Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elina Yankova-Tsvetkova | + 3829 word(s) | 3829 | 2021-10-27 05:13:13 | | | |
| 2 | Vivi Li | -79 word(s) | 3750 | 2021-11-10 03:05:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yankova-Tsvetkova, E. Reproductive Potential in Primula veris L. (Primulaceae). Encyclopedia. Available online: https://encyclopedia.pub/entry/15837 (accessed on 07 February 2026).
Yankova-Tsvetkova E. Reproductive Potential in Primula veris L. (Primulaceae). Encyclopedia. Available at: https://encyclopedia.pub/entry/15837. Accessed February 07, 2026.
Yankova-Tsvetkova, Elina. "Reproductive Potential in Primula veris L. (Primulaceae)" Encyclopedia, https://encyclopedia.pub/entry/15837 (accessed February 07, 2026).
Yankova-Tsvetkova, E. (2021, November 09). Reproductive Potential in Primula veris L. (Primulaceae). In Encyclopedia. https://encyclopedia.pub/entry/15837
Yankova-Tsvetkova, Elina. "Reproductive Potential in Primula veris L. (Primulaceae)." Encyclopedia. Web. 09 November, 2021.
Copy Citation
Primula veris (Primulaceae) is a valuable medicinal plant. P. veris is a Euro-Siberian Temperate element distributed throughout most of temperate Europe and Western Asia. As with most species of the genus Primula, it is entomophilous with a great affinity between its flowers. Insect pollinators belong to Hymenoptera (mainly bumblebees and bees), Lepidoptera and Diptera.
embryology
Primula
Primula veris
reproduction
reproductive potential
1. Introduction
The genus Primula L. includes six subgenera with 37 sections [1], and is the largest in the family Primulaceae. The number of species belonging to it, indicated by different authors, varies from about 400 up to more than 500 [1][2]. In Europe, the genus Primula is represented by only 34 species, included in four sections [1]. The genus was an object of the pioneer Darwin’s scientific treatise on the floral morphology, in particular heterostyly and reproductive biology [3]. He paid particular attention to the distyly in it. Heterostyly (reciprocal herkogamy) is confirmed for 28 Angiosperms families [4][5], and in particular, the distyly, as its most common type, is found in 26 of them [6], including Primulaceae.
Primula veris L. (cowslip, cowslip primrose) (syn. Primula officinalis Hill) belongs to the section Primula. The latter is phylogenetically quite isolated from the other sections in the genus and includes a total of seven species [7]. This section comprises six distylous and one homostylous species which all are diploids (2n = 2x = 22) with basic chromosome number x = 11 [8][9]. In Bulgarian flora, the genus Primula is represented by eight species [10][11]. Specifically, the section Primula is represented by three species: P. veris, P. elatior and P. vulgaris.
The section Primula is used as a model for a broad area of studies: evolutionary [12], ecological and conservation [13], molecular and genetic [14][15], phytochemical and pharmacological [16][17], as well as studies related to hybridization [1][18], reproductive biology and pollination ecology [5][19], reproductive capacity [19], genetics, ecological basis and genetic control of the distyly [9][14][20], etc.
P. veris is a Euro-Siberian Temperate element [21] distributed throughout most of temperate Europe and Western Asia [22]. As with most species of the genus Primula, it is entomophilous with a great affinity between its flowers. Insect pollinators belong to Hymenoptera (mainly bumblebees and bees), Lepidoptera and Diptera [20][23].
P. veris has a long history of medicinal use. It is a species with an economic significance that is also used as a decorative and melliferous plant. In the European Red List of Medicinal Plants, P. veris is included under the category (LC) “Least Concern” [24]. Together with P. elatior, this species is listed in the European Pharmacopoeia as a source of Primula roots [25]. In Bulgaria, P. veris is included in the Biodiversity Act, Annex 4 [26], in particular in the list of species of medicinal plants under a special regime of protection and regulated use, as well as in the Medicinal Plants Act [27] in which P. veris, P. elatior and P. vulgaris are cited in the “List of Medicinal Plants Falling under the Provisions of this Act”. P. veris is a species collected as a herb with a commercial designation from its natural habitats, outside the territories of the Bulgarian national parks, determined by the Bulgarian Ministry of Environment and Water (MEW). The above-ground parts (exclusively flowers) and roots of P. veris are permitted to be harvested but only in accordance with the annual quotas of MEW.
Phenolic compounds, including flavonoids, phenolic acids, phenolic glycosides and triterpen saponins, are the main active compounds in Primula flowers and roots [28][29].
Many authors [17][30] have shown that the flowers and roots of P. veris and closely related species, specifically P. elatior, are used for the production of herbal teas and as dietary supplements. They exhibit various pharmacological activities, particularly secretolytic, expectorant, anti-inflammatory, diuretic, antimicrobial, antifungal, and sedative [28][29]. In official medicine, P. veris is used for treating bronchial catarrhs of the respiratory tract, pertussis, asthma, colds and influenza [17][31].
Contrary to the intensive studies undertaken on the members of the genus Primula in all aspects mentioned above, the embryological studies involve a small number of species. The data in them are usually scarce or incomplete.
