) Mechanical disaggregation with the Rigeneracons® leads to the generation of micrograft suspensions collected from the device with a syringe. (D) The biomaterial is generated by soaking a scaffold with the micrografts.
3.4. Surgery and Grafting Procedure
In the three studies [16][17][18] included for the augmentation of the sinus floor, a simple flap and a lateral bone window were used; after membrane elevation, in two studies, a periosteal sample was collected. According to Baena et al. [16], a control group was assessed, and non-sintered porous hydroxyapatite (Alos®, Allmed srl, Lissone, Italy) or bovine bone (Bio-oss®, Geistlich Biomaterials, Wolhusen, Switzerland) was used alone. Before the closure for primary intention of the flap, a collagen membrane (Bio-Gide®, Geistlich Biomaterials, Wolhusen, Switzerland) was used in the test and control; thus, the flap was repositioned with a single suture line.
Ferrarotti et al. [19] showed the use of micrografts combined with a minimally-invasive surgical procedure (MIST), and in association with a collagen sponge (Condress®, Istituto Gentili, Milano, Italy), the elevation of the flap was kept at a minimum to ensure cloth stability and to facilitate the regenerative procedure in the test and control sites. Aimetti et al. [20] showed the same surgical procedures with the minimally invasive flap and the use of pulp progenitor cells combined with a collagen sponge (Condress®, Istituto Gentili, Milan, Italy). The molars from which the pulp samples were extracted were washed with chlorhexidine at 0.2% (CHX) for 60 s, and after crown separation, pulp was collected and used to generate micrografts using the Rigeneracons®. All the defects were treated with scaling and root planing before the insertion of the sponge, and after the placement and complete filling of the infrabony defect, primary wound closure was achieved with horizontal interrupted mattress sutures (Gore-Tex®, WL Gore & Associates Inc., Newark, DE, USA). Graziano et al. [21]in their case report used a sample of periodontal ligament from an extracted third molar after a washing period of 60 s with CHX at 0.2%. Scaling and root planing were achieved by the use of manual Gracey curettes and ultrasonic instruments in the test and control groups; thus, all the inflammatory tissue was removed. Collagen sponge (Gingistat®, GABA, Rome, Italy) was used in combination with micrografts or alone in the control site.
In the studies by D’Aquino et al. in 2009 [22] and 2016 [23], respectively, an impacted third molar was used to extract pulp tissue, or a periosteal sample was collected from the inner layer of an access flap. When simple extraction was not possible in one go, a crown and root separation was performed. The socket obtained by the extraction of the third molar was then filled with a collagen sponge (Gingistat®, GABA, Rome, RM, Italy) embedded with progenitor cells. Primary closure was achieved by an interrupted suture.
3.5. Qualitative Analysis
Clinical, histological, and radiographic evaluation was performed with dissimilar follow-up periods according to the procedures of each study. The clinical parameters were assessed during follow-ups at 1 week and 3, 6, and 12 months regarding periodontal regeneration with the use of a periodontal probe (PCP 15/11.5, Hu-Friedy, Chicago, IL, USA). The parameters evaluated were the presence or absence of plaque (PI), presence or absence of bleeding on probing (BoP) [24], periodontal depth (PD), recession (REC), and clinical attachment level (CAL) [24].
Histological evaluations were made after 40, 60, 90, and 120 days for socket preservation, whereas for sinus lift, the histological analysis was performed 4 months after the surgery. For periodontal regeneration, no histological data were found. Radiographic analysis was performed in all the studies with a periapical X-ray or a cone-beam. Follow-up was at 4 months for sinus floor augmentation; 3, 6, and 12 months for periodontal procedures; and 45 or 90 days for socket preservation after dental implant insertion.
3.6. Results
The micrografts in all the studies were combined with a scaffold such as collagen sponge or hydroxyapatite. In the control group, a collagen sponge or hydroxyapatite alone was used (Table 1).
