
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Javad Sharifi-Rad | + 1674 word(s) | 1674 | 2021-11-04 09:55:31 | | | |
| 2 | Peter Tang | Meta information modification | 1674 | 2021-11-11 10:30:20 | | |
Video Upload Options
Epigallocatechin gallate (EGCG) is the main bioactive component of catechins predominantly present in various types of tea. EGCG is well known for a wide spectrum of biological activities as an anti-oxidative, anti-inflammatory, and anti-tumor agent. The effect of EGCG on cell death mechanisms via the induction of apoptosis, necrosis, and autophagy has been documented.
1. Introduction
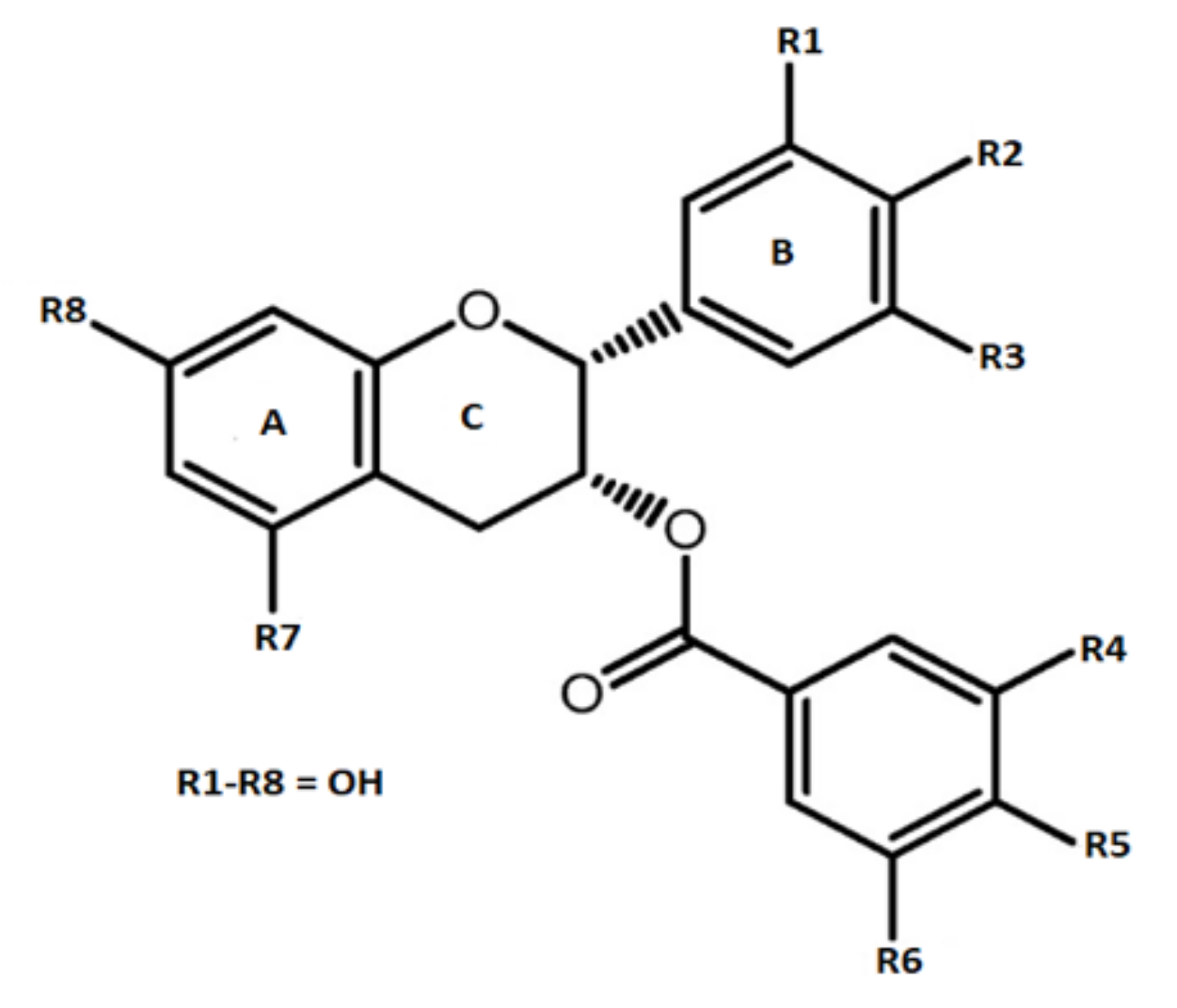
2. Occurrence of Epigallocatechin-3-Gallate in Different Foods
|
Description |
Content |
Reference |
|---|---|---|
|
Japanese green tea |
18.1–23.1 mg/g |
[44] |
|
Long-jing tea |
32.9–35.5 mg/g |
|
|
Jasmine tea |
29.8–31.0 mg/g |
|
|
Pu-erh tea |
16.9–19.19.1 mg/g |
|
|
Iron Buddha tea |
0.12–0.30 mg/g |
|
|
Oolong tea |
11.8–12.2 mg/g |
|
|
Ceylon tea |
7.4–8.9 mg/g |
|
|
Green tea |
4.62 mg/100 mL |
[45] |
|
Black tea |
1.35 mg/100 mL |
|
|
Ban-cha (tea leaves) |
12.2–27.3 mg/g DM |
[46] |
|
Fukamushi-cha (tea leaves) |
13.8–18.6 mg/g DM |
|
|
Yame-cha (tea leaves) |
19.7–32.9 mg/g DM |
|
|
Uji-cha tea leaves) |
22.2–35.9 mg/g DM |
|
|
Sayama-cha (tea leaves) |
21.5–37.2 mg/g DM |
|
|
Uji-matt-cha (Powder tea) |
20.1–29.6 mg/g DM |
|
|
Gyokuro-cha (Powder tea) |
29.9–37.2 mg/g DM |
|
|
Sen-cha (tea bags) |
12.9–23.6 mg/g DM |
|
|
Green tea (Bagged leave form) |
54.3–153.0 mg/g dry tea |
[47] |
|
Green tea (Loose leave form) |
56.5–205.0 mg/g dry tea |
|
|
White tea (Bagged leave form) |
46.0–154.0 mg/g dry tea |
|
|
White tea (Loose leave form) |