2. Embryological Features
Some embryological features on species of the family Primulaceae, including P. officinalis (syn. P. veris), were reported by [32]. Since then, studies on the embryology of P. veris are almost lacking. Data for Bulgarian populations of the species were obtained for the first time in the present work.
2.1. Anther, Microsporogenesis and Male Gametophyte Development
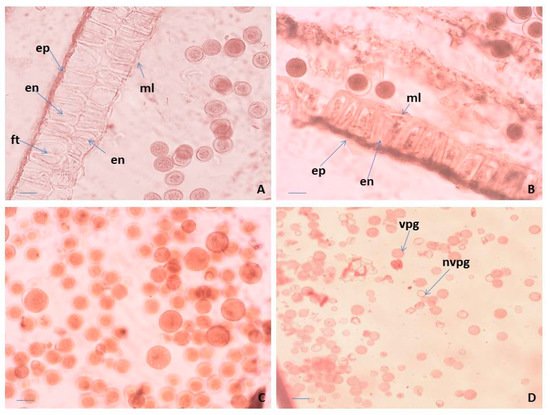
The anthers in P. veris are tetrasporangiate, a typical feature in all representatives of the family Primulaceae [33][34]. The anther wall forms following the Dicotyledonous-type [35] and consists of epidermis, endothecium, a middle layer and glandular (secretory) tapetum. Initially, it is difficult to distinguish morphologically the layers of the anther wall from each other. During the ontogenesis of the anther, they undergo significant changes and finally become clearly visible. The cells of the epidermis enlarge and lengthen tangentially, whereas these of the endothecium also extend but radially. In addition, formation of a second endothecium layer is observed, which has not been recorded so far in other Primula species. Thus, initially, the four-layered wall of the anthers in P. veris becomes five-layered. After forming microspore tetrads in the anthers, both endothecium layers develop fibrous thickenings (Figure 1A). During the anther ontogenesis, the cells of the middle layer elongate tangentially to such an extent that this layer becomes visible as a very thin strip between the endothecium and the tapetum. The tapetum cells in P. veris are one-nucleate. In contrast, in other Primulaceae members, namely in Lysimachia hybrida and L. quadrifolia [35], initially the one-nucleate tapetum cells become two-nucleate as a result of endomitosis, retaining their integrity. The glandular (secretory) anther tapetum, typical for the family Primulaceae [34], does not transform to an amoeboid one and remains cellular up to the end of the anther development.

Figure 1. Anther and male gametophyte development. (A) Anther wall and mature pollen in an anther locule of “pin” floral morph, (B) anther wall and mature pollen, (C) mature pollen in an anther locule of “thrum” floral morph, and (D) viable and unviable pollen after acetocarmine testing. ep—epidermis; en—endothecium; ft—fibrous thickenings of the endothecium cells; ml—middle layer; vpg—viable mature pollen; nvpg—unviable mature pollen. Scale bar = 20 μm (A,B); 50 μm (C); 100 μm (D).
Regarding the structure of the anther wall, in P. bayernii it is more massive than in P. veris, namely six-layered, consisting of epidermis, endothelium, two middle and two tapetum layers [36][37]. In the mature anthers of P. veris, some of the layers degenerate, and the wall contains only epidermis, endothecium with preserved integrity and remnants of the middle layer (Figure 1B). As a result of the normal course of meiosis in pollen mother cells (PMCs) and simultaneous microsporogenesis, predominantly tetrahedral microspore tetrads are formed in the anthers of P. veris. Mature pollen grains are bicellulate when released from the anther and morphologically identical or heterogeneous in size within the same anther locule (Figure 1A,C). In general, for almost all representatives of Primulaceae, it is stated that pollen grains are two-nucleate when dispersed [34][38].
Observations on the morphology of pollen from natural and cultivated Polish populations of P. veris show that pollen grains of the “thrum” (short-styled) morphs are more variable in size than those of “pin” (long-styled) morphs [39]. In the present study, this is illustrated in Figure 1A,C. In addition, it is important to note that in P. veris, a distylous species, the differences in the floral morphology do not influence the success of the insect pollination [20].
2.2. Ovule, Megasporogenesis and Female Gametophyte Development
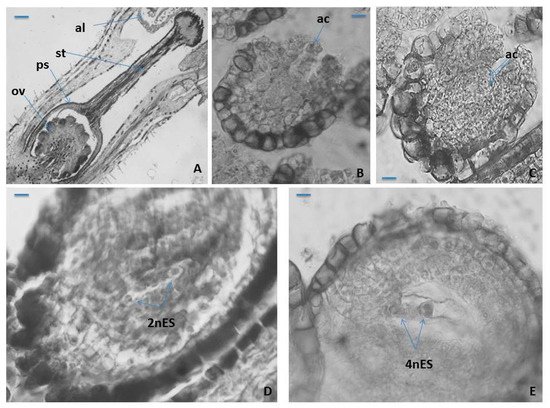
The ovary in P. veris is superior, unilocular, in which approximately 20 ovules with free central placentation being formed and developed (Figure 2A). In P. vulgaris [40] and P. nutans [41], the ovule number in an ovary does not differ (it is almost the same) between “pin” and “thrum” floral morphs. In the pistil of P. veris a solitary style and papilous capitate stigma (with) were observed (Figure 2A). The developed ovules in P. veris are anatropous, tenuinucellate, bitegmic with two-layered outer integument and more massive (four- to five-layered) inner integument (Figure 2A). The same type of ovule was reported for most Primulaceae representatives [33][34][38] and in particular for P. officinalis and Cortusa matthioli [32]. It has been established that the ovules in P. amoena are not anatropous but hemianatropous [42]. In another member of Primulaceae, namely Anagallis pumila, a gradual change to apotropous and even campylotropous ovule types have been observed [43].

