These results imply, in accordance with many high-impact studies, that curcuminoids and flavonoids have a strong ability to suppress metastasis. Based on their effects on cancer cells and tumour tissues, possible therapeutic strategies implementing curcuminoids and flavonoids can be developed for the repression of NSCLC metastasis, normalization of the tumour microenvironment, repression of EMT, and targeting of migrating cancer cells. The effects of curcumin and flavonoids on these phenomena are described in detail in the following subsections.
2. Effects of Curcumin and Flavonoids on Tumour-Associated and -Infiltrating Cells: Suppression of CTC Support
The tumour microenvironment contains tumour support/tumour-associated cells, such as macrophages, lymphocytes, fibroblasts, and endothelial cells, and an extracellular matrix with signalling molecules
[20][21]. While “healthy” stromal cells can repress carcinogenesis, interactions of cancer cells with the tumour stroma have a strong effect on tumour development, progression, and resistance. In addition, it was observed that circulating tumour cells (CTCs) cannot migrate on their own and rather migrate in clusters with tumour-supporting/tumour-associated cells, the metastatic activity of which is an order of magnitude higher than that of CTCs alone
[22][23][24]. Therefore, targeting this circulating microenvironment
[25] is an intensively studied method of cancer treatment.
The fact that curcumin administration in patients with cancer, including lung cancer, is strongly associated with a decreased level of inflammatory factors (interleukin 6 (IL-6), interleukin 8 (IL-8), and tumour necrosis factor alpha (TNF-α)) implies its strong potential for metastasis suppression
[1]. High IL-6 activity is correlated with poor prognosis and lung-cancer-related symptoms such as fatigue, thromboembolism, cachexia, and anaemia
[26]. In lung cancer, high IL-6 activity is associated with overactivated signal transducer and activator of transcription 3 (STAT3) signalling (one mechanism of TKI resistance)
[27][28], which can lead to IL-6 overproduction and inflammation associated with tumour resistance and development
[29]. Tumour inflammation is induced by reciprocal interactions of tumour cells and tumour-associated macrophages (TAMs, the most abundant immune cells in NSCLC), followed by stimulation of TAM polarization to the M2 phenotype and repression of the M1 phenotype
[30]. The M1 (antitumour) TAM phenotype is associated with good prognosis, and the M2 phenotype (stimulated by IL-6, IL-8, and other inflammatory factors) is associated with shorter OS
[31]. According to a study by Almatroodi et al., the expression of M2 markers (CD68 and CD163) was increased in NSCLC tumour tissue compared to a control (non-tumour tissue from the same patient)
[32]. However, expression of M1 markers was decreased in patients with adenocarcinoma and squamous carcinoma; serum levels of interleukin 1 beta (IL-1β), interleukin 4 (IL-4), IL-6, and IL-8 were higher in patients with large-cell carcinoma than in healthy controls.
Some studies imply that the anticancer effect of curcumin may be associated with repression of the M2 TAM phenotype
[33][34][35]. For example, a sublethal dose of nanoformulated curcumin (c
max 0.61 µmol/l in mouse plasma) or curcumin combined with epicatechin gallate and resveratrol can revert the M2 TAM phenotype to a tumouricidal phenotype with a potent immune antitumour response, leading to tumour eradication
[33]. The decreased levels of inflammatory factors (IL-6, IL-8, and TNF-α) strongly associated with curcumin administration (180 mg/day; ~c
max 0.5 µmol/l in human plasma
[36]) in patients with cancer, including those with lung cancer
[1], imply a possible reduction of the M2 TAM phenotype. Higher levels inflammatory factors can increase the M2 TAM phenotype
[37][38]. Reduction of their levels in a mouse model of lung cancer led to upregulation of the M1 TAM phenotype
[37]. Zou et al. reported that curcumin application to patients with lung cancer leads to a transformation of Treg cells into Th1 cells and an increase in IFN-γ
[39]. It is known that the secretion of IFN-γ by Th1 cells leads to macrophage polarization into the M1 phenotype
[40]. Nevertheless, the effect of curcumin on the macrophage phenotype in NSCLC patients must be evaluated in other clinical studies.
Myeloid-derived suppressor cells (MDSCs) are cells of the immune system that can play important roles in metastatic spread
[41]. Activated MDSCs (e.g., activated by vascular endothelial growth factor (VEGF) or IL-6) induce suppression of innate and adaptive immune systems and thereby the host antitumour response. The blood level of MDSCs is a predictive marker. For example, Augustyn et al. reported that serum levels of cancer-associated macrophage-like cells (CAMLs; multinuclear myeloid cells) can significantly influence the treatment outcome of NSCLC patients (those with advanced cancer treated with chemoradiotherapy and atezolizumab)
[42]. The authors found that the levels after the chemoradiotherapy cycle correlated with the metastatic disease status and survival.
Interestingly, curcumin administration in a mouse model of carcinoma led to the maturation of MDSCs (loss of immunosuppressive effects) in spleen and tumour tissues
[43]. Additionally, the levels of CD4+ and CD8+ T cells were restored. In MDSCs, this effect was associated with suppression of reactive oxygen species (ROS), arginase (Arg-1), and inducible nitric oxide synthase (iNOS). Lio reported that curcumin could support anticancer immunity by repressing the expression of PD-L1 in cancer cells
[44].
An important immunosuppressive effect of MDSCs is the induction of CD4+ T cell differentiation to Treg cells. Via cytokines (e.g., transforming growth factor beta 1 (TGF-β1)), Treg cells suppress cancer-specific effector immune cells (CD8+ T cells) and decrease the antitumour capacity of the host
[45]. According to a study by Zou et al., patients with lung cancer have significantly higher Treg cell levels
[39]. Curcumin administration at 1.5 g per day significantly decreased Treg cells and increased Th1 cells in the peripheral system. An in vitro study showed that curcumin converted Treg cells obtained from patients into Th1 cells (which induce cancer cell apoptosis)
[46] via repression of FOXP3. Experiments in a mouse model with lung metastasis suggested that this strategy could prolong the survival of patients with metastatic disease
[47].