38.9–144.0 mg/g dry tea |
|
|
Green tea (Infusions) |
117 to 442 mg/l |
[48] |
|
Typhoo, |
7.9 mg/230 mL (mg/serving) |
[49] |
|
Tesco standard blend, |
4.7 mg/230 mL (mg/serving) |
|
|
Tesco premium blend, |
5.6 mg/230 mL (mg/serving) |
|
|
Sainsbury red blend, |
8.5 mg/230 mL (mg/serving) |
|
|
Sainsbury gold blend, |
11.8 mg/230 mL (mg/serving) |
|
|
PG Tips, |
7.1 mg/230 mL (mg/serving) |
|
|
Tetley |
5.7 mg/230 mL (mg/serving) |
|
|
Apples, Fuji, raw, with skin |
1.93 mg/100 g edible portion |
[50] |
|
Apples, Golden Delicious, raw, with skin |
0.19 mg/100 g edible portion |
|
|
Apples, Granny Smith, raw, with skin |
0.24 mg/100 g edible portion |
|
|
Apples, Red Delicious, raw, without skin |
0.46 mg/100 g edible portion |
|
|
Apples, Red Delicious, raw. with skin |
0.13 mg/100 g edible portion |
|
|
Avocados, raw, |
0.15 mg/100 g edible portion |
|
|
Blackberries, raw (Rubus spp.) |
0.68 mg/100 g edible portion |
|
|
Cranberries, raw |
0.97 mg/100 g edible portion |
|
|
Peaches, raw |
0.30 mg/100 g edible portion |
|
|
Pears, raw |
0.17 mg/100 g edible portion |
|
|
Plums, black diamond, with peel, raw |
0.48 mg/100 g edible portion |
|
|
Raspberries, raw |
0.54 mg/100 g edible portion |
|
|
Nuts, hazelnuts or filberts |
1.06 mg/100 g edible portion |
|
|
Nuts, pecans |
2.30 mg/100 g edible portion |
|
|
Nuts, pistachio nuts, raw |
0.40 mg/100 g edible portion |
|
|
Tea, black, brewed, prepared with tap water |
9.36 mg/100 g edible portion |
|
|
Tea, black, brewed, prepared with tap water, decaffeinated |
1.01 mg/100 g edible portion |
|
|
Tea, fruit, dry |
415.0 mg/100 g edible portion |
|
|
Tea, green, brewed |
64.0 mg/100 g edible portion |
|
|
Tea, green, brewed, decaffeinated |