Figure 2. Ovule and female gametophyte development. (A) An ovary with ovules, style and stigma, (B) unicellular archesporium in a young ovule, (C) two-cellular archesporium in an ovule, (D) two-nucleate Polygonum-type ES and (E) Four-nucleate Polygonum-type ES. ov—ovule; ps—ovary; st—style; al—anther locule; ac—archesporium cell; mt—megaspore tetrad; 2nES—two-nucleate ES; 4nES—four-nucleate ES. Scale bar = 100 μm (A); 50 μm (B,C); 20 μm (D,E).
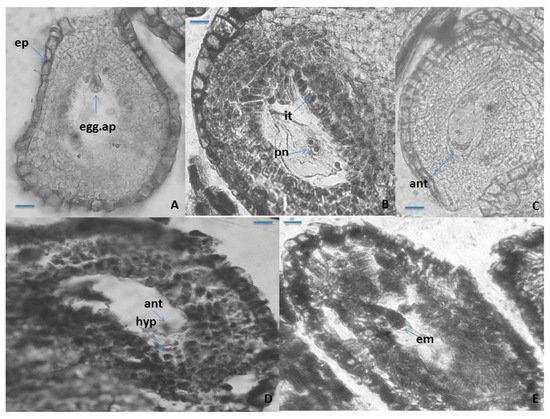
The cells of the epidermal layer of the outer integument in P. veris and of the endothelium have strongly thickened walls. During the ovule ontogenesis and the formation of the embryo sac (ES) in it, the cells of the innermost layer of the inner integument lengthen radially becoming tabular in form, forming so-called endothelium (integumentary tapetum) [44], which after the degeneration of nucellus surrounds the ES (Figure 2B). Hypodermally, in the nucellus of an ovule, unicellular archesporium forms (Figure 1B), but rarely, two archesporium cells are also observed (Figure 2C). The formation of two archesporium cells, instead of one, has also been occasionally found in the ovules of another Primulaceae member, namely Anagallis pumila [43]. Anderberg’s statement [38] that the multicellular female archaespore is typical of the whole family Primulaceae raises doubts since in the embryological studies conducted so far, such feature has not been found, including in P. veris.
Contrary to Anderberg’s assertion, in important treatises on Angiosperm embryology, for the family Primulaceae, the formation of only one archaesporium cell in the ovule is indicated [33][34].
In P. veris, as in other Primula species, namely P. bayernii [36], P. algida and P. amoena [42], the archesporium cell functions directly as megaspore mother cell (MMC), and because of that, no cover cell formation is observed. After the meiosis occurring in MMC, a linear megaspore tetrad forms in the ovule and from its chalazal megaspore Polygonum (monosporic)-type ES (Figure 2D,E) develops, which is typical for the family Primulaceae [33][34]. Except for the linear megaspore tetrads, formation of T-shaped tetrads in some ovules of Anagallis pumila has also been observed [43].
The mature seven-cellular Polygonum-type ES consists of the following elements: an egg apparatus, with small size of its three cells (egg cell and two synergids), with the typical shape and location of the nuclei in them (Figure 3A); central cell of the ES, results of the fusion of the two polar nuclei about the time of fertilization (Figure 3B), and three ephemeral antipodals, that degenerate before the fertilization (Figure 3C). The ephemerous nature of antipodals is shown as a typical feature for the whole family Primulaceae [33][34][37][38]. The spherical inclusions in the epidermal cells of the anther wall and in the epidermal cells of the outer integument of the ovule (Figure 1B and Figure 3A), observed in some other representatives of Primulaceae, are probably tannins [37]. It was observed in P. veris that under the antipodals, from cells of the internal integument an additional structure in the chalaza is formed, namely a hypostase (Figure 3D). This is a newly established feature that has not been reported so far for the embryology of the genus Primula or of the family Primulaceae. The embryo and endosperm formations begin after fertilization of the egg cell and the central cell of the ES, respectively, and the endospermogenesis precedes embryogenesis. This was found in all studied members of the family, and differences were found only between the time of the first division of the zygote and the number of the already formed free endosperm nuclei. The embryogenesis in P. veris runs after the Caryophyllad-type (Figure 3E), which is typical for the family Primulaceae [33][34][38], and the embryo has a long suspensor at its globular stage of development (Figure 3E). Initially, the endosperm consists of free nuclei, but during embryogenesis, it transforms into a cellular one. It has been observed that cytokinesis in P. veris and other embryologically studied Primula species occurs only after different number of free endosperm nuclei have been formed, for example, 32–46 endosperm nuclei in P. algida and 64–128 in P. amoena

Figure 3. Ovule and development of the female gametophyte. (A–C) Anatropous ovule with mature Polygonum-type ES, (D) antipodals and hypostase in the ES cavity, and (E) young globular embryo with suspensor in the ES cavity. ep—epidermis; egg.ap—egg apparatus; it-integumentary tapetum pn—polar nuclei; ant—antipodals; hyp—hypostase; em—embryo. Scale bar = 20 μm (A–E).