Cancer-associated fibroblasts (CAFs) constitute a major portion of the reactive tumour stroma and play a crucial role in tumour progression
[48]. They initiate angiogenesis (via overproduction of VEGF), promote tumour progression, and support invasiveness
[49]. In addition, some studies suggest that there is an association between CAFs and characteristics of the stem-cell-like phenotypes of NSCLC cells, such as chemoresistance and overproduction of inflammatory factors
[50][51][52]. Sung et al. reported that netrin-1 secretion by CAFs leads to overexpression of IL-6 and IL-8 by cancer cells
[53]. In a mouse model, administration of the netrin-1 antibody significantly repressed tumour growth. On the other hand, solid tumours after radiotherapy can display increased CAFs
[54]. Cho et al. found that the survival of quiescent cancer cells induced via oncogenic signalling factors (e.g., IL-1β, IL-8, TGF-β1, and epidermal growth factor (EGF)) stimulated fibroblast migration to cancer cells and their transformation into CAFs
[55]. Some studies imply that CAF metastatic effects could also be associated with CTC migration and survival
[17][56][57]. Otero et al. observed CAF−CTC clusters in blood samples from patients with metastatic cancers such as NSCLC
[56]. The interaction of such clusters with cancer cells via direct contact or signalling factors can induce drug resistance and cell proliferation and thereby enhance their metastatic potential
[17].
Ba et al. reported that the administration of 10 µM curcumin modulated the phenotype of CAFs into one of peritumour fibroblast-like cells via downregulation of the expression of alpha smooth muscle actin (α-SMA), a marker of the CAF phenotype
[58]. This transformation led to inhibition of the secretion of procarcinogenic cytokines, including TGF-β1, matrix metalloproteinase 2 (MMP-2), and stromal-cell-derived factor-1 (SDF-1). In accordance with the above findings, Wang et al. showed that curcumin-treated CAFs lost the ability to induce the metastatic potential of cancer cells (a primary cell line derived from patients with oral squamous cell carcinoma) compared to nontreated CAFs
[59]. Their ability to interact with cancer cells via gap junctions was also reduced. Another study indicating that curcumin suppresses CAF communication with cancer cells was published by Kreutz et al.
[60]. They found that administration of curcumin (30 μM) in coculture with CAFs and cancer cells suppressed TNF-α signalling and survival pathways. However, in single-cell-type cultures, these effects were not observed. Luo et al. reported that CAFs obtained from NSCLC patients (stages I–III) induced EMT of NSCLC cells (A549 and H1299)
[61]. This pattern was associated with a metabolic transition of NSCLC cells to aerobic glycolysis in association with the connexin 43 gap junction. Subsequently, overactivation of PI3K/protein kinase B (Akt) and MAPK/ERK signalling and increased mobility and invasiveness of cancer cells were observed. The expression of CAF markers in tumour tissue (α-SMA, lactate dehydrogenase isoform B, and connexin 43) was also strongly correlated (
p < 0.0001) with poor prognosis, and sometimes shorter OS and PFS.
Tumour endothelial cells (TECs) support nutritional transport to tumour tissue by inducing angiogenesis (via VEGF) and assist in leukocyte infiltration
[62]. Higher levels of TECs can lead to chemoresistance and higher metastatic activity. In a mouse NSCLC model, targeting the vascular endothelial growth factor receptor (VEGFR) and EGFR pathways overcame TKI resistance and suppressed angiogenesis
[63][64]. Similarly, Lee et al. showed that targeting TECs could repress the paclitaxel resistance of NSCLC brain metastases
[65]. Unlike normal cells, TECs display wide and leaky junctions, multiple transendothelial channels, and abnormal shunts, which contribute to the high permeability of the tumour vasculature. This phenotype transition is stimulated by VEGF
[66][67].
High levels of B cell lymphoma 2 (Bcl-2), a key antiapoptotic protein, were shown to promote
[66][67] tumour cell proliferation and invasion
[66][68]. A study in a mouse model indicated that this ability is not dependent on the tumour mass. Another important metastatic TEC function was reported by Yadav et al.
[69]. They found that endothelial cells overexpressing Bcl-2 (EC-Bcl-2) can display a higher affinity for cancer cells via overexpressed E-selectin and can decrease the apoptosis of CTCs. In the mouse model, coadministration of cancer cells with EC-Bcl-2 led to significantly higher metastatic activity. This implies that tumour-associated endothelial cells can enhance the survival of tumour cells in the blood and chaperone them to distant sites. However, their function can be significantly repressed by curcumin, and more effectively repressed by curcumin in combination with flavonoids, such as EGCG
[70]. Such applications can lead to repression of angiogenesis via decreased VEGF production
[71][72][73], blocking monocyte binding via downregulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-
kB) signalling
[74]. A model of the therapeutic effects of curcumin and flavonoids on the tumour microenvironment and tumour development is shown in
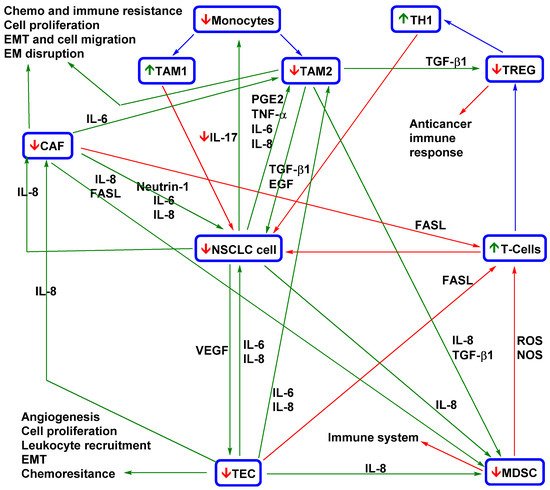
Figure 1.
