
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prasun Jalal | + 1539 word(s) | 1539 | 2021-11-10 10:36:26 | | | |
| 2 | Jason Zhu | Meta information modification | 1539 | 2021-11-15 03:23:06 | | |
Video Upload Options
Most immunosuppressive medications have been incriminated in renal, cardiovascular, and neurological complications, relapse of viral hepatitis, and recurrence of HCC and other cancers. Efforts to minimize immunosuppression are directed toward decreasing medication side effects, increasing cost effectiveness, and decreasing economic burden without increasing the risk of rejection.
1. Introduction
Liver transplantation (LT) is an expensive procedure, with the greatest cost in the first year and a considerable economic burden in the following years. A study from the USA for the estimation of the economic burden of LTs from 2008 to 2013 estimated that Medicare coverage reimbursed USD 185 thousand per recipient in the first year post-LT. The average cost after the first year was USD 154 thousand per recipient. The cost of re-transplantation was USD 388 thousand per-patient, and the costs in the second year post-LT were USD 28 thousand [1][2]. In a study by Serper and colleagues, nearly one-fifth of the recipients made tradeoffs for the medications post-LT that led to decreased compliance and increased hospital admissions. Insurance type, presence of co-morbid conditions, health literacy, and number of medications were associated with increased medication tradeoffs, decreased frequency of administration, or delayed purchase of the medications [3].
In addition to an increased economic burden, chronic use of immunosuppressive drugs leads to significant morbidities and metabolic side effects. While calcineurin inhibitors (CNIs) exhibit considerable nephrotoxicity and neurotoxicity, steroids are diabetogenic, and mycophenolic acid derivatives (MMFs) have adverse hematologic effects. Mammalian target of rapamycin inhibitors (MTORis) have been implicated in the development of interstitial lung disease. Different classes of immunosuppressive medications are associated with an increased infection rate. Immunosuppressive drugs used for rejection pose a high risk of demyelination and tuberculosis reactivation [4]. The long-term side effects of these drugs, including malignancies, opportunistic infections, metabolic disorders, and organ toxicities, represent the main clinical concern in formulating an immunosuppression protocol [5].
The 1-year survival post-LT improved to 85% after the introduction of CNIs in addition to the improvement of the recipients’ selection and surgical techniques [6]. The overall survival is still multifactorial; medications’ side effects, the rate of original disease recurrence, and the presence of comorbidities all play an important role when choosing the optimal immunosuppression protocol. Minimization of the side effects of the drugs without exposing patients to an increased risk of rejection is the ultimate goal for successful immunosuppressive protocols [7].
2. Immunosuppression Protocols: The Past and the Present
| Year of Discovery/FDA Approval | Indication | Immunosuppressive Agent | Mode of Action | Main Adverse Effect |
|---|---|---|---|---|
| 1972/1983 TAC in 1994 |
Maintenance | CNI | Blocks protein transcription in response to Il-2 and prevents cellular proliferation |
Neurotoxicity Nephrotoxicity Diabetogenic |
| 1893/1995 | Maintenance | MMF | Interferes with DNA synthesis | Pancytopenia Abdominal discomfort |
| 1997 | AMR | Rituximab | Anti-CD 20 | Reactivation of TB |
| 2007 | Maintenance | MTORi | Inhibits cytokine receptor signal transduction | Hyperlipidemia Pedal edema Oral ulcers HAT |
| 1998/2008 | Induction | Basiliximab | IL2 receptor blocker | Hypersensitivity |