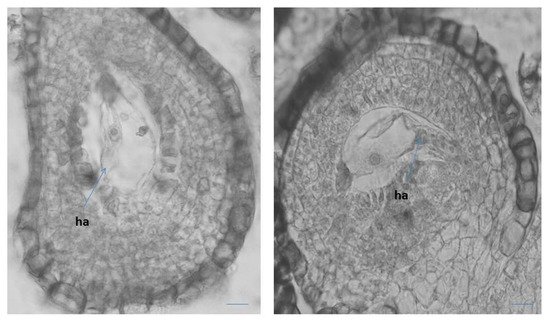
It has been observed that after the degeneration of the antipodals (before fertilization), the lower end of the central cell enlarges considerably, forming an outgrowth with dense cytoplasm haustorizing to the chalaza (Figure 4). Similar haustorization of the central cell of the ES was also observed in two other Primula species—P. algida and P. amoena [42]. In Primula amoena, after the endosperm has transformed from nuclear to cellular, the formation of endosperm haustorium is observed, as well as several lateral haustoria penetrating into the endothelial cells [42]. The formation of such structures was not detected in the present study of P. veris. In addition, the formation of endosperm haustorium is not included as a characteristic feature of the Primulaceae family in well-known monographs on the embryology of flowering plants [33][34][38].

Figure 4. Central cell in the ES with outgrowth like haustorization (ha) to the chalaza of the ovule. Scale bar = 20 μm.
This study shows that P. veris is a diploid, sexually reproducing (amphimictic) species that reproduces mainly by seeds. However, rarely it may reproduce vegetatively during the growing season by the formation of lateral rosettes. This has already been announced for the species [45], and is confirmed by our observations (in situ and ex situ). Results of a study on the distyly and its influence on the size of the Belgian populations of P. veris [46] show that in large populations, the short-styled morph is usually more “female based”, and the long-styled morph may rather manifest as a pollen donor. The same authors [46] also point out that these two morphs may behave differently with respect to restrictions arising in fragmented populations. In Bulgarian flora, P. veris is represented by such populations.
It is important to note that in our study of the generative sphere of P. veris, neither apomixis nor formation of additional embryos (i.e., polyembryony) was observed. As a result, the species is considered sexually reproducing, which correlates with its diploid status. However, polyembryony is found in other Primula species. Therefore, in P. auricula the additional 2–3 globular embryos are formed by the zygote [47] and this type of polyembryony is described as “cleavage polyembryony” [48]. In P. amoena, besides the zygotic embryo from the fertilized egg cell, an apomictic embryo also forms [42] from the synergid, without fertilization (apogamy).
3. Pollen Viability
Pollen viability (fertility) is an important factor in whether a population will undergo effective pollination and subsequent sexual reproduction to ensure the survival of each plant species. Different terms in pollen testing criteria based on the stages of pollen development in which it is tested are suggested [49]. In our study, we use the term “viability”, which is defined as “having the capacity to live, grow, germinate or develop”, which refers to the assessment of the viability of mature pollen [50].
According to the level of staining, the pollen viability in P. veris was estimated after acetocarmine testing: red-colored pollen grains were determined as viable, and colorless ones as nonviable (Figure 1D). As a result, the following percentage of pollen viability was found: 98.05% ± 2.2 for the Pirin Mts (the Ilindentsi village) population and 95.84% ± 5.7 for the population from the Golo Bardo Mts.
The pollen viability is one of the main parameters for the evaluation of plant reproductive potential. The viable (fertile) mature pollen is that which, when falling on the stigma under normal conditions, would start growing a pollen tube and finally discharge its male gametes in the embryo sac in an ovule to perform fertilization. A comparative study on “pin” and “thrum” floral morphs in P. paliurni showed that the pollen viability is significantly affected by the temperature and humidity [51]. In the plants from a population of this species, these authors [51] found that the pollen of the “thrum” morph showed significantly higher viability than the pollen of the “pin” floral morph. While in P. bayernii, pollen sterility in the “pin” flowers was found [36], the data from our study in P. veris shows that in both “pin” and “thrum” flowers, fertile pollen predominantly forms. The latter is an important factor for the efficiency of the subsequent processes of pollination and fertilization, and hence, the sexual reproduction of this species.
4. Seed Viability
A viable seed is a seed that is capable of germinating and producing a “normal” seedling [52]. A related term is ‘seed viability’, which describes the share of viable seeds and is usually used synonymously with ‘germination capacity’ (germinability). This definition includes dormant but viable seeds, in which case the dormancy must be broken before viability can be assessed if germination is achieved. Therefore, a non-viable seed fails to germinate even under optimal conditions, including treatments for the removal of dormancy. Seed germination is “the emergence and development from the seed embryo, those essential structures, which, for the kind of seed in question, are indicative of the ability to produce a normal plant under favourable conditions” [53].