Figure 1. Simplified model of curcumin and flavonoids effects on the NSCLC microenvironment
[75][1][33][43][44][47][53][55][59][60][71][72][73][74][76][77][78][79][80][81][82]. A necessary part of igenesis and CTC spreading is the interaction of NSCLC cells with tumour-associated cells. NSCLC cells recruit monocytes via IL-17 into tumour tissue. Signalling factors (e.g., IL-6, IL-8, TNF-α, and PGE2) produced in the tumour microenvironment stimulate monocyte differentiation into TAM2 (which support NSCLC cell proliferation, EMT, chemoresistance and immune resistance, and EM disruption). MDSCs recruited via IL-8 and TGF-β1 repress the cytotoxic effects of T cells against NSCLC cells and induce their differentiation into Treg cells that are responsible for the suppression of the host immune response. VEGF-recruited TECs affect EMT, chemoresistance, and the proliferation of NSCLC cells, recruit MDSCs and induce angiogenesis. IL-8-activated CAFs decrease the anticancer immune response (with the support of TAM2 and MDSCs) and repress T cells. CAFs stimulate EMT and induce NSCLC cell proliferation, migration, and drug resistance. Tumour-associated cells help sustain the tumour microenvironment and aggressive metastatic phenotype. TAM2, CAFs, and TECs are co-inducers of EMT and thereby CTC spreading, chemoresistance, and immune resistance. MDSCs and Treg cells repress the host immune response and thereby support CTC survival in the blood. Nevertheless, the anticancer effect of curcuminoids and flavonoids is not dependent on targeting of NSCLC cells, as they repress other important parts of the complex tumour ecosystem. The application of such agents is associated with stimulation of the immune system (higher levels of TAM1 and T cells; lower levels of Th1 cells, TAM2, and Treg cells; and lower recruitment of monocytes and MDSCs). Curcuminoids and flavonoids also decrease the levels of CAFs and TECs and repress their interaction with NSCLC cells. In addition, the application of curcuminoids and flavonoids leads to a decrease in the proliferation, survival, chemoresistance, immunoresistance, and migration ability of NSCLC cells. CAF, cancer-associated fibroblast; EM, extracellular matrix; EMT, epithelial–mesenchymal transition; EGF, endothelial growth factor; FasL, Fas ligand; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin 8; PGE2, prostaglandin E2; NOS, nitric oxide species; ROS, reactive oxygen species; TAM1, tumour-associated macrophage M1; TAM2, tumour-associated macrophage M2; TEC, tumour endothelial cell; TGF-β1, transforming growth factor beta 1; Th1, T helper 1; Treg, regulatory T; TNF-α, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor. Green arrow = induction/activation of factor/phenomenon/cell; red arrow = repression/inhibition of factor/phenomenon/cell; blue arrow = differentiation of immune cells;
↑ = curcumin/flavonoids activation/induction;
↓ = curcumin/flavonoids repression/inhibition.
CTC spreading from tumours is not an isolated phenomenon but a central part of the complex process underlying the development of metastases. Tumour-associated cells can support CTC metastatic activity in several ways. As part of the tumour microenvironment, they can induce an aggressive metastatic phenotype with high production of CTCs (e.g., an EMT phenotype), protect CTCs in the bloodstream, and assist in metastasis formation (see the next subchapter). These phenomena suggest that repressing tumour-associated cells could be an important part of CTC targeting. For example, higher lymphocyte infiltration in breast cancer patients was associated with higher CTC counts and metastatic relapse
[83]. Additionally, higher Treg cell levels and neutrophil-to-lymphocyte ratios can induce CTC spreading
[84][85]. CTCs in clusters with CAFs display higher survival in the bloodstream
[17]. Osmundski et al. found that TAM-associated macrophages can stimulate an aggressive phenotype of prostrate CTCs, including high adherence and plasticity
[86]. In breast cancer, a decrease in CD8+ T cells and IFN-γ can lead to an increase in the CTC count
[87]. On the other hand, activated NK cells can repress metastasis via CTC killing
[88].
In addition, the application of curcuminoids and flavonoids can significantly lower CTC counts
[89][90]. Because these agents are multifunctional, their effects on tumour-associated cells should also be considered.
The above results show that curcuminoids and flavonoids repress CTC spreading induced by the tumour microenvironment and metastatic activity by targeting tumour-associated cells, including circulating cells. Relevant in vivo clinical trials show that the application of curcuminoids and flavonoids could greatly enhance NSCLC treatment
[1][13][91]. However, the above data were mostly obtained from animal models of various oncological diseases. Therefore, more clinical trials are required to design therapeutic applications.
3. Effect of Curcumin and Flavonoid Applications on EMT and Metastasis Formation
Two important phenomena that are strongly associated with CTC spreading in lung cancer are EMT and mesenchymal–epithelial transition. First, EMT (induction of the TAM M2 phenotype, CAFs, and TECs) causes polarity loss and cell/matrix adhesion of cancer cells, aids digestion of the extracellular matrix, and supports migratory properties (e.g., actin polymerization)
[92]. In the next step, cancer cells are taken up in the bloodstream and become CTCs. CTCs are expected to undergo transitions. In the metastatic site or in the bloodstream, CTCs undergo mesenchymal−epithelial reverting transition (MErT) and obtain increased cell adhesion, and the most aggressive of them can form metastatic tumours
[93]. CTC spreading can also be stimulated by macrophages, which provide immunoprotection and growth promotion for the formation of metastases
[29]. Manjunath et al. found that a higher number of CTCs with expression of PD-L1 and mesenchymal markers (vimentin and N-cadherin) was significantly associated with reduction of PFS
[94]. Another important role of EMT in metastatic spread could be stimulation of resistance of CTCs to treatment. For example, PD-L1-expressing CTCs obtained from NSCLC patients displayed spindle-like elongated morphology, which corresponded to EMT-associated nivolumab resistance
[95][96]. PD-L1-negative CTCs were mostly small and regularly shaped (round). Raimondi et al. reported the coexpression of vimentin with PD-L1 in these cells
[96].
Relevant studies imply that curcumin and flavonoids can repress the mesenchymal phenotype. Liang et al. reported that curcumin administration (100 mg/kg) reversed tobacco-smoke-induced EMT alterations in a mouse model
[97]. This process was associated with increased expression of epithelial markers (E-cadherin) and decreased expression of mesenchymal markers (vimentin and N-cadherin). Similarly, Chang et al. found that quercetin repression of EMT via Akt downregulation was strongly associated with a reduction in bone metastasis in an NSCLC mouse model
[98].
Knowledge about MErT is still limited. Several studies suggest that E-cadherin overexpression, which enables CTCs to adhere to target tissue and survive within ectopic metastatic microenvironments, is key in the MErT process
[93][99][100][101]. MErT can also be significantly influenced by the presence of TAMs. Yang et al. reported that the M1 TAM phenotype can support MErT by stimulating E-cadherin expression and thereby enhance cancer cell colonization
[102]. Infiltrating cancer cells that penetrate healthy tissues can likely survive in the dormant state even with a lower metabolic load. Their eradication is complicated.