26.0 mg/100 g edible portion |
|
|
Tea, green, large leaf, Quingmao, dry leaves |
7380 mg/100 g edible portion |
|
|
Tea, oolong, brewed |
34.48 mg/100 g edible portion |
|
|
Tea, white, brewed |
46.0 mg/100 g edible portion |
|
|
Tea, white, dry leaves |
4245 mg/100 g edible portion |
|
|
Carob flour |
109.46 mg/100 g edible portion |
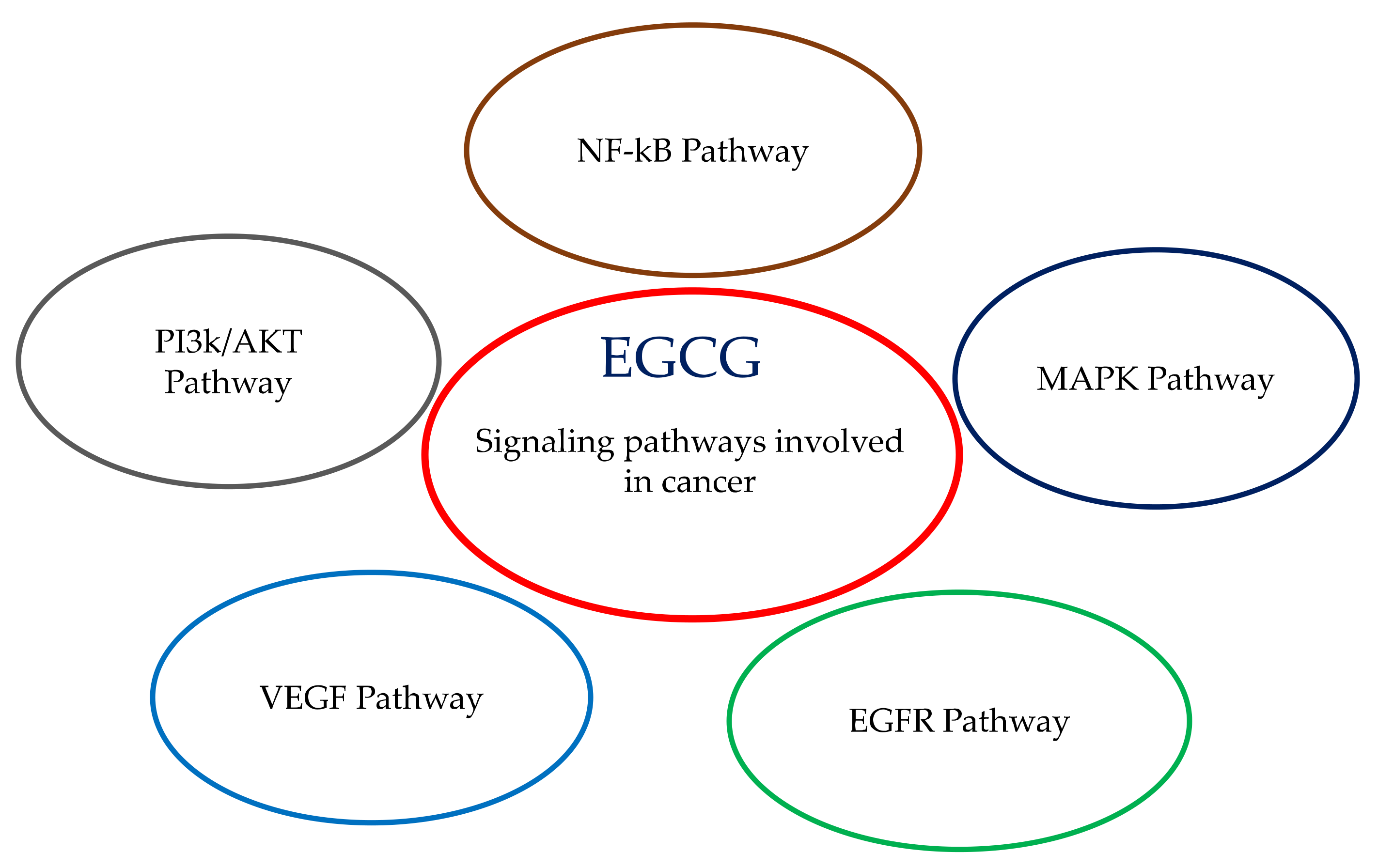
3. Signaling Pathways Involving EGCG: Proliferation, Differentiation, Apoptosis, Inflammation, Angiogenesis and Metastasis
References
- Gupta, S.; Saha, B.; Giri, A.K. Comparative antimutagenic and anticlastogenic effects of green tea and black tea: A review. Mutat. Res. Rev. Mutat. Res. 2002, 512, 37–65.
- Hu, G.; Zhang, L.; Rong, Y.; Ni, X.; Sun, Y. Downstream carcinogenesis signaling pathways by green tea polyphenols: A translational perspective of chemoprevention and treatment for cancers. Curr. Drug Metab. 2014, 15, 14–22.
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99.
- Min, K.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24.
- Nagle, D.G.; Ferreira, D.; Zhou, Y.-D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855.
- Sutherland, B.A.; Rahman, R.M.; Appleton, I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 2006, 17, 291–306.
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int. J. Mol. Sci. 2011, 12, 5592–5603.
- Das, S.; Tanwar, J.; Hameed, S.; Fatima, Z. Antimicrobial potential of epigallocatechin-3-gallate (EGCG): A green tea polyphenol. J. Biochem. Pharmacol. Res. 2014, 2, 167–174.
- Wink, M. Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2010; p. xiii.
- Cechinel-Filho, V. Plant Bioactives and Drug Discovery: Principles, Practice, and Perspectives; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. vii.