In the present study, the assessment of seed (embryo) viability was made after testing with tetrazolium solution in the two studied populations (both in situ and ex situ collected seeds) of P. veris (Table 1 and Table 2). The evaluation was performed on the basis of the criteria for interpretation of the tetrazolium test results, according to the intensity of staining and localization of unstained parts in the embryo [54]: the embryos stained entirely in dark red, brighter red or pink and partially stained embryos (only the root tip colored in red) are defined as viable; colorless or partially stained embryos (only the top of the embryo–cotyledons are colored in red), as well as empty seeds are considered non-viable (Figure 5 and Figure 6). The color differences observed, together with the knowledge of seed features and function, permit an assessment of the presence, location, and nature of weaknesses within embryo tissues [54].

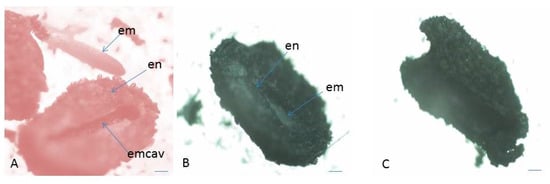
Figure 5. Assessment of seed (embryo) viability in P. veris after TZ testing. (A) Seed with endosperm and viable embryo, (B) seed with endosperm and unviable embryo, (C) empty seed (without embryo) unnviable. em—embryo; en—endosperm; emcav—ES cavity. Scale bar = 100 μm.

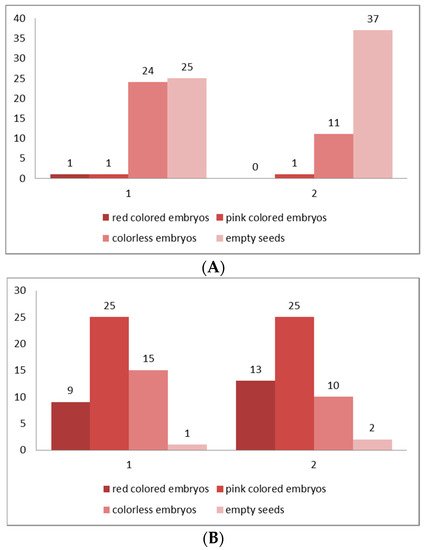
Figure 6. (A,B) Classification of the embryos viability in four classes according to the intensity of their staining after the results of TZ testing. (A) From seeds collected from the natural populations (in situ), (B) from seeds collected from the plants at the experimental plots (ex situ). Class I—seeds with viable embryo; completely (100%) colored in dark red; Class II—seeds with viable embryo; colored in pink; Class III—seeds with colorless embryo, unviable; Class IV—empty seeds without embryo. 1—Golo Bardo Mt population; 2—the Ilindentsi village population.
Table 1. Assessment of the seed (embryo) viability after TZ testing for seeds collected from the natural populations (in situ).
| Population | Number of Tested Seeds |
Number of Viable Embryos |
Viable Embryos % |
Unviable Embryos % |
|---|---|---|---|---|
| Golo Bardo Mt | 50 | 2 | 4 | 96 |
| Ilindentsi village | 50 | 1 | 2 | 98 |
Table 2. Assessment of the seeds (embryos) viability after TZ testing for seeds collected from the plants at the experimental plots (ex situ).
| Population | Number of Tested Seeds |
Number of Viable Embryos |
Viable Embryos % |
Unviable Embryos % |
|---|---|---|---|---|
| Golo Bardo Mt | 50 | 34 | 68 | 32 |
| Ilindentsi village | 50 | 38 | 76 | 24 |
In distylous species such as P. veris, only cross-pollination between the two genetically determined individuals (“pin” and “thrum” morphs) produces a successful seed set [55]. The established exclusively low seed viability for the natural populations (in situ)—4% for Golo Bardo Mt population and 2% for the Ilindentsi village population (Table 1)—is probably due to the seed dormancy, and to various restrictions in the conditions in the natural habitats, which negatively influence the processes of reaching seeds maturity and their further development in plants. The results of studies on foreign populations of P. veris have also shown an exclusively low (0.01%) seed viability [19][56]. The higher seed viability (over 60%) (Table 2) established in ex situ cultivated plants from the two studied populations shows a positive influence of the controlled conditions on the formation and maturation of P. veris. It is a prerequisite for the successful growth of the species in culture.
The exclusively low seed viability assumes a slow rate of germination. It decreases the successful growth of the seedling progeny in P. veris, and one of the probable reasons for this may be seed dormancy [57]. It has been shown that the low seed viability, which is a reason for the poor germination rate, is due to dormancy [58]. The seed dormancy in P. veris continues during the last several months of its development [57][59]. The non-mature seeds are strictly dormant at the time of dispersal and to activate germination a cold stratification is required [60].
5. Genetic Diversity
In total, 63% of the primers scored for the initial pilot study of Primula veris were polymorphic. The mean number of bands per primer was 10.7. These data are similar to those obtained by Crema et al. [61] for the endemic species Primula apennina, but there the authors used many more primers (twenty-five primers).
The percentage of polymorphic bands was the highest in the population of Western Rhodopes (74), which is due probably to the larger number of the studied individuals. The same percentage in the population of Petrohan was 72, in the population of Slavyanka 69, and in the population of Gorno Harsovo (Rila) 67. The number of polymorphic bands is only approximately characteristic of the genetic diversity, and is analogous to the mean number of alleles in other markers (for example, isozymes). Much more informative are the characteristics of genetic diversity [62]. When using this index, again the highest diversity was demonstrated by the population of Kraishte (Western Rhodopes) (0.256), followed by Slavyanka (0.238), Gorno Harsovo (0.201), and the lowest level of diversity was recorded in the population of Petrohan, which is the northernmost of all of the populations.