| 2011 | Induction or maintenance | Balatacept | Blocks CD80 co-stimulation ligand | Post-TX lymphoproliferative disorder |
| Induction, Maintenance, and Rejection | Corticosteroids | Affects the production of several inflammatory mediators and multiple cytokines, including IL-1, IL-2, IL-3, IL-6, TNF-a, IFN-Y, leukotrienes, and prostaglandins | Diabetes Hypertension Osteoporosis Hirsutism Weight gain |
|
| 2014 | Chronic rejection | Alemtuzumab (Campath 1H) | Anti-CD52 on nucleated cells of BM | Hirsutism Post-TX Lymphoproliferative disorder |
| 2017 | Induction or rejection | ATG | Anti-CD3 on lymphocytes | Anaphylaxis, post-TX lymphoproliferative disorders |
3. Strategies for Immune Suppression Withdrawal/Minimization
| Study | Population | Type | Duration of Withdrawal | Total Subjects | Successful Withdrawal Arm | Follow Up |
|---|---|---|---|---|---|---|
| Feng/iWITH Study [20] | Pediatrics | Multicenter | 36–48 weeks | 2909 | 37.5%% of 88 subjects | 1, 2, 3, 4 years |
| Levitsky [21] | Adults | Single center | >3 years post-LTWithdrawal over 6 months | 1255 | >50% of 15 subjects | 1 year |
| Benitez [22] | Adults | Multicenter | >3 years post-LTWithdrawal over 6–9 months | 500 | 40% of 98 subjects | Up to 3 years |
| Pons [23] | Adults | Single center | >2 years post-LT | 490 | 42% of 12 subjects | 2 years |
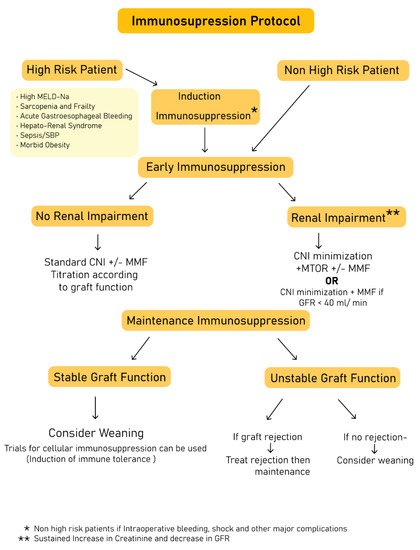
4. Deliberation on Strategies to Improve Immune Suppression Practice
References
- Neuberger, J. Follow-up of liver transplant recipients. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101682.
- Scientific Registry of Transplant Recipients. Available online: https://srtr.transplant.hrsa.gov/annual_reports/2016/Economics.aspx#Econ_3_LI_tx_medicare_cov_1_b64 (accessed on 20 August 2021).
- Serper, M.; Reese, P.P.; Patzer, R.R.; Levitsky, J.; Wolf, M.S. The prevalence, risk factors, and outcomes of medication trade-offs in kidney and liver transplant recipients: A pilot study. Transpl. Int. 2018, 31, 870–879.
- Leighton, J.; Wilson, C. Modern immunosuppression. Surgery 2020, 38, 368–374.
- Tasdogan, B.E.; Ma, M.; Simsek, C.; Saberi, B.; Gurakar, A. Update on immunosuppression in liver transplantation. Euroasian J. Hepato Gastroenterol. 2019, 9, 96–101.
- Zarrinpar, A.; Busuttil, R.W. Liver transplantation: Past, present and future. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 434–440.
- Geissler, E.K.; Schlitt, H.J. Immunosuppression for liver transplantation. Gut 2009, 58, 452–463.
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940.
- Liu, C.L.; Fan, S.T.; Lo, C.M.; Chan, S.C.; Ng, I.O.; Lai, C.L.; Wong, J. Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: A protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transplant. 2004, 10, 728–733.
- Magliocca, J.F.; Knechtle, S.J. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl. Int. 2006, 19, 705–714.
- Vincenti, F.; Mendez, R.; Pescovitz, M.; Rajagopalan, P.R.; Wilkinson, A.H.; Butt, K.; Laskow, D.; Slakey, D.P.; Lorber, M.I.; Garg, J.P.; et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am. J. Transplant. 2007, 7, 1770–1777.
- Hutchinson, J.A.; Brem-Exner, B.G.; Riquelme, P.; Roelen, D.; Schulze, M.; Ivens, K.; Grabensee, B.; Witzke, O.; Philipp, T.; Renders, L.; et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl. Int. 2008, 21, 742–754.
- Tanimine, N.; Ohira, M.; Tahara, H.; Ide, K.; Tanaka, Y.; Onoe, T.; Ohdan, H. Strategies for deliberate induction of immune tolerance in liver transplantation: From preclinical models to clinical application. Front. Immunol. 2020, 11, 1615.
- De Simone, P.; Carrai, P.; Coletti, L.; Ghinolfi, D.; Petruccelli, S.; Filipponi, F. Modification of immunosuppressive therapy as risk factor for complications after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 199–209.