- Grotewold, E. The Science of Flavonoids; Springer: New York, NY, USA, 2008; p. vii.
- Fraga, C.G. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology; Wiley: Hoboken, NJ, USA, 2010.
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting Multiple Signaling Pathways by Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate. Cancer Res. 2006, 66, 2500–2505.
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867.
- James, K.; Whitesell, B. An Encyclopedia of Chemicals, Drugs & Biologicals, 5nd ed.; Chapman & Hall: New York, NY, USA, 1997.
- Istenič, K.; Korošec, R.C.; Ulrih, N.P. Encapsulation of (−)-epigallocatechin gallate into liposomes and into alginate or chitosan microparticles reinforced with liposomes. J. Sci. Food Agric. 2016, 96, 4623–4632.
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539.
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151.
- Zhong, Y.; Shahidi, F. Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533.
- Bhushani, J.A.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382.
- Du, L.-L.; Fu, Q.-Y.; Xiang, L.-P.; Zheng, X.-Q.; Lu, J.-L.; Ye, J.-H.; Li, Q.-S.; Polito, C.A.; Liang, Y.-R. Tea Polysaccharides and Their Bioactivities. Molecules 2016, 21, 1449.
- Liang, R.; Chen, L.; Yokoyama, W.; Williams, P.A.; Zhong, F. Niosomes Consisting of Tween-60 and Cholesterol Improve the Chemical Stability and Antioxidant Activity of (−)-Epigallocatechin Gallate under Intestinal Tract Conditions. J. Agric. Food Chem. 2016, 64, 9180–9188.
- Paximada, P.; Echegoyen, Y.; Koutinas, A.A.; Mandala, I.G.; Lagaron, J.M. Encapsulation of hydrophilic and lipophilized catechin into nanoparticles through emulsion electrospraying. Food Hydrocoll. 2017, 64, 123–132.
