
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Norman Toro | + 2630 word(s) | 2630 | 2021-11-08 04:12:16 | | | |
| 2 | Conner Chen | Meta information modification | 2630 | 2021-11-10 04:39:23 | | |
Video Upload Options
Chalcocite is the most abundant secondary copper sulfide globally, with the highest copper content, and is easily treated by conventional hydrometallurgical processes, making it a very profitable mineral for extraction. Among the various leaching processes to treat chalcocite, chloride media show better results and have a greater industrial boom. Chalcocite dissolution is a two-stage process, the second being much slower than the first. During the second stage, in the first instance, it is possible to oxidize the covellite in a wide range of chloride concentrations or redox potentials (up to 75% extraction of Cu). Subsequently, CuS2 is formed, which is to be oxidized. It is necessary to work at high concentrations of chloride (>2.5 mol/L) and/or increase the temperature to reach a redox potential of over 650 mV, which in turn decreases the thickness of the elemental sulfur layer on the mineral surface, facilitating chloride ions to generate a better porosity of this.
1. Effect on Chloride Concentration
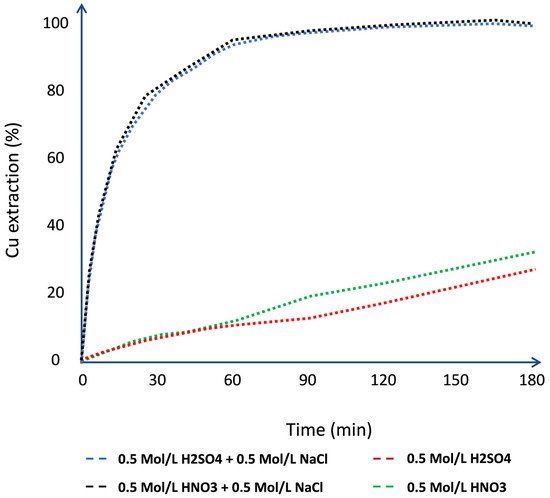
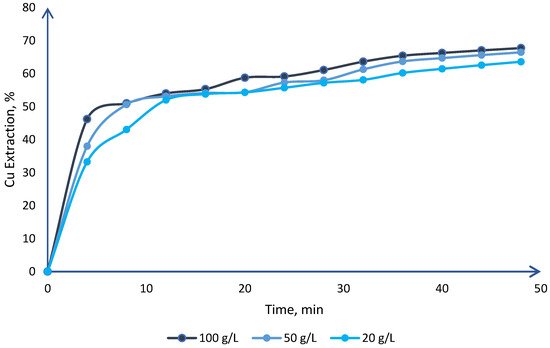
2. Effect on Stirring Speed
3. Effect on Acid Concentration
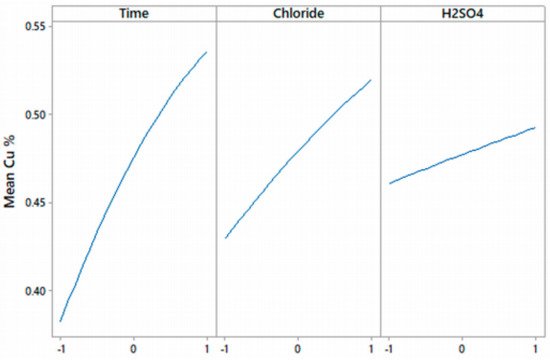
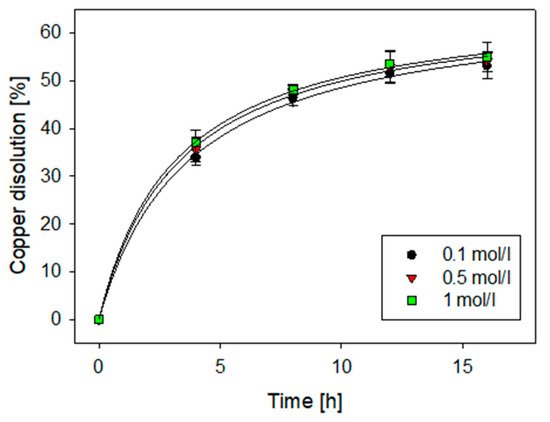
| Experimental Parameters | Low | Medium | High |
|---|---|---|---|
| Time (h) | 4 | 8 | 12 |
| Cl− concentration (g/L) | 20 | 50 | 100 |
| H2SO4 (mol/L) | 0.5 | 1 | 2 |
4. Particle Size Effect
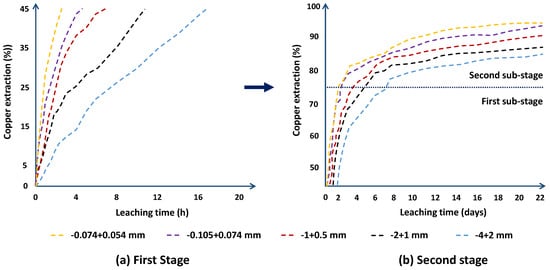
5. Effect of Temperature
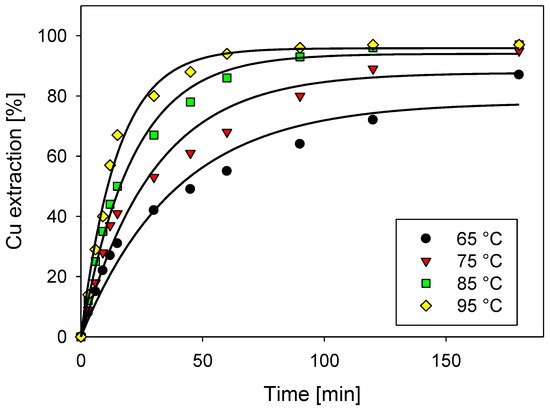
6. Effect of Redox Potential
7. Effect of Oxidizing Agents
7.1. Air
7.2. Ferric Ions
7.3. MnO2
References