Survival of dormant cancer cells can occur through induction of the hypoxia phenotype and NRF2 and their activation by Wnt/β-catenin signalling
[103][104]. In the case of micrometastasis, cancer cell development and survival are strongly dependent on higher ROS levels
[105]. Curcuminoids and flavonoids can repress oxidative stress and inflammatory factors in healthy tissues and cells
[106][107][108][109] and are potent inhibitors of Wnt/β-catenin signalling
[110][111]. For example, Tabasum et al. reported that fisetin (10 µmol/l) inhibited the expression of β-catenin, NF-κB, EGFR, and STAT3 and decreased stem cell phenotype markers (CD44 and CD133) and TKI resistance in NSCLC cell lines (A549 and H1299)
[110]. Targeting STAT3 was found to block TEC activation by cancer cells in metastatic brain sites and thereby suppress metastasis formation
[112]. In addition, curcumin and flavonoid administration (apigenin, flavone, and 4′,7-dihydroxyflavone) can modulate the hypoxic phenotype of NSCLC cells
[113][114].
Importantly, the iron chelation ability of flavonoids may play a significant role in repressing micrometastasis. Fryknäs et al. reported that iron chelators are potent agents for targeting quiescent cancer cells
[115]. The suppressive effects of curcumin and flavonoids on metastatic spread and development are shown in
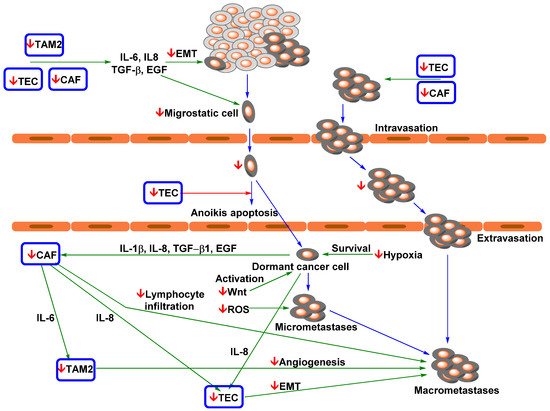
Figure 2.
Figure 2. Simplified model of curcumin and flavonoids action on NSCLC metastasis
[75][76][97][98][103][104][105][110][111][112][113][114][116][117][118][119][120][121][122][123]. 1. NSCLC cells with a mesenchymal phenotype partially induced by TAM2, TECs, and CAFs migrate from tissue into blood vessels. 2. CTCs are transported (via passive transport) to distant metastatic site(s), and TECs can serve as guardians against anoikis apoptosis. 3. Infiltrating NSCLC cells revert to an epithelial phenotype via MErT (which possibly occurs in blood). 4. Dormant cancer cells activated by Wnt signalling start recruiting CAFs and TECs and form micrometastases. 5 Micrometastases stabilised by oxidative stress recruit tumour-associated cells to form macrometastases, at which point metastatic NSCLC can be diagnosed in patients. TECs and CAFs can support the formation of highly aggressive and metastatic CTC clusters. CAF, cancer-associated fibroblast; EMT, epithelial–mesenchymal transition; EGF, endothelial growth factor; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin 8; PGE2, prostaglandin E2; ROS, reactive oxygen species; TAM2, tumour-associated macrophage M2 phenotype; TEC, tumour endothelial cell; TGF-β1, transforming growth factor beta 1; VEGF, vascular endothelial growth factor. Green arrow = induction/activation of factor/phenomenon; red arrow = repression/inhibition of factor/phenomenon; blue arrow = transition between individual steps of metastatic spread;
↓ = curcumin/flavonoids repression/inhibition.
In this process, in addition to targeting signalling pathways of NSCLC cells, curcumin can modulate processes in tumour-associated cells. The presence of TAM2 in the tumour microenvironment of lung adenocarcinoma contributes to an invasive phenotype and induces the expression of EMT-related markers
[124]. Zhang et al. reported that curcuminoids are able to reverse the macrophage phenotype from TAM2 to TAM1 by inhibiting STAT3 signalling
[125]. In glioblastoma, curcumin treatment elicited activation of antitumour STAT1 signalling and the expression of iNOS in TAMs
[34]. Mukherjee et al. found that a sublethal dose of TriCurin (curcumin in combination with resveratrol and epicatechin gallate; ~ nM concentration in plasma) in a mouse model changed TAMs to the M1 phenotype and was associated with strong repression of tumour growth
[35]. Nevertheless, Murakami et al. found that curcumin inhibited lipopolysaccharide-induced expression of inducible forms of both nitric oxide synthase and cyclooxygenase in RAW 264.7 cells (murine macrophages)
[126]. This finding could mean that curcumin could also target micrometastases by lowering inflammatory processes and oxidative stress.
Zeng et al. found that curcumin induces endoplasmic reticulum stress, loss of mitochondrial potential, cell cycle arrest at the G2−M transition, and apoptosis in prostate CAFs. The sensitivity of natural fibroblasts was approximately three times lower than that in treated CAFs
[127]. Ba et al. reported that a sublethal curcumin dose (10 µM) decreased the expression of α-SMA, MMP-2, SDF-1, and TGF-β1, and Smad2/3 phosphorylation/activation by approximately half
[58]. Curcumin can also suppress CAF-induced EMT of cancer cells. Du et al. found that in CAFs, monoamine oxidase A (a mitochondrial enzyme) induces mammalian target of rapamycin (mTOR)/hypoxia-inducible factor 1 alpha (HIF-1α) signalling
[128]. In prostate cancer cells, this CAF phenotype can induce ROS production and C-X-C motif chemokine receptor 4 (CXCR4) and IL-6 receptor expression and thereby cell migration and invasion. Nevertheless, curcumin application suppresses the activation of HIF-1α signalling and subsequently suppresses EMT in cancer cells. In a mouse model of pancreatic cancer, it was observed that curcumin-induced EMT suppression via CAF targeting could repress cancer metastasis
[59].