The Shannon’s polymorphic index showed similar trends and again the highest values had the population from the Western Rhodopes, followed by Petrohan, Gorno Harsovo, and the lowest value was recorded in the population of Slavyanka.
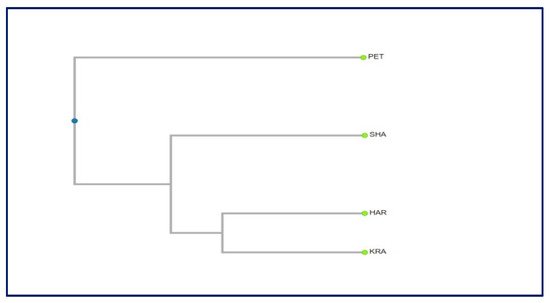
The dendrogram constructed based on the genetic distances among populations revealed that there is no relationship between the geographic and genetic distances (Figure 7). The population Petrohan (Western Stara planina) can be clearly distinguished, and the other three populations are closer to each other. The lack of clear spatial subdivision in the grouping of populations is probably due to the small sample size. It would be worth including other populations in the study, and then we could expect to get more clear relationships and trends in the distribution of the genetic diversity and to draw more definite conclusions.

Figure 7. Dendrogram based on the genetic distances among the studied populations.
As in P. apennina [61] and P. heterochroma [63], the level of genetic variability in Primula veris was high both at species and at population levels (Table 3). Therefore, it can be concluded that the high genetic diversity is common for Primula species. It correlates with their outcrossing nature, which is a prerequisite for high level of genetic diversity within populations and lower differentiation among populations [64].
Table 3. Natural populations included in the genetic studies and polymorphism and diversity.
| Population | Geographic Coordinates | Altitude (m) | Percent of Polymorphic Bands | Gene Diversity He (±SD) |
Average Intra-Population Diversity HS (±SD) |
|---|---|---|---|---|---|
| Shabran (Slavyanka Mts) | 41°23′19″ N 23°36′30″ E |
1900 | 69 | 0.238 ± 0.155 | 0.236 ± 0.249 |
| Kraishte (Western Rhodopes) | 41°57′28″ N 23°39′12″ E |
1180 | 74 | 0.256 ± 0.108 | 0.302 ± 0.248 |
| Petrokhan (Western Stara planina) | 43°7′39″ N 23°7′28″ E |
1450 | 72 | 0.192 ± 0.134 | 0.256 ± 0.154 |
| Gorno Harsovo (Rila Mts) | 42°00′47″ N 23°12′30″ E |
820 | 67 | 0.201 ± 0.164 | 0.238 ± 0.203 |
References
- Richards, J. Primula L., 2nd ed.; Timber Press: Portland, OR, USA, 2003; 386p.
- Zhang, L.B.; Kadereit, J.W. Classification of Primula sect. Auricula (Primulaceae) based on two molecular data sets (ITS, AFLPs), morphology and geographical distribution. Bot. J. Linn. Soc. 2004, 146, 1–26.
- Darwin, C.R. The Different Forms of Flowers on Plants of the Same Species; John Murray: London, UK, 1877; p. 351.
- Barrett, S.C.H.; Jesson, L.K.; Baker, A.M. The evolution and function of stylar polymorphisms in flowering plants. Ann. Bot. 2000, 85, 253–265.
- Brys, R.; Jacquemyn, H. The impact of individual inaccuracy of reciprocal herkogamy on legitimate pollen deposition and seed set in a distylous self-incompatible herb. J. Ecol. 2020, 108, 81–93.
- Naiki, A. Hetrostyly and the possibility of its breakdown by polyploidization. Plant Species Biol. 2012, 27, 3–29.
- Schmidt-Lebuhn, A.N.; de Vos, J.M.; Keller, B.; Conti, E. Phylogenetic analysis of Primula section Primula reveals rampant non-monophyly among morphologically distinct species. Mol. Phylogenet. Evol. 2012, 65, 23–34.
- Clapham, A.R.; Tutin, T.G.; Moore, D.M. Flora of the British Isles, 3rd ed.; Cambridge University Press: Cambridge, UK, 1987; 688p.
- Nowak, M.D.; Russo, G.; Schlapbach, R.; Huu, C.N.; Lenhard, M.; Conti, E. The draft genome of Primula veris yields insights into the molecular basis of heterostyly. Genome Biol. 2015, 16, 12.
- Peev, D. Genus Primula L. In Flora of the Republic of Bulgaria; Velchev, V., Ed.; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1982; Volume 8, pp. 324–336. (In Bulgarian)
- Assyov, B.; Petrova, A.; Dimitrov, D.; Vassilev, P. Conspectus of the Bulgarian Vascular Flora. Distribution Maps and Floristic Elements, 4th ed.; Assyov, B., Petrova, A., Eds.; Bulgarian Biodiversity Foundation: Sofia, Bulgaria, 2012; pp. 331–332.
