Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rosa Amoroso | + 2289 word(s) | 2289 | 2021-10-27 05:11:57 | | | |
| 2 | Conner Chen | Meta information modification | 2289 | 2021-11-04 02:46:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Amoroso, R. Deep eutectic solvents in pharmaceutical synthesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/15687 (accessed on 07 February 2026).
Amoroso R. Deep eutectic solvents in pharmaceutical synthesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/15687. Accessed February 07, 2026.
Amoroso, Rosa. "Deep eutectic solvents in pharmaceutical synthesis" Encyclopedia, https://encyclopedia.pub/entry/15687 (accessed February 07, 2026).
Amoroso, R. (2021, November 03). Deep eutectic solvents in pharmaceutical synthesis. In Encyclopedia. https://encyclopedia.pub/entry/15687
Amoroso, Rosa. "Deep eutectic solvents in pharmaceutical synthesis." Encyclopedia. Web. 03 November, 2021.
Copy Citation
DES are mixtures of two or more compounds, able to form liquids upon mixing, with lower freezing points when compared to the individual constituents (eutectic mixtures). DES have been utilized in organic synthesis as green media thanks to the high potential to replace the classical solvents. In fact, since the DES are characterized by a network of hydrogen bonds, they have the possibility to dissolve solutes that can form hydrogen bonds and stabilize transition states. Furthermore, during the purification procedures, the addition of water to a DES (very soluble in water) causes the precipitation of organic products, facilitating the workup and avoiding the use of solvents for the extraction.
NADES
choline chloride
pharmaceutical synthesis
1. Choline-Based DES as Solvents
The thiazolidin-4-one system is a privileged heterocycle present in many synthetic analogs with a wide range of pharmacological activities [1]. In particular, the 2-iminothiazolidin-4-one pharmacofore has been reported to exhibit anticancer properties that can be associated with the affinity for some biotargets [2]. Among the several synthetic routes available for the synthesis of the multifunctionalized 2-iminothiazolidin-4-ones, Mobinikhaledi and Amiri modified the classical pathway based on condensation between thioureas with chloroacetyl chloride, by introducing a one-pot three-component reaction in NADES, obtaining a variety of 5-arylidene-2-imino-4-thiazolidinones [3]. As shown in Scheme 1, they investigated the reaction of thioureas, chloroacetyl chloride, and aromatic aldehydes in ChCl/urea. The best results were obtained with condensation of N,N’-diphenylthiourea, chloroacetyl chloride, and benzaldehyde to give 5-benzylidene-3-phenyl-2(phenylimino) thiazolidin-4-one in 94% yield.
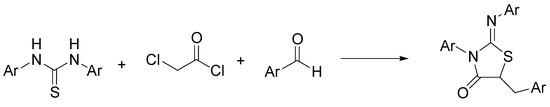
Scheme 1. Condition reactions: ChCl/urea (12), 30 min, 80 °C (Ar = aryl).
The bis(indolyl)methanes (BIM) are indole derivatives with a broad spectrum of biological and pharmacological activities, present in various natural products and possessing important biological activity