
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rohan Dassanayake | + 2220 word(s) | 2220 | 2021-08-10 06:16:47 | | | |
| 2 | Peter Tang | Meta information modification | 2220 | 2021-11-12 03:25:36 | | |
Video Upload Options
Synthetic dyes have become an integral part of many industries such as textiles, tannin and even food and pharmaceuticals. Industrial dye effluents from various dye utilizing industries are considered harmful to the environment and human health due to their intense color, toxicity and carcinogenic nature.
1. Introduction
2. Different Types of Dyes
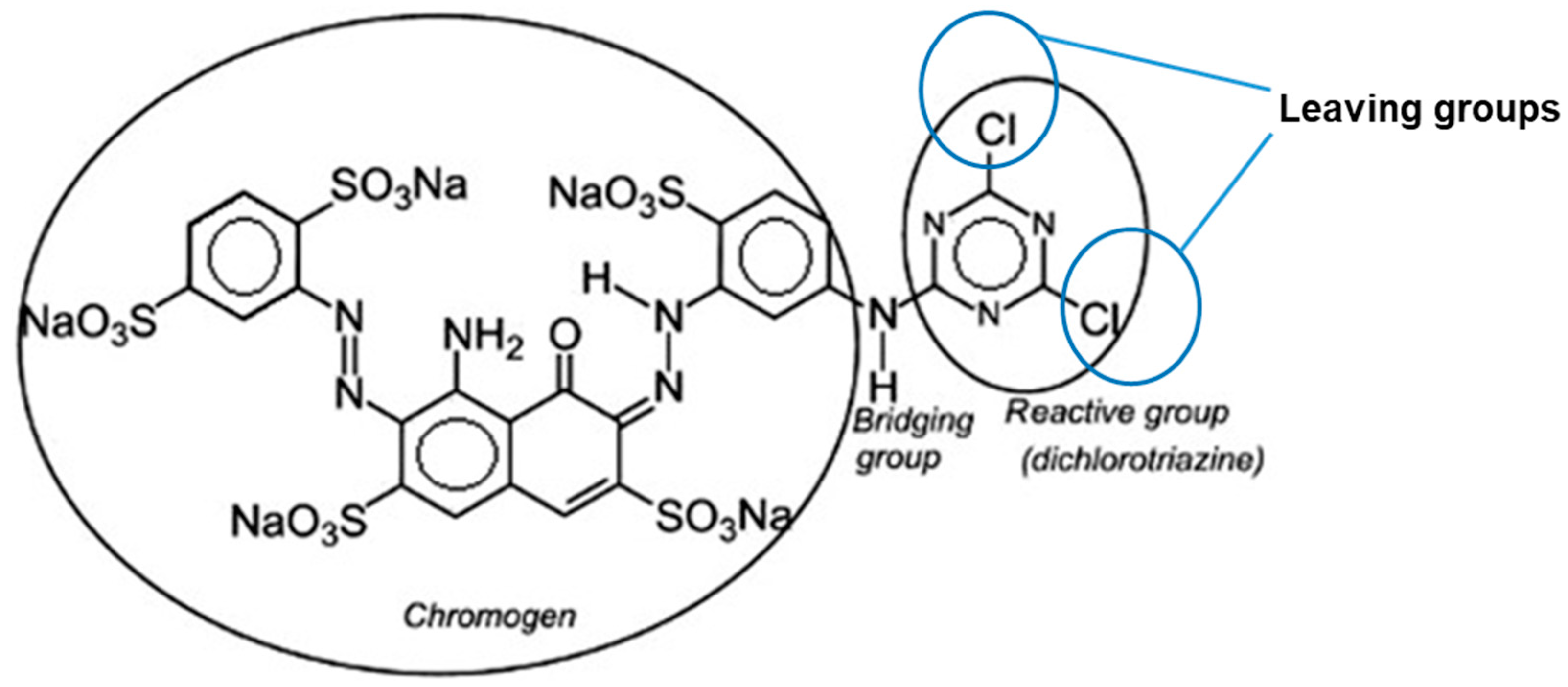
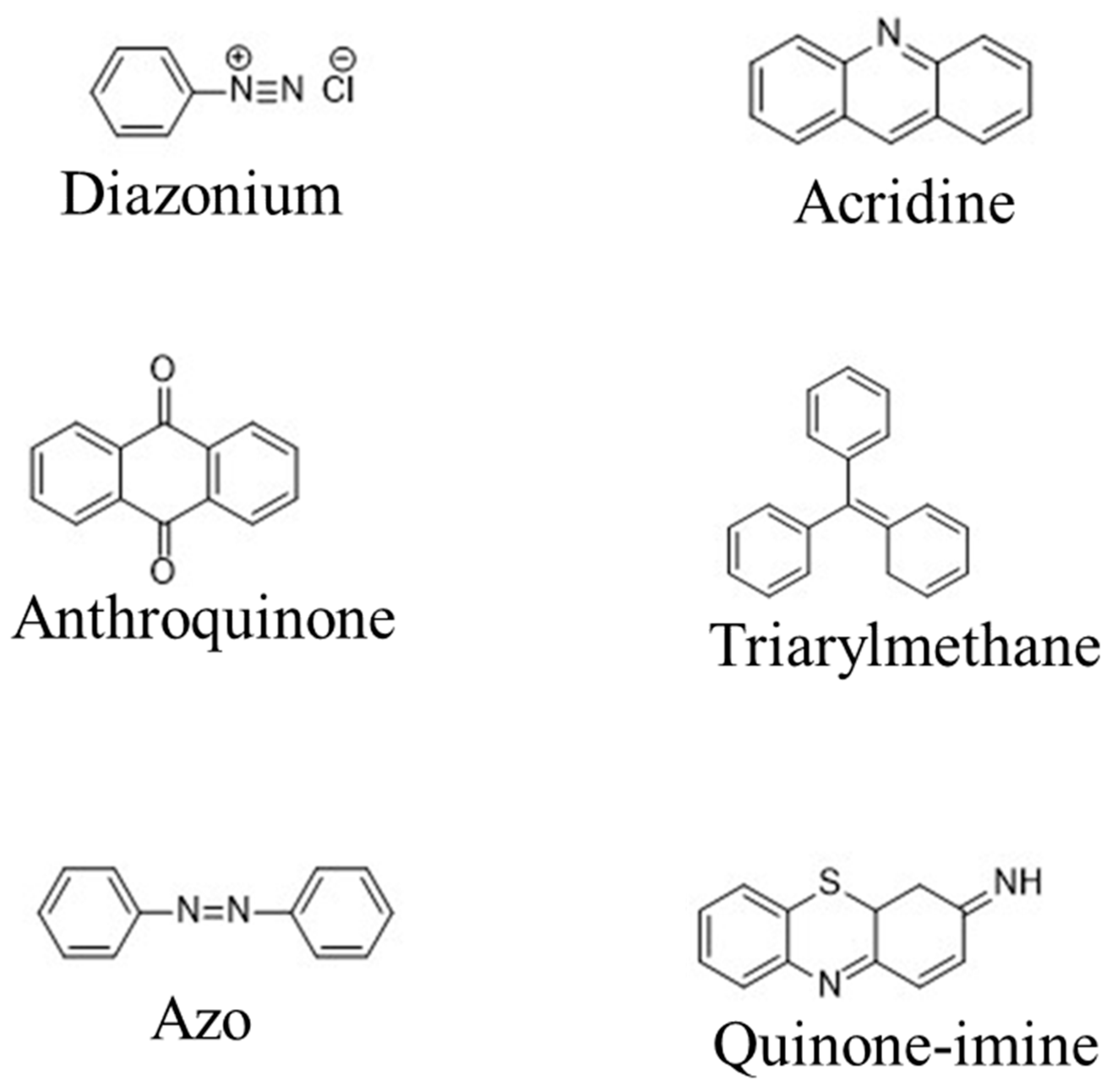
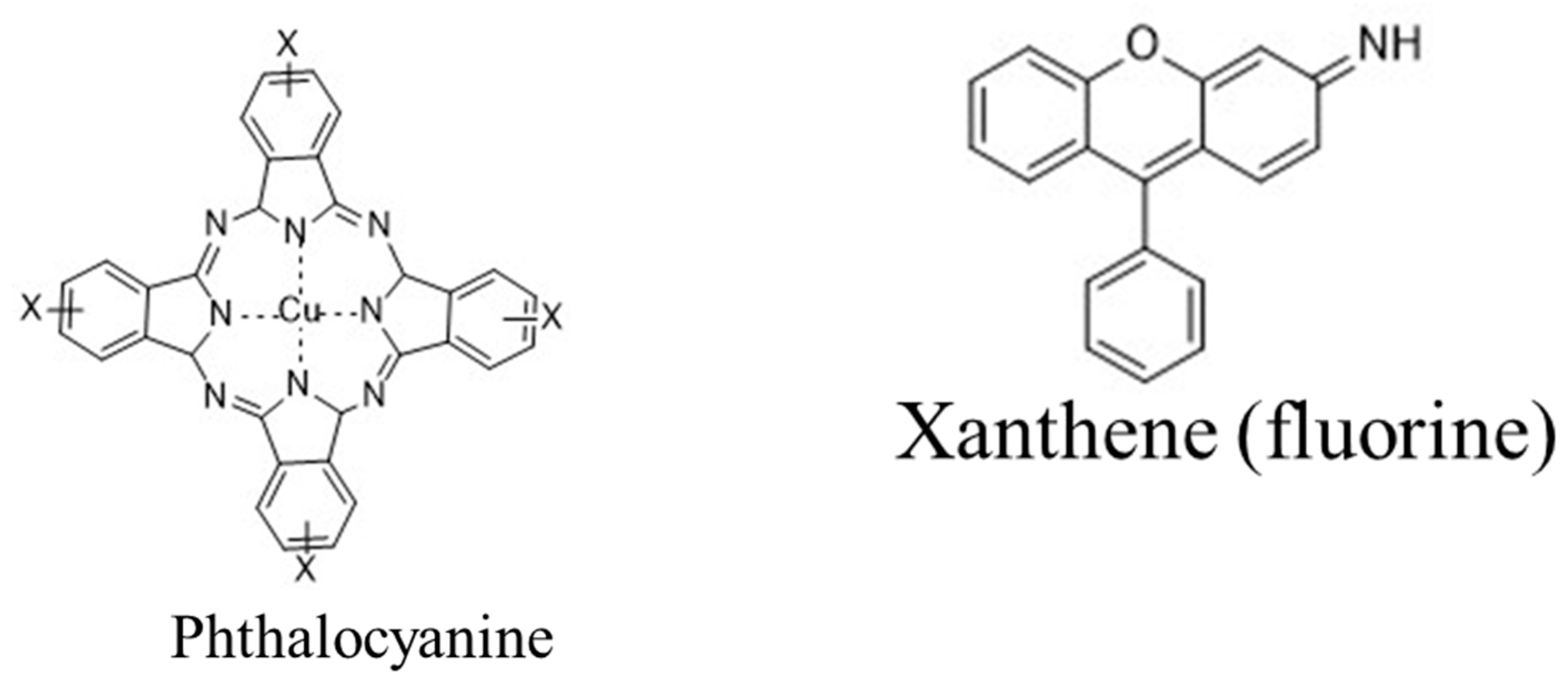
|
Type |
Water Solubility |
Applications |
Common Application Method |
Example |
|---|---|---|---|---|
|
Acid |
Soluble |
Cosmetics, food, leather, modified acrylics, nylon, paper, printing ink, silk and wool |
In dye baths with neutral to acidic conditions |
Acid Yellow 36 |
|
Azo |
Soluble/insoluble |
Acetate, cellulose, cotton, rayon and polyester |
Coupling component used to impregnate fiber and a solution of stabilized diazonium salt is used for treatment |