- Torres Albornoz, D.A. Copper and Manganese Extraction Through Leaching Processes, Universidad Politécnica de Cartagena. 2021. Available online: https://repositorio.upct.es/bitstream/handle/10317/9373/data_C.pdf?sequence=1&isAllowed=y (accessed on 1 October 2021).
- Toro, N.; Briceño, W.; Pérez, K.; Cánovas, M.; Trigueros, E.; Sepúlveda, R.; Hernández, P. Leaching of pure chalcocite in a chloride media using sea water and waste water. Metals 2019, 9, 780.
- Cheng, C.Y.; Lawson, F. The kinetics of leaching chalcocite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268.
- Miki, H.; Nicol, M.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327.
- Fisher, W.W.; Flores, F.A.; Henderson, J.A. Comparison of chalcocite dissolution in the oxygenated, aqueous sulfate and chloride systems. Miner. Eng. 1992, 5, 817–834.
- Hashemzadeh, M.; Dixon, D.G.; Liu, W. Modelling the kinetics of chalcocite leaching in acidified cupric chloride media under fully controlled pH and potential. Hydrometallurgy 2019, 189, 105114.
- Saldaña, M.; Rodríguez, F.; Rojas, A.; Pérez, K.; Angulo, P. Development of an empirical model for copper extraction from chalcocite in chloride media. Hem. Ind. 2020, 74, 285–292.
- Pérez, K.; Toro, N.; Saldaña, M.; Salinas-Rodríguez, E.; Robles, P.; Torres, D.; Jeldres, R.I. Statistical Study for Leaching of Covellite in a Chloride Media. Metals 2020, 10, 477.
- Hashemzadeh, M.; Dixon, D.G.; Liu, W. Modelling the kinetics of chalcocite leaching in acidified ferric chloride media under fully controlled pH and potential. Hydrometallurgy 2019, 186, 275–283.
- Hernández, P. Estudio del Equilibrio Sólido-Líquido de Sistemas Acuosos de Minerales de Cobre Con Agua de Mar, Aplicado A Procesos de Lixiviación, Universidad de Antofagasta. 2013. Available online: https://intranetua.uantof.cl/docs/carreras/tesis_pia_hernandez.pdf (accessed on 1 October 2021).
- Pérez, K.; Jeldres, R.; Nieto, S.; Salinas-Rodríguez, E.; Robles, P.; Quezada, V.; Hernández-Ávila, J.; Toro, N. Leaching of pure chalcocite in a chloride media using waste water at high temperature. Metals 2020, 10, 1–9.