Table 1. Results extracted from test and control sites with follow-up periods of 4 months for sinus lift procedures, 6 and 12 months for periodontal regeneration, and 3 and 4 months for socket preservation.
|
Sinus Lift |
||
|
Baena et al. 2017 [16] |
Micro-grafts group |
Bone substitutes |
|
Histological |
VMT=58.5 ± 2.5%;NMT=41.4 ± 5.6%;NVMT=N/A |
Hidroxiapatite alone VMT=20.2 ± 3.1%;NMT=5.5 ± 1.6%;NVMT=N/A; Bovine bone aloneVMT=48 ± 2.5%;NMT=20.5 ± 3.1%;NVMT=31.5 ± 2.3% |
|
Radiographical |
High mineralization |
High mineralization |
|
Periodontal regeneration |
||
|
Ferrarotti et al. 2018 [19] |
Micro-grafts group |
Collagen sponge group |
|
Clinical |
PD=8.3 ± 1.2mm;CAL=10.0 ± 1.6mm; |
PD=7.9 ± 1.3mm;CAL=9.4 ± 1.5mm; |
|
Radiographical |
IBD=6.4 ± 1.4mm |
IBD=5.6 ± 1.0mm |
|
Graziano et al. 2013 [21] |
Micro-grafts group |
Collagen sponge group |
|
Clinical |
PD reduction from 12 to 3mm |
PD reduction from 11 to 7mm |
|
Radiographical |
High mineralization |
Low mineralization |
|
Socket preservation |
||
|
d'Aquino et al. 2009 [22] |
Micro-grafts group |
Collagen sponge group |
|
Clinical |
CAL=6.2±2.3mm |
CAL=4.4±1.2 mm |
|
Histological |
lamellar architecture |
immature Bone |
|
Radiographical |
High mineralization |
Low mineralization |
|
d'Aquino et al. 2016 [23] |
Micro-grafts group |
Collagen sponge group |
|
Clinical |
Horizontal reduction= 25% Vertical reduction=0.60% |
Horizontal reduction= 30% Vertical reduction=1.5% |
|
Histological |
calcified matrix |
organic matrix |
|
Radiographical |
High mineralization |
Low mineralization |
4. Conclusion
By using Rigenera® technology, all the characteristics of MSCs are preserved, ensuring viability and proliferation, which are not always guaranteed in an enzymatic process. Another aspect is that with the Rigeneracons®, the donor and acceptor sites are the same, avoiding the possible complications associated with non-autologous micrografts [13]. Moreover, the Rigeneracons® system is closed, thus avoiding contamination and unpredictability of the cell material. PCs are considered to be adult stem cells due to their origin, unlike embryonal or perinatal stem cells which are not easy to isolate or to harvest. Furthermore, to our knowledge, the behaviors of various types of these stem cells in oral regeneration, and how they might enhance the regenerative process, are not yet clear. On the other hand, it is clear that progenitor cells or MSCs have a limited life span, leading to a short period of regeneration when they are transplanted [25][26]. The future of oral regeneration might be in the use of MSCs/PCs associated with a scaffold. Furthermore, according to the literature, there are reservoirs of PCs in the oral cavity that are able to differentiate in various directions depending on the tissue to be repaired [27][28].
References
- Grassia, ; Nucci, L. New Materials in Oral Surgery. Materials 2020, 13, 1034.
- Zhang, ; Wu, W.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J.; Meng, Z.; Zhu, J.; Ge, Z.; Cui, W. Advanced biomaterials for repairing andreconstruction of mandibular defects. Mater. Sci. Eng. C 2019, 103, 109858.
- Dimitriou, ; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from theiliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15.
- Nkenke, ; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W.; Morbidity of harvesting of bone grafts from theiliac crest for preprosthetic augmentation procedures: A prospective study. Int J. Oral Maxillofac Surg. 2004, 33, 157–163.
- Sanz, ; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari; E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; Ellingsen, J. E.; et al.Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th EuropeanWorkshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91.
- Haugen, J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92.S–102.S.
- Stevens, M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25.
- Chen, M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang,Y. J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defectsusing autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33.
- Bajestan, N.; Rajan, A.; Edwards, S.P.; Aronovich, S.; Cevidanes, L.; Polymeri, A.; Travan, S.; Kaigler, D. Stem cell therapy for reconstruction ofalveolar cleft and trauma defects in adults: A randomized controlled, clinical trial. Clin. Implant. Dent. Relat Res. 2017, 19, 793–801.