Bluish Red azo dye |
|
Basic |
Soluble |
Inks, medicine, modified nylon, modified polyester, paper, polyacrylonitrile, polyester, silk, tannin, mordanted cotton and wool |
In dye baths with acidic conditions |
Methylene Blue |
|
Direct |
Soluble |
Cotton, leather, nylon, rayon, silk and paper |
In dye baths with neutral or slightly alkaline conditions with additional electrolyte. |
Direct Orange 26 |
|
Disperse |
Insoluble |
Acetate, acrylic fibers, cellulose, cellulose acetate, nylon, polyamide, polyester, polyester–cotton and plastic |
Padded on cloth and either baked or thermo-fixed at high pressure and temperature or low temperature carrier methods |
Disperse Blue 27, Disperse Red 4, Disperse Yellow 3 |
|
Fluorescent brighteners |
All fibers, oils, paints, plastics and soaps as well as detergents |
Mass dispersion, solution or suspension |
4,4′-bis (ethoxycarbonylvinyl) stilbene |
|
|
Food, drug, and cosmetics |
Food, drug, and cosmetics |
Mixing |
Food Yellow 4 and tartrazine |
|
|
Mordant |
Anodized aluminum, natural fibers, leather and wool |
Along with chromium salts |
Mordant Red 11 |
|