- Torres, D.; Trigueros, E.; Robles, P.; Leiva, W.H.; Jeldres, R.I.; Toledo, P.G.; Toro, N. Leaching of Pure Chalcocite with Reject Brine and MnO2 from Manganese Nodules. Metals 2020, 10, 1426.
- Hashemzadeh, M.; Liu, W. The response of sulfur chemical state to different leaching conditions in chloride leaching of chalcocite. Hydrometallurgy 2020, 192, 105245.
- Schlesinger, M.; King, M.; Sole, K.; Davenport, W. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780080967899.
- Ruiz, M.C.; Honores, S.; Padilla, R. Leaching kinetics of digenite concentrate in oxygenated chloride media at ambient pressure. Metall. Mater. Trans. B 1998, 29, 961–969.
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl–H2SO4–O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268.
- Velásquez-Yévenes, L. The Kinetics of the Dissolution of Chalcopyrite in Chloride Media, Murdoch University. 2009. Available online: https://researchrepository.murdoch.edu.au/id/eprint/475/ (accessed on 1 October 2021).
- Dutrizac, J.E. The leaching of sulphide minerals in chloride media. Hydrometallurgy 1992, 29, 1–45.
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088.
- Toro, N.; Pérez, K.; Saldaña, M.; Jeldres, R.I.; Jeldres, M.; Cánovas, M. Dissolution of pure chalcopyrite with manganese nodules and waste water. J. Mater. Res. Technol. 2019, 9, 798–805.
- Naderi, H.; Abdollahy, M.; Mostoufi, N. Kinetics of chemical leaching of chalcocite from low-grade copper ore: Size-distribution behavior. J. Min. Environ. 2015, 6, 109–118.
- Phyo, H.A.; Jia, Y.; Tan, Q.; Zhao, S.; Liang, X.; Ruan, R.; Niu, X. Effect of particle size on chalcocite dissolution kinetics in column leaching under controlled Eh and its implications. Physicochem. Probl. Miner. Process. 2020, 56, 676–692.
- Toro Villarroel, N.R. Optimización de Parámetros Para la Extracción de Elementos Desde Minerales en Medios Ácido, Universidad Politécnica de Cartagena. 2020. Available online: https://repositorio.upct.es/handle/10317/8504 (accessed on 1 October 2021).
- Chang, K.; Zhang, Y.; Zhang, J.; Li, T.; Wang, J.; Qin, W. Effect of temperature-induced phase transitions on bioleaching of chalcopyrite. Trans. Nonferrous Met. Soc. China 2019, 29, 2183–2191.
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32.
- Ruan, R.; Zou, G.; Zhong, S.; Wu, Z.; Chan, B.; Wang, D. Why Zijinshan copper bioheapleaching plant works efficiently at low microbial activity—Study on leaching kinetics of copper sulfides and its implications. Miner. Eng. 2013, 48, 36–43.
- Ruiz, M.C.; Abarzúa, E.; Padilla, R. Oxygen pressure leaching of white metal. Hydrometallurgy 2007, 86, 131–139.
- Niu, X.; Ruan, R.; Tan, Q.; Jia, Y.; Sun, H. Study on the second stage of chalcocite leaching in column with redox potential control and its implications. Hydrometallurgy 2015, 155, 141–152.
- Liu, W.; Granata, G. The Effect of Aeration on Chalcocite Heap Leaching. In Extraction; Davis, B., Moats, M., Wang, S., Gregurek, D., Kapusta, J., Battle, T., Schlesinger, M., Alvear, G., Jak, E., Goodall, G., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018.
- Devi, N.B.; Madhuchhanda, M.; Rao, K.S.; Rath, P.C.; Paramguru, R.K. Oxidation of chalcopyrite in the presence of manganese dioxide in hydrochloric acid medium. Hydrometallurgy 2000, 57, 57–76.
- Kowalczuk, P.B.; Manaig, D.O.; Drivenes, K.; Snook, B.; Aasly, K.; Kleiv, R.A. Galvanic leaching of seafloor massive sulphides using MnO2 in H2SO4-NaCl media. Minerals 2018, 8, 235.
- Torres, D.; Ayala, L.; Jeldres, R.I.; Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals 2020, 10, 107.