- Wang, X.; Xie, Y.; Ge, H.; Chen, L.; Wang, J.; Zhang, S.; Guo, Y.; Li, Z.; Feng, X. Physical properties and antioxidant capacity of chitosan/epigallocatechin-3-gallate films reinforced with nano-bacterial cellulose. Carbohydr. Polym. 2018, 179, 207–220.
- Zhong, Y.; Ma, C.-M.; Shahidi, F. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. J. Funct. Foods 2012, 4, 87–93.
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628.
- Wang, R.; Zhou, W.; Wen, R.-A.H. Kinetic Study of the Thermal Stability of Tea Catechins in Aqueous Systems Using a Microwave Reactor. J. Agric. Food Chem. 2006, 54, 5924–5932.
- Zeng, L.M.J.; Li, C.; Luo, L.Y. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18.
- Fan, F.-Y.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.-H.; Liang, Y.-R. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354.
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J. Agric. Food Chem. 2005, 53, 9478–9484.
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12.
- Wang, R.; Zhou, W.; Jiang, X. Reaction Kinetics of Degradation and Epimerization of Epigallocatechin Gallate (EGCG) in Aqueous System over a Wide Temperature Range. J. Agric. Food Chem. 2008, 56, 2694–2701.
- Suzuki, M.; Sano, M.; Yoshida, R.; Degawa, M.; Miyase, T.; Maeda-Yamamoto, M. Epimerization of Tea Catechins and O-Methylated Derivatives of (−)-Epigallocatechin-3-O-gallate: Relationship between Epimerization and Chemical Structure. J. Agric. Food Chem. 2003, 51, 510–514.
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent advances in anticancer activities and drug delivery systems of tannins. Med. Res. Rev. 2017, 37, 665–701.
- Thangapandiyan, S.; Miltonprabu, S. Epigallocatechin gallate effectively ameliorates fluoride-induced oxidative stress and DNA damage in the liver of rats. Can. J. Physiol. Pharmacol. 2013, 91, 528–537.
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; MacKenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696.
- Shang, W.; Lu, W.; Han, M.; Qiao, J. The interactions of anticancer agents with tea catechins: Current evidence from preclinical studies. Anti-Cancer Agents Med. Chem. 2014, 14, 1343–1350.
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 894–903.
- Pal, S.; Dey, S.K.; Saha, C. Inhibition of Catalase by Tea Catechins in Free and Cellular State: A Biophysical Approach. PLoS ONE 2014, 9, e102460.
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821.
- Ross, S.A. Evidence for the relationship between diet and cancer. Exp. Oncol. 2010, 32, 137–142.
- Wu, F.; Sun, H.; Kluz, T.; Clancy, H.A.; Kiok, K.; Costa, M. Epigallocatechin-3-gallate (EGCG) protects against chromate-induced toxicity in vitro. Toxicol. Appl. Pharmacol. 2012, 258, 166–175.
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99.
- Lee, B.-L.; Ong, C.-N. Comparative analysis of tea catechins and theaflavins by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2000, 881, 439–447.
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337.
- Shishikura, Y.; Khokhar, S. Factors affecting the levels of catechins and caffeine in tea beverage: Estimated daily intakes and antioxidant activity. J. Sci. Food Agric. 2005, 85, 2125–2133.
- Rusak, G.; Komes, D.; Likić, S.; Horžić, D.; Kovač, M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. 2008, 110, 852–858.
- Reto, M.; Figueira, M.E.; Filipe, H.M.; Almeida, C.M.M. Chemical Composition of Green Tea (Camellia sinensis) Infusions Commercialized in Portugal. Plant Foods Hum. Nutr. 2007, 62, 139–144.
- Rechner, A.; Wagner, E.; Van Buren, L.; Van De Put, F.; Wiseman, S.; Rice-Evans, C. Black tea represents a major source of dietary phenolics among regular tea drinkers. Free Radic. Res. 2002, 36, 1127–1135.
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; US Department of Agriculture: Beltsville, MD, USA, 2011.