- Cillo, U.; De Carlis, L.; Del Gaudio, M.; De Simone, P.; Fagiuoli, S.; Lupo, F.; Tisone, G.; Volpes, R.; Avolio, A.; Bitetto, D.; et al. Correction to: Immunosuppressive regimens for adult liver transplant recipients in real-life practice: Consensus recommendations from an Italian Working Group. Hepatol. Int. 2021, 14, 930–943.
- Kwon, H.; Jeon, J.; Kim, D.; Jang, H.; Sung, H.; Han, D.; Park, J.; Lee, J.; Huh, W.; Kim, S.; et al. Clinical impact of a protocolized kidney donor follow-up system. Transplant. Proc. 2019, 51, 692–700.
- Shaked, A.; DesMarais, M.R.; Kopetskie, H.; Feng, S.; Punch, J.D.; Levitsky, J.; Reyes, J.; Klintmalm, G.B.; Demetris, A.J.; Burrell, B.E.; et al. Outcomes of immunosuppression minimization and withdrawal early after liver transplantation. Am. J. Transplant. 2019, 19, 1397–1409.
- Feng, S.; Bucuvalas, J.C.; Mazariegos, G.V.; Magee, J.C.; Sanchez-Fueyo, A.; Spain, K.M.; Lesniak, A.; Kanaparthi, S.; Perito, E.; Venkat, V.L.; et al. Efficacy and safety of immunosuppression withdrawal in pediatric liver transplant recipients: Moving toward personalized management. Hepatology 2021, 73, 1985–2004.
- Levitsky, J.; Burrell, B.E.; Kanaparthi, S.; Turka, L.A.; Kurian, S.; Sanchez-Fueyo, A.; Lozano, J.J.; Demetris, A.; Lesniak, A.; Kirk, A.D.; et al. Immunosuppression withdrawal in liver transplant recipients on sirolimus. Hepatology 2020, 72, 569–583.
- Benítez, C.; Londoño, M.-C.; Miquel, R.; Manzia, T.M.; Gonzalez-Abraldes, J.; Lozano, J.J.; Martínez-Llordella, M.; López, M.; Angelico, R.; Bohne, F.; et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013, 58, 1824–1835.
- Pons, J.A.; Revilla-Nuin, B.; Baroja-Mazo, A.; Ramírez, P.; Martínez-Alarcón, L.; Sánchez-Bueno, F.; Robles, R.; Rios, A.; Aparicio, P.; Parrilla, P. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplanation 2008, 86, 1370–1378.
- Neuberger, J.M.; Mamelok, R.D.; Neuhaus, P.; Pirenne, J.; Samuel, D.; Isoniemi, H.; Rostaing, L.; Rimola, A.; Marshall, S.; Mayer, A.D.; et al. delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: The ‘ReSpECT’ study. Am. J. Transplant. 2009, 9, 327–336.
- De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Saliba, F.; Jonas, S.; Sudan, D.; Fung, J.; Fischer, L.; et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am. J. Transplant. 2012, 12, 3008–3020.
- Saliba, F.; De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Jonas, S.; Sudan, D.; Fischer, L.; Duvoux, C.; et al. Renal function at two years in liver transplant patients receiving everolimus: Results of a randomized, multicenter study. Am. J. Transplant. 2013, 13, 1734–1745.
- Fischer, L.; Saliba, F.; Kaiser, G.M.; De Carlis, L.; Metselaar, H.J.; De Simone, P.; Duvoux, C.; Nevens, F.; Fung, J.J.; Dong, G.; et al. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: Follow-up results from a randomized, multicenter study. Transplantation 2015, 99, 1455–1462.
- De Simone, P.; Saliba, F.; Dong, G.; Escrig, C.; Fischer, L. Do patient characteristics influence efficacy and renal outcomes in liver transplant patients receiving everolimus? Clin. Transplant. 2015, 30, 279–288.
- De Simone, P.; Fagiuoli, S.; Cescon, M.; De Carlis, L.; Tisone, G.; Volpes, R.; Cillo, U. Use of everolimus in liver transplantation. Transplantation 2017, 101, 239–251.
- Rodríguez-Perálvarez, M.; Guerrero-Misas, M.; Thorburn, U.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Maintenance immunosuppression for adults undergoing liver transplantation: A network meta-analysis. Cochrane Database Syst. Rev. 2017, 2017, 356.
- Tang, Q.; Vincenti, F. Transplant trials with tregs: Perils and promises. J. Clin. Investig. 2017, 127, 2505–2512.