- Keller, B.; de Vos, J.M.; Conti, E. Decrease of sexual organ reciprocity between heterostylous primrose species, with possible functional and evolutionary implications. Ann. Bot. 2012, 110, 1233–1244.
- Brys, R.; Jacquemyn, H.; Endels, P.; Van Rossum, F.; Hermy, M.; Triest, L.; De Bruyn, L.; Blust, G.D.E. Reduced reproductive success in small populations of the self-incompatible Primula vulgaris. J. Ecol. 2004, 92, 5–14.
- Van Rossum, F.; Triest, L. Within-population genetic variation in the distylous Primula veris: Does floral morph anisoplethy matter in fragmented habitats? Perspectives in Plant Ecology. Evol. Syst. 2006, 7, 263–273.
- Berisha, N.; Millaku, F.; Gashi, B.; Krasniqi, E.; Novak, J. Initial determination of DNA polymorphism of some Primula veris L. populations from Kosovo and Austria. Physiol. Mol. Biol. Plants 2015, 21, 117–122.
- Müller, A.; Ganzera, M.; Stuppner, H. Analysis of phenolic glycosides and saponins in Primula elatior and Primula veris (primula root) by liquid chromatography, evaporative light scattering detection and mass spectrometry. J. Chromatogr. A 2006, 1112, 218–223.
- Bączek, K.; Przybył, J.L.; Mirgos, M.; Kosakowska, O.; Szymborska-Sandhu, I.; Węglarz, Z. Phenolics in Primula veris L. and P. elatior (L.) Hill Raw Materials. Int. J. Anal. Chem. 2017, 1, 2871579.
- Martins, B.C.; Oberprieler, C.; Hellwig, F.H. A phylogenetic analysis of Primulaceae s.l. based on internal transcribed spacer (ITS) DNA sequence data. Plant Syst. Evol. 2003, 237, 75–85.
- Brys, R.; Jacquemyn, H.; Endels, P.; Hermy, M.; De Blust, G. The relationship between reproductive success and demographic structure in remnant populations of Primula veris. Acta Oecol. 2003, 24, 247–253.
- Deschepper, P.; Brys, R.; Jacquemyn, H. The impact of flower morphology and pollinator community composition on pollen transfer in the distylous Primula veris. Bot. J. Linn. Soc. 2017, 186, 414–424.
- Preston, C.D.; Hill, M.O. The geographical relationships of British and Irish vascular plants. Bot. J. Linn. Soc. 1997, 124, 1–120.
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals, A Handbook of Practice on a Scientific Basis, 3rd ed.; Medpharm Scientific Publisher: Stuttgart, Germany, 2004; 704p.
- Ornduff, R. Pollen flow in a population of Primula vulgaris Huds. Bot. J. Linn. Soc. 1979, 78, 1–10.
- Allen, D.; Bilz, M.; Leaman, D.J.; Miller, R.M.; Timoshyna, A.; Window, J. European Red List of Medicinal Plants; Publications Office of the European Union: Luxembourg, 2014; 62p.
- European Pharmacopoeia. Primula root (Primulae radix). In European Directorate for the Quality of Medicines and Health Care (EDQM), 5th ed.; Council of Europe: Strasbourg, France, 2006; p. 1588.
- Biological Diversity Act. 2002. Promulgated, State Gazette SG, No. 77/9.08.2002, Last amended, in SG No. 58/26.07.2016 (Bg). Available online: http://eea.government.bg/bg/legislation/biodiversity/ZBR_en_26_07_2016.pdf (accessed on 2 September 2021).
- Medicinal Plants Act. 2000. Promulgated State Gazette (SG), issue 29/7.04.2000. Last amended in SG, Number 98. 28 November 2014; (In Bulgarian). Available online: http://eea.government.bg/bg/legislation/biodiversity/ZLR_en.pdf (accessed on 2 September 2021).
- EMA (European Medicines Agency). Assessment Report on Primula veris L. and/or Primula elatior (L.) Hill, Flos. EMA/HMPC/136583/2012; European Medicines Agency: Amsterdam, The Netherlands, 2012.
- EMA (European Medicines Agency). Assessment Report on Primula veris L. and/or Primula elatior (L.) Hill, Radix. EMA/HMPC/113577/2012; European Medicines Agency: Amsterdam, The Netherlands, 2012.
- Tarapatskyy, M.; Gumienna, A.; Sowa, P.; Capusta, I.; Puchalski, C. Bioactive Phenolic Compounds from Primula veris L.: Influence of the Extraction Conditions and Purification. Molecules 2021, 26, 997.
- Pamukov, D.; Ahthardzhiev, H. Natural Pharmacy; Zemizdat: Sofia, Bulgaria, 1989; 327p. (In Bulgarian)
- Dahlgren, K.V.O. Zytologische und embryologische Studien über die Reihen Primulales und Plumpaginales. Kongl. Svenska Vetensk.-Akad. Handl. 1916, 56, 360–363.
- Davis, G.L. Systematic Embryology of the Angiosperms; John Wiley: New York, NY, USA, 1966; 528p.
- Poddubnaya-Arnoldi, V.A. Characteristics of Flowering Plants Families According to the Cytoembryological Features; Nauka: Moscow, Russia, 1982. (In Russian)
- Lersten, N.R.; Eilers, L.J. Binucleate Tapetum in Two Species of Lysimachia (Primulaceae). Proc. Iowa Acad. Sci. 1974, 81, 93–94.