|
Oxidation bases |
Cotton, fur and hair |
The substrate is oxidized with aromatic amines and phenols |
Direct Blue |
|
|
Reactive |
Soluble |
Cellulosic, cotton, nylon, silk and wool |
Reaction between functional group on fiber and reactive group on dye. Covalently bonding under heat and alkaline pH |
Reactive Blue 5 |
|
Solvent |
Insoluble |
Fats, gasoline, inks, lacquers, lubricants, oils, plastics, stains, varnishes and waxes |
Substrate dissolution |
Solvent Red 26, Solvent Blue 35 |
|
Sulfur |
Cotton, leather, paper, polyamide fibers, rayon, silk and wood |
Aromatic substrate vatted with sodium sulfide and reoxidized to insoluble sulfur-containing products on fiber |
Sulfur Black 1 |
|
|
Vat |
Insoluble |
Cotton, cellulosic, polyester–cotton, rayon and wool |
Water-insoluble dyes solubilized by reducing with sodium hydrogen sulfite, then exhausted on fiber and reoxidized |
Vat Blue 4 (Indathrene) |
3. Biopolymer-based Dye Removal Technologies
References
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In Water Chemistry; Eyvaz, M., Yüksel, E., Eds.; Intech Open: London, UK, 2020.
- Acharya, S. Cationization of Cotton Fabric for Improved Dye Uptake. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 2012.
- Siva, R. Status of natural dyes and dye-yielding plants in India. Curr. Sci. 2007, 92, 916–925.
- Saini, R.D. Textile Organic Dyes: Polluting effects and Elimination Methods from Textile Waste Water. Int. J. Chem. Eng. Res. 2017, 9, 975–6442.
- Cañamares, M.V.; Reagan, D.A.; Lombardi, J.R.; Leona, M. TLC-SERS of mauve, the first synthetic dye. J. Raman Spectrosc. 2014, 45, 1147–1152.
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118.
- Rauf, M.A.; Salman Ashraf, S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 2012, 209, 520–530.
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697.
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of amine-modified zeolitic imidazolate framework-8, ultrasound-assisted dye removal and modeling. Ultrason. Sonochem. 2017, 39, 550–564.
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 326, 1145–1158.
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3906390233.
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile Dyeing Wastewater Treatment. Adv. Treat. Text. Effl. 2011.