Recruitment of TECs by cancer cells is associated with activation of JAK/STAT3 signalling via IL-8. Nevertheless, application of curcumin in combination with EGCG (50 mg/kg per day) in a mouse model of colorectal carcinoma led to suppression of JAK/STAT3 signalling in endothelial cells and thereby their recruitment by cancer cells
[70]. Similarly, the therapeutic effect of curcumin in glioblastoma also causes a reduction in TEC recruitment
[129]. TEC presence was found to correlate with EMT and metastasis in various cancer models
[130][131][132]. These effects, however, may be due to recruitment of leukocytes by TECs. NSCLC patients can have significantly increased blood levels of TNF-α
[133]. Kumar et al. reported that endothelial cells induced TNF-α expression via activation of products of NF-KB signalling (adhesion factors; e.g., intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1, and endothelial leukocyte adhesion molecule-1)
[74]. Nevertheless, curcumin can effectively revert this phenotype of endothelial cells and subsequently monocyte adhesion.
In addition, some studies suggest that curcumin also repressed cluster formation. Taftaf et al. reported that depletion of ICAM-1 in a mouse patient-derived xenograft (PDX) model of triple-negative breast cancer significantly inhibited cluster formation, tumour cell transendothelial migration, and lung metastasis
[134]. Yang et al. found that curcumin inhibited the IL-6/STAT3 pathway in NCI-H446 and NCI-1688 small carcinoma cells, leading to suppression of VEGF, MMP-2, MMP-7, and ICAM-1
[135]. Nevertheless, in A549 cells, IL-1β can induce ICAM-1 expression via the NF-kB and Src/PDGFR/PI3K/Akt pathways
[136][137]. However, in the presence of curcumin, ICAM-1 expression is repressed
[137].
One of the key factors that control CTC acceptance and possible micrometastasis formation is induction and stimulation of the supportive microenvironment (called pre-metastatic niche
[138]) in distance organs by primary tumour
[139]. Tumour cells display an ability to selectively modify the microenvironment of distant organs via extracellular vehicles (e.g., exomes). They are nano-sized membranous structures liberated from cells into extracellular space
[140]. Exosomes transport proteins, RNA, DNA, miRNA, and lipids. Exosomes can mediate communication between different cell types from various tissue and organs.
Numerous high-impact studies imply that tumour exosomes strongly support tumourgenesis and metastatic formation
[139]. Ma et al. reported that exosomes isolated from NSCLC patients displayed significantly higher levels of some miRNAs (e.g., miR-3157-3p, miR-3613-5p, and miR-3921) against healthy controls
[141]. In addition, metastatic patients have higher expression of miR-3157-3p (exosomes and tumour tissues) than comparative nonmetastatic ones. A549 cells with transferred miR-3157-3p display an increase in protein levels of VEGF, MMP2, and MMP9, and their exosomes can induce vascular permeability of endothelial cells and angiogenesis. In the mice model, exosomes with miR-3157-3p stimulate higher microvessel density and larger tumour tissue.
It was proven that curcuminoids and flavonoids are potent suppressor of MMPs actives and VEGF signalling
[142][143]. This suggests that they could suppress the role of exosomes in the formation of the metastases. Kaplan et al. found that bone-marrow-derived haematopoietic progenitor cells (BMDCs) with expression of vascular endothelial growth factor receptor 1 (VEGFR1) significantly participate in the formation of pre-metastatic sites in mice with Lewis lung carcinoma
[138]. VEGFR1 repression by specific antibody suppresses BMDCs clusterisation and prevents tumour metastasis. Their cluster formation in the pre-metastatic site was associated with MMP9 induction. Nevertheless, their effect on tumour-derived exosomes was much more complicated. It was observed that curcumin-exposed cancer cells liberated exosomes. It has been shown that treatment of cancer cells with different doses of curcumin leads to the release of exosomes containing curcumin
[144].
Unlike classical tumour exomes, curcumin-induced exosomes display anticancer effects in recipient cells and reduce tumour growth. For example, exosomes produced by H1299 cells (10 μM curcumin for 48 h) display upregulation of the transcription factor 21 (TCF21) via downregulation of DNA Methyltransferase 1
[145]. The level of the TCF21 mRNA is inversely correlated with poor prognosis in patients with lung adenocarcinoma
[146] and the aggressivity of NSCLC cell lines
[145]. In the presence of exosomes derived from curcumin-pre-treated H1299 cells, BEAS-2B cells displayed a significant decrease in proliferation, colony formation, and migration.
Exosomes derived from K562 leukemic cells increase production of IL8 and VCAM1 in endothelial cells; nevertheless, after curcumin treatment (20 μM for 24 h), the obtained exosomes displayed opposite effects and inhibited tube formation and vascular permeability in the endothelial cells
[147]. In K562 cells, curcumin caused a decrease of cellular miR-21, while it increased miR-21 selective packaging in exosomes. Its decrease can induce PTEN expression (target of miRNA-21)
[148]. In the endothelial cells, higher levels of miR-21 can supress their angiogenic capacity, directly targeting RhoB, a critical regulator of actin dynamics
[149].
Exosomes derived from the TS/A cell line (murine mammary adenocarcinoma) displayed a strong inhibition effect on cytotoxicity of the IL-2 activated NK cells
[150]. Nevertheless, exosomes isolated from the cells pre-treated with polyphenols (curcumin, baicalin, or genistein, 1 µM for 36 h) cause a significant reduction of immunosuppression of NK cells and increase their cytotoxicity against cancer cells. Other polyphenols, including biochanin A and quercetin, had no significant effects.
The cited studies prove that curcuminoids and flavonoids are effective agents for the repression of CTC spreading because they target EMT in primary tumours. We can assume that they also suppress macrometastasis formation by targeting the activation and survival of dormant cancer cells. In addition, the formation of macroscopic metastasis is accompanied by a second EMT event
[103][121], which is a possible target for curcuminoids and flavonoids. However, knowledge of this phenomenon is very limited, and the presented model of metastasis formation needs to be validated in NSCLC.
4. Effect of Curcumin and Flavonoids on the Migration of Cancer Cells
Another discussed strategy for metastasis suppression is targeting the mobility of cancer cells. At present, some high-impact studies strongly recommend targeting cytoskeletal dynamics as an optimal method to suppress metastatic activity
[151].
Chen et al. reported that curcumin administration to the 801D (human large-cell lung carcinoma) cell line led to significant inhibition of EGF- or TGF-β1-induced lung cancer cell migration and invasion
[152]. These inhibitory effects of curcumin were related to the inhibition of Rac1/PAK1 signalling pathways, MMP-2/9 expression, and actin cytoskeleton rearrangement. In a mouse model, curcumin (60 mg/kg) displayed a comparable effect on tumour volume and metastatic potential to cisplatin (8 mg/kg).