- Gachechiladze, M.I. The embryology of Primula bayernii (Primulaceae). Bot. J. 1993, 78, 93–96. (In Russian)
- Mameteva, T.B. Family Primulaceae. In Comparative Embryology of Flowering Plants; Yakovlev, M.S., Ed.; Nauka: Leningrad, Russia, 1983; pp. 241–243. (In Russian)
- Anderberg, A.A. Primulaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 6, pp. 313–319.
- Morozowska, M.; Idzikowska, K. Morphological differentiation of Primula veris L. pollen from natural and cultivated populations. Acta Soc. Bot. Pol. 2004, 73, 229–232.
- Kálmán, K.; Medvegy, A.; Mihalik, E. Quantitative characteristics of the gynoecium in Primula vulgaris. Acta Biol. Szeged. 1999, 43, 5–14.
- Petrova, S.E.; Kozhin, M.N. Heterostyly in Primula nutans ssp. finmarchica (Jacq.) Á. Löve & D. Löve (Primulaceae) from three northern coenopopulations (Kandalaksha Bay, White Sea). Wulfenia 2018, 25, 231–241.
- Akhalkatsi, M.; Gvaladze, G.; Taralashvili, N. Embryology of Primula algida and Primula amoena (Primulaceae). Bull. Georg. Aca. Sci. 1998, 157, 98–101.
- Raju, M.V.S. Embryology of Anagallis pumila Swartz. Proc. Indian Acad. Sci. Sect. B 1952, 36, 34–42.
- Kapil, N.R.; Tiwari, S.C. The integumentary tapetum. Bot. Rev. 1978, 44, 457–490.
- Brys, R.; Jacquemyn, H. Biological Flora of the British Isles: Primula veris L. J. Ecol. 2009, 97, 581–600.
- Van Rossum, F.; De Sousa, S.C.; Triest, L. Morph-specific differences in reproductive success in the distylous Primula veris in a context of habitat fragmentation. Acta Oecol. 2006, 30, 426–433.
- Veillet-Bartoszewska, M. La polyembryonie chez le Primula auricula L. Bull. Soc. bot. Fr. 1957, 104, 473–475.
- Maheshwari, P. Polyembryony in Angiosperms. Palaeobotanist 1952, 1, 319–329.
- Dafni, A.; Firmage, D. Pollen viability and longevity: Practical, ecological and evolutionary implications. Plant Syst. Evol. 2000, 222, 113–132.
- Lincoln, R.J.; Boxshall, G.A.; Clark, P.F. A Dictionary of Ecology, Evolution and Systematics; Cambridge University Press: New York, NY, USA, 1982; 313p.
- Aronne, G.; Iovane, M.; Strumia, S. Temperature and humidity affect pollen viability and may trigger distyly disruption in threatened species. Ann. Bot. 2021, 11, 77–82.
- Copeland, L.O.; McDonald, M.F. Seed Viability and Viability Testing. In Principles of Seed Science and Technology, 4th ed.; Copeland, L.O., McDonald, M.F., Eds.; Kluwer Academic Publisher: New York, USA, 2001; pp. 124–139.
- AOSA. Tetrazolium Testing Handbook; Contribution No. 29; Association of Official Seed Analysts: Lincoln, NE, USA, 2000; Available online: http://gsem.weebly.com/uploads/9/3/5/1/9351412/tetrazolium_testing_handbook__2001-2002__-_part_i.pdf (accessed on 2 September 2021).
- Moore, R.P. Handbook on Tetrazolium Testing; International Seed Testing Association: Zürich, Switzerland, 1985; 99p.
- Richards, A.J. Primulas of the British Isles; Shire Publications: Princes Risborough, UK, 1989; 24p.
- Jacquemyn, H.; Brys, R. Population growth rates of the forest herb Primula elatior increase with stand age in post-agricultural forests. Ecology 2008, 89, 3480–3489.
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology. A Functional Approach to Common British Species; Unwin Hyman: London, UK, 1988; 679p.
- Thompson, K.; Bakker, J.P.; Bekker, R.M. The Soil Seed Bank of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1988.
- Antrobus, S.; Lack, A.J. Genetics of colonizing and established populations of Primula veris. Heredity 1993, 71, 252–258.
- Milberg, P. Germination ecology of the polycarpic grassland perennials Primula veris and Trollius europaeus. Ecography 1994, 17, 3–8.
- Crema, S.; Christofolini, G.; Rossi, M.; Conte, L. High genetic diversity detected in the endemic Primula apennina Widmer (Primulaceae) using ISSR fingerprinting. Plant Syst. Evol. 2009, 280, 29–36.
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323.
- Noroozisharafa, A.; Hatamzadeha, A.; Samizadeh Lahiji, H.; Bakhshi, D. Genetic diversity of endangered primrose (Primula heterochroma Stapf.) accessions from Iran revealed by ISSR and IRAP markers. Sci. Hortic. 2015, 190, 173–178.
- Rossetto, M.; Weaver, P.K.; Dixon, K.W. Use of RAPD analysis indevising conservation strategies for the rare and endangered Grevillea scapigera (Proteaceae). Mol. Ecol. 1995, 4, 321–329.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
10 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