- Chequer, F.D.; De Oliveira, G.R.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Textile Dyes: Dyeing Process and Environmental Impact. In Eco-Friendly Textile Dyeing and Finishing; Intech Open: London, UK, 2013; pp. 151–176.
- Joshi, M.; Bansal, R.; Purwar, R. Colour removal from textile effluents. Indian J. Fibre Text. Res. 2004, 29, 239–259.
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085.
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971.
- Isah, U.A.; Abdulraheem, G.; Bala, S.; Muhammad, S.; Abdullahi, M. Kinetics, equilibrium and thermodynamics studies of C.I. Reactive Blue 19 dye adsorption on coconut shell based activated carbon. Int. Biodeterior. Biodegrad. 2015, 102, 265–273.
- Weber, E.J.; Stickney, V.C. Hydrolysis kinetics of Reactive Blue 19-Vinyl Sulfone. Water Res. 1993, 27, 63–67.
- Fatima, M.; Farooq, R.; Lindström, R.W.; Saeed, M. A review on biocatalytic decomposition of azo dyes and electrons recovery. J. Mol. Liq. 2017, 246, 275–281.
- Oblak, R.; Kete, M.; Štangar, U.L.; Tasbihi, M. Alternative support materials for titania photocatalyst towards degradation of organic pollutants. J. Water Process Eng. 2018, 23, 142–150.
- Khan, R.; Patel, V.; Khan, Z. Bioremediation of dyes from textile and dye manufacturing industry effluent. In Abatement of Environmental Pollutants: Trends and Strategies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–125.
- Rodríguez-Couto, S.; Osma, J.F.; Toca-Herrera, J.L. Removal of synthetic dyes by an eco-friendly strategy. Eng. Life Sci. 2009, 9, 116–123.
- Zouboulis, A.I.; Moussas, P.A.; Tzoupanos, N.D. Biosorbents based on lignin used in biosrption processes from wastewater treatment: A review. In Lignin; Nova Science Publishers: Hauppauge, NY, USA, 2018; pp. 289–324.
- Broadbent, A.D. Basic Principles of Textile Coloration; Society of Dyers and Colorists: Bradford, UK, 2001; ISBN 090195676727.
- Pal, P. Chapter 6 - Industry-Specific Water Treatment: Case Studies. In Industrial Water Treatment Process Technology; Pal, P., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 243–511. ISBN 978-0-12-810391-3.
- Cotillas, S.; Llanos, J.; Cañizares, P.; Clematis, D.; Cerisola, G.; Rodrigo, M.A.; Panizza, M. Removal of Procion Red MX-5B dye from wastewater by conductive-diamond electrochemical oxidation. Electrochim. Acta 2018, 263, 1–7.
- Orzel, J.; Daszykowski, M.; Grabowski, I.; Zaleszczyk, G.; Sznajder, M.; Walczak, B. Simultaneous determination of Solvent Yellow 124 and Solvent Red 19 in diesel oil using fluorescence spectroscopy and chemometrics. Talanta 2012, 101, 78–84.
- Zazouli, M.A.; Kalankesh, L.R. Removal of precursors and disinfection by-products (DBPs) by membrane filtration from water; a review. J. Environ. Health Sci. Eng. 2017, 15, 25.
- Steter, J.R.; Barros, W.R.P.; Lanza, M.R.V.; Motheo, A.J. Electrochemical and sonoelectrochemical processes applied to amaranth dye degradation. Chemosphere 2014, 117, 200–207.
- Pedro Silva, J.; Sousa, S.; Rodrigues, J.; Antunes, H.; Porter, J.J.; Gonçalves, I.; Ferreira-Dias, S. Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains. Sep. Purif. Technol. 2004, 40, 309–315.
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818.
- Kyzioł, A.; Kyzioł, K. Surface Functionalization With Biopolymers via Plasma-Assisted Surface Grafting and Plasma-Induced Graft Polymerization—Materials for Biomedical Applications. In Biopolymer Grafting Applications; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–151. ISBN 978-0-12-810462-.