Flavonoid administration could lead to repression of cancer cell mobility. Incubation of A549 cells with quercetin resulted in dose-dependent disorganization of the actin cytoskeleton
[153].
The incorporation of curcumin in cancer treatment regimens aimed at erasing primary tumours may aid targeting and suppress metastases. High-impact studies of animal models strongly imply a high potential of this approach. For example, the combination of cisplatin and curcumin led to a significant reduction in lymph node metastasis and primary tumour size
[122]. Application of isorhamnetin in combination with cisplatin and carboplatin led to inhibition of cancer cell migration
[154]. This effect was associated with the induction of microtubule depolymerization by isorhamnetin.
In addition to suppressing cancer metastasis, curcumin also has a cytostatic effect. For example, Mirza et al. reported that curcumin administration could lead to specific killing of circulating metastatic cells
[123]. The authors reported that cancer cells obtained from peripheral blood of patients with lung adenocarcinoma displayed a cancer-stem-cell-related phenotype (expression of P-glycoprotein 1 (CD44), prominin-1 (CD133), and aldehyde dehydrogenase). These cells also had strong chemoresistance; for example, gemcitabine, even at concentrations higher than 100 μM, did not cause any significant cytotoxicity. Treated cells showed a high response to a low concentration of nanoformulated curcumin (IC
50 = 10 µM). This effect was coupled with inhibition of the DNA repair mechanism and DNA damage. In contrast, the cytotoxic effect against healthy cells (peripheral blood mononuclear cells) was lower. Possible effects of curcumin on NSCLC cells are shown in
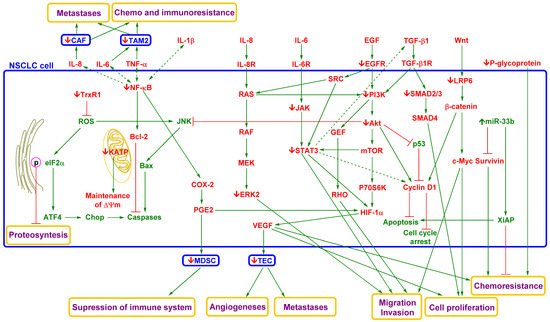
Figure 3.
Figure 3. Simplified model of the effects of curcumin on NSCLC cells
[10][82][116][117][118][119][122][123][152][155][156][157][158][159][160]. NSCLC is associated with dysregulation of numerous signalling and regulatory pathways. Some signalling factors (e.g., IL-6, IL-8, EGF, TGF-β1, and Wnt) produced by cancer or tumour-associated cells can induce migration and invasion phenotypes of NSCLC cells and thereby support CTC spreading. These pathways are interconnected, and dysregulation of one can induce dysregulation of another and modulate therapeutic targeting. Nevertheless, curcumin targets multiple signalling pathways to repress this phenomenon. Its effect is associated with repression of ERK2, JAK, STAT3, EGFR, PI3K, Akt, SMAD 2/3, and β-catenin. In addition, curcumin induces oxidative stress in the endoplasmic reticulum by inhibiting TrxR1 and mitochondrial-dependent and mitochondrial-independent apoptotic pathways and repressing drug resistance. Reduced levels of signalling factors produced by NSCLC cells, such as IL-6, 8, VEGF, and PGE2, lead to decreased activity of tumour-associated/tumour-infiltrating cells and thereby decreased support of NSCLC cell metastasis, proliferation, and survival in the tumour microenvironment. Curcumin’s effects on the tumour microenvironment also include activation of the immune system and suppression of angiogenesis and chemoresistance and immune resistance. Akt, protein kinase B; Bcl-2, B cell lymphoma 2; CAF, cancer-associated fibroblast; COX-2, cyclooxygenase-2; EGF, endothelial growth factor; EGFR, endothelial growth factor receptor; ERK2, extracellular signal-regulated kinase 2; GEF, guanine nucleotide exchange factor; HIF-1α, hypoxia-inducible factor 1α; JAK, Janus tyrosine kinase; JNK, c-Jun N-terminal kinase; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-6R, interleukin 6 receptor; IL-8, interleukin 8; IL-8R, interleukin 8 receptor; KATP, ATP-sensitive potassium channel; LRP-6, low-density lipoprotein receptor-related protein 6; MAPK, mitogen-activated protein kinase; MDSC, myeloid-derived suppressor cell; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; P70S6K, ribosomal protein S6 kinase beta-1; p-eIF2α, phosphorylated eukaryotic translation initiation factor 2 subunit 1; PGE2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription 3; SRC, intracytoplasmic tyrosine kinase; TAM2, tumour-associated macrophage M2 phenotype; TEC, tumour endothelial cell; TGF-β, transforming growth factor beta; TGF-β1R, transforming growth factor beta 1 receptor; TNF-α, tumour necrosis factor alpha; Trx1, thioredoxin reductase 1; VEGF, vascular endothelial growth factor; ΔΨm, mitochondrial membrane potential. Green arrow = induction/activation of factor/phenomenon/cell, dotted (indirect); red arrow = repression/inhibition of factor/phenomenon/tumour-supporting cell; green factor = anticarcinogenic factor; red factor = carcinogenic factor;
↑ = curcumin activation/induction;
↓ = curcumin repression/inhibition.
In NSCLC, curcumin represses some important signalling pathways. It inhibits the activation/phosphorylation of JAK and STAT3 (part of the EGF and IL-6 signalling pathways)
[161]. STAT3 is constitutively activated in approximately 50% of NSCLC primary tumours and NSCLC cell lines and is associated with poor prognosis
[162][163][164]. Jiang et al. reported that in A549 cells, curcumin application led to not only decreased EGFR expression but also reduced EGFR activity via induction of ubiquitin-activating enzyme E1-like
[165]. In human samples (NSCLC tumour tissues and adjacent tissues from NSCLC patients (stages I-IIIa)), there was an inverse correlation between curcumin administration and the activity of the EGFR/AKT/NF-κB pathway.