- Barbier, ; Ramos, E.; Mendiola, J.; Rodriguez, O.; Santamaria, G.; Santamaria, J.; Arteagoitia, I.; Autologous dental pulp mesenchymal stem cells forinferior third molar post-extraction socket healing: A split-mouth randomised clinical trial. Med. Oral Patol Oral Cir. Bucal. 2018, 23, 469–477.
- Cheng, ; Chen, B.P.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: ASystematic Review. Surg Infect. (Larchmt). 2017, 18, 722–735.
- Trovato, ; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New MedicalDevice Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J. Cell Physiol. 2015,2 30, 2299–2303.
- Monti, ; Graziano, A.; Rizzo, S.; Perotti, C.; Del Fante, C.; d’Aquino, R.; Redi, C. A.; Rodriguez Y Baena, R. In Vitro and In Vivo Differentiation ofProgenitor Stem Cells Obtained After Mechanical Digestion of Human Dental Pulp. J Cell Physiol. 2017, 232, 548–555.
- Giaccone, ; Brunetti, M.; Camandona, M.; Trovato, L.; Graziano, A. A New Medical Device, Based on Rigenera Protocol, in the Management ofComplex Wounds. J. Stem Cells Res. Rev. Rep. 2014, 1, 3–5.
- Ceccarelli, ; Graziano, A.; Benedetti, L.; Imbriani, M.; Romano, F.; Ferrarotti, F.; Aimetti, M.; Cusella de Angelis, G. M. Osteogenic Potential ofHuman Oral-Periosteal Cells (PCs) Isolated From Different Oral Origin: An In Vitro Study. J. Cell Physiol. 2016, 231, 607–612.
- Baena, R.; D’Aquino, R.; Graziano, A.; Trovato, L.; Aloise, A.C.; Ceccarelli, G.;Cusella, G.; Pelegrine, A. A.; Lupi, S. M. Autologous periosteum-derived micrografts and PLGA/HA enhance the bone formation in sinus lift augmentation. Front. Cell Dev. Biol. 2017, 1–7.
- Brunelli, ; Motroni, A.; Graziano, A.; D’Aquino, R.; Zollino, I.; Carinci, F. Sinus lift tissue engineering using autologous pulp micro-grafts: A casereport of bone density evaluation. J. Indian Soc. Periodontol. 2013, 17, 644–647.
- Lupi, M. Rodriguez y Baena, A.; Todaro, C.; Ceccarelli, G.; Rodriguez y Baena, R. Maxillary sinus lift using autologous periosteal micrografts: A newregenerative approach and a case report of a 3-year follow-up. Case Rep. Dent. 2018, 2018, 3023096.
- Ferrarotti, ; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrograftscontaining dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850.
- Aimetti, ; Ferrarotti, F.; Gamba, M.N.; Giraudi, M.; Romano, F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous DentalPulp Stem Cells: A 1-Year Follow-Up Case Series. Int J. Periodontics Restorative Dent. 2018, 38, 51–58.
- Graziano, ; Carinci, F.; Scolaro, S.; D’Aquino, R. Periodontal tissue generation using autologous dental ligament micro-grafts: Case report with 6months follow-up. Ann. Oral Maxillofac. Surg. 2013, 1, 20.
- d’Aquino, ; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. . Human mandible bone defect repair bythe grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009, 18, 75–83.
- d’Aquino, ; Trovato, L.; Graziano, A.; Ceccarelli, G.; Cusella de Angelis, G.; Marangini, A.; Nisio, A.; Galli, M.; Pasi, M.; Finotti, M.; et al.Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. J. Transl Sci. 2016, 2, 125–129.
- Lang, P.; Joss, A.; Orsanic, T.; Gusberti, FA.; Siegrist, B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986, 13, 590–596.
- Abdel Meguid, ; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell Physiol. 2018, 233, 1825–1835.
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Patini, R.; Ferlito, S.; Periodontitis and Tooth Loss Have Negative Systemic Impact on Circulating Progenitor Cell Levels: A Clinical Study. Genes (Basel). 2019;10,1022.
- Mao, J.; Robey, P.G.; Prockop, D.J. Stem cells in the face: Tooth regeneration and beyond. Cell Stem Cell. 2012, 11, 291–301.
- Sanz, R.; Carrión, F.S.; Chaparro, A.P. Mesenchymal stem cells from the oral cavity and their potential value in tissue engineering. Periodontol 20002015, 67, 251–267.