In athymic nude mice bearing NCI-H460 tumours, curcumin-induced inhibition of JAK and STAT3 phosphorylation led to reduced tumour weight and improved the survival rate of mice. In addition, STAT3-regulated promoter activation of VEGF, Bcl-xL, and cyclin D1 was also repressed after treatment
[161]. Targeting STAT3 thus represents another promising approach for reducing CTC counts. Zhang et al. reported that suppression of the JAK/STAT3 pathway reduced CTC seeding in primary tumours (with a nude mouse model of human osteosarcoma)
[166]. Mautsaka et al. found that the CTC EGFR expression relative to baseline was strongly associated with regorafenib resistance in patients with refractory metastatic colorectal cancer
[167].
One of key factors of NSCLC pathogenesis is NF-κB. Details on its role were described by Dimitrakopoulos et al.
[168]. NF-κB has been found to control inflammation, proliferation, survival, apoptosis, angiogenesis, EMT, metastasis, stemness, metabolism, and therapy resistance
[169][170]. For example, Sun et al. reported that activation of the PI3K/Akt/NF-κB/tyrosine kinase B (TrkB) pathway led to resistance of cancer cells to anoikis and thereby higher metastatic activity in a mouse model of hepatocellular carcinoma
[171].
An interesting approach is using microRNAs (miRNAs) as therapeutics. The striking advantage of miRNAs is that a single miRNA has the potential to simultaneously suppress several oncogenic pathways because it can target multiple genes. Naidu et al. reported targeting of the miR-23b cluster, or miR-125a-5p, which silenced KRAS and NF-κB signalling and resulted in significant repression of the tumourigenicity of CTCs from NSCLC patients in a mouse model
[172]. Similarly, Lin et al. showed that TNF-α-stimulated degradation of IkappaB-alpha and translocation of NF-κB into the nucleus subsequently induce MMP-9 expression in A549 cells
[173]. Nevertheless, curcumin application led to the suppression of IκB-α phosphorylation and thereby NF-κB activation
[174]. Lee et al. reported that interferon alpha (IFN-α)-induced activation of NF-κB and COX-2 was inhibited by curcumin in A549 cells
[175]. Accordingly, curcumin inhibition of the migratory and invasive abilities of NSCLC cells seems to be mediated through the NF-kB/MPP pathway according to studies in a mouse model
[176][177].
On the other hand, CTC survival can be associated with deacetylase sirtuin 1 (SIRT1) expression, which leads to suppression of NF-κB and ROS activity in some oncological diseases, such as breast cancer
[178]. However, the role of SIRT1 expression is not clear, as it can have oncogenic and antitumour effects. For example, repression of cancer cell migration and angiogenesis via activation of both intrinsic (caspase-9) and extrinsic (caspase-8) apoptotic pathways was found to be associated with SIRT1 activation by curcumin in squamous cell carcinoma of the head and neck
[179]. Nevertheless, in human colon cancer cells, the repressive effect of curcumin on cell viability and migration caused inactivation of SIRT1 via covalent modification of the cysteine 67 residue
[180].
A great benefit of curcumin application is its targeting of hypoxia-related metabolism in NSCLC. Cancer cells, which are located further from blood vessels due to their faster metabolism, have lower oxygen levels. Therefore, hypoxic tumours display significantly lower oxygen pressure (10 mmHg or less)
[181]. Hypoxia-activated HIF-1α can stimulate carcinogenesis via induction of numerous signalling pathways (TGF-β1, EGF, Wnt, and Notch), transcription factors (Snail, Slug, Twist, and Zeb1/2) and other factors
[182][183]. These effects lead to the induction of a cancer stem cell phenotype with features such as immune and drug resistance, a mesenchymal phenotype, and metastatic activity. In metastases, HIF-1α levels have been found to be significantly increased compared with those in primary breast tumours
[184]. This finding suggests the importance of HIF-1α levels in CTC spreading.
Hypoxic CTCs have a greater chance of survival in the bloodstream because of their PD-L1 expression
[185]. Nevertheless, the influence of hypoxia on CTC metastatic activity is complicated. Donato et al. reported that in a breast cancer model under hypoxic conditions, liberated CTC clusters contained cells with a hypoxic phenotype
[186]. In contrast, in normoxia, liberated tumour cells have a normoxic phenotype. However, knockout of HIF-1α did not repress cluster formation. Moreover, knockout of VEGF (an angiogenic factor induced by HIF-1α) led to tumour shrinkage but also supported cluster formation.
It has been proven that curcuminoids could represent suitable structural motifs for targeting HIF-1α hypoxia-related signalling. For example, Li et al. reported that curcumin significantly decreased the expression of HIF-1α and VEGF in a mouse model of lung cancer (A549 cells)
[177]. Ye et al. reported curcumin-induced repression of HIF-1α, which led to downregulation of P-glycoprotein expression and increased chemosensitivity of A549 cells
[187]. Similarly, Fan et al. observed decreased levels of HIF1α, p-mTOR/mTOR, VEGF, and VEGFR in a mouse model of Lewis lung carcinoma
[188].
An important anticancer effect of curcumin could be its repression of Smad 2/3 phosphorylation. Datta et al. reported that curcumin represses Smad 2/3 phosphorylation in TGF-β1-dependent H358 and A549 (NSCLC) cell lines
[189]. However, no difference in curcumin-induced toxicity or changes in tumourigenicity were reported between the TGF-β1-dependent cells and ACC-LC-176 cells (an NSCLC line independent of TGF-β1). Nevertheless, some results obtained from studies of other cancer types imply that curcumin can inhibit TGF-β1-induced EMT, invasion, and IL-6 expression, and thereby cancer metastasis
[116][190][191][192][193][194].
Targeting the Wnt/β-catenin pathway is an intensively studied method in cancer treatment. Wang found that the suppressive effect of curcumin on β-catenin expression was mostly caused by the induction of oxidative stress in NSCLC cells (A549)
[111]. Lu et al. reported that administration of curcumin to NSCLC cells (95D and A549) reduced the overexpression of metastasis-associated protein 1, which caused a decrease in Wnt/β-catenin signalling (downregulation of β-catenin, cyclin D1, and MMP-7)
[195]. Wen et al. reported that targeting Wnt/β-catenin signalling could lead to a reduction in CTC counts in oncology patients
[196].
Flavonoids represent potent agents for the suppression of cancer spreading. They can decrease the activity and expression of numerous factors associated with NSCLC metastatic activity, such as NF-κB, STAT3, Akt, β-catenin, VEGF, HIF-1α, EGFR, and mTOR. For example, QSAR modelling strongly implies that EGCG is a potent EGFR binder
[197]. Minnelli et al. reported that the interaction energies were 98, 54, and 75 kcal/mol for wild-type EGFR, T790M/L858R-mutated EGFR, and ELREA (EGFR with deletion of five amino acids in exon 19), respectively
[198]. For comparison, the obtained values for erlotinib were 71 (wild-type EGFR), 39 (T790M/L858R-mutated EGFR), and 97 kcal/mol (ELREA). Liu et al. found that EGCG is a dual inhibitor of PI3Kα (IC
50 = 0.69 µM) and mTOR (IC
50 = 0.12 µM), an inhibitor of EMT, and can overcome gefitinib resistance
[199]. Zhang et al. found that EGCG not only reduces the active form of NF-κB but also directly impacts this factor (K
D = 4.8 × 10
−5 M)
[200]. Rawangkan et al. reported that EGCG pretreatment of Lu99 cells (an NSCLC cell line) strongly decreased PD-L1 expression and induction by EGF and IFN-γ
[201].
Both EGFR mutations and PD-L1 overexpression enhance CTC and metastatic cell spreading in NSCLC. CTCs with EGFR mutations and PD-L1-expressing CTCs have been shown to correlate with shortened OS, disease progression, formation of metastases, and therapeutic failure (
Section 3.1 and
Section 3.2). As such, agents with low toxicity, such as flavonoids, could represent promising tools for decreasing CTC counts and thereby suppress metastasis.
The application of flavonoids can, via various independent mechanisms, repress NSCLC cell survival, proliferation (e.g., modulation of cyclin-dependent kinases, caspase induction, activation of apoptotic factors, and repression of survival) and migration; for example, flavonoids can inhibit MMPs. Suzuki et al. described EGCG binding with the β- and γ-chains of fibrinogen, which represses fibrinogen interaction with NSCLC cancer cells (e.g., LL2-Lu3 cells) and thereby impairs their metastatic spread
[202].
Although curcumin and flavonoids display various toxic effects on cancer cells, they are surprisingly less toxic to normal cells. A possible explanation could be their effect on the redox homeostasis of cells
[203]. Higher ROS levels are strongly associated with characteristics of carcinogenesis, such as increased mutations and dysregulation of signalling cascades (MAPK, PI3K/Akt, Nrf2, AP-1, NF-κB, STAT3, and p53)
[204][205]. Curcumin and flavonoids are potent antioxidants and can protect normal cells against the carcinogenesis and apoptosis induced by ROS. On the other hand, ROS generation is part of numerous therapeutic strategies
[205][206], and the application of antioxidants can be counterproductive. Some clinical trials have found that β-carotene and retinol can promote tumour growth and metastasis in cancer patients
[207][208]. Godman et al. found that their application is associated with a higher risk of NSCLC in female patients
[208].
However, hypermetabolism of cancer cells results in higher production of ROS and antioxidant capacity. Curcumin dysregulates redox balance by disrupting mitochondrial homeostasis via oxidative stress. It causes opening of the mitochondrial permeability transition pore, mitochondrial swelling, loss of mitochondrial membrane potential, and inhibition of ATP synthesis
[209][210]. Curcumin can also supports the mitochondrial apoptotic pathway by inducing overexpression of pro-apoptotic Bax protein and reduced expression of Bcl-2 in NSCLC cells
[211]. Moreover, the cytotoxic effect of curcumin or its analogues can be detected via the accumulation of curcuminoids in the ER and the upregulation of the ER stress-related unfolded protein response, which leads to inhibition of protein synthesis and cell cycle arrest
[211][212]. In addition, curcuminoid application leads to increased intracellular ROS levels and increased SOD and γ-GCS activity
[111]. Additionally, flavonoids such as fisetin can induce oxidative stress in cancer cells
[213].
Another possible explanation for the high cell selectivity of curcuminoids and flavonoids is their effects on cellular hypoxia. These agents can significantly induce higher toxicity in hypoxic cancer cells
[214][215]. However, in the case of normal cells, their application has protective effects and leads to restoration of normal metabolism
[215][216]. For example, in ischaemic muscles (which have a decrease in oxygen level due loss of blood flow), curcumin helps tissue restoration
[188].
Cancer cells have higher concentrations of and need iron ions
[217]. Targeting iron homeostasis with flavonoid chelators such as quercetin has been intensively studied in anticancer treatment and is usually highly specific for cancer cells
[218]. The anticancer effects of curcuminoids are also associated with iron chelation
[219][220]. Nevertheless, higher levels of transition metal ions can lead to higher levels of flavonoids and curcumin metal complexes. These complexes also likely have their own biological/anticancer activities
[221]. For example, Tan et al. found that the anticancer effect of iron−flavonoid complexes is associated with DNA targeting
[222]. Chen et al. reported that hyperoside activity against hypoxic A549 cells was significantly higher in the presence of iron ions
[223]. Similarly, iron−curcumin complexes (IC
50 = 8 μM) displayed higher cytotoxicity against MDA-MB-231 breast cancer cells than curcumin (IC
50 = 24 μM)
[224].
In addition, antimetastatic effects can be achieved by using a noncytotoxic dose
[110][225]. Tabasum et al. observed that fisetin (10 μM) decreased the expression of NF-κB, STAT3, and β-catenin, and repressed EMT in A549 and H1299 cells
[110].
The above findings imply that curcumin and flavonoids are prospective agents for incorporation in NSCLC therapy with effective targeting against cancer cell migration. However, oncogenic signalling pathways display strong redundancy; therefore, inhibiting one signalling pathway may not be enough. Incorporating multiple targeted agents, such as curcuminoids, flavonoids, or their combination, in therapeutic regimens could effectively avoid this phenomenon. This strategy could control the growth of tumours or shrink their volume with a reduced risk of metastasis. Many high-impact studies have shown that these compounds are potential agents for combination therapy
[226][227][228][229][230]. Their application can significantly increase the efficiency of classically used therapies (e.g., chemotherapy, TKIs, immunotherapy, and radiotherapy) and repress tumour resistance. However, the anticancer effects of curcuminoids and flavonoids discussed above were mostly seen in in vivo and in vitro studies. A meaningful assessment of their potential therapeutic effects is not possible without other clinical trials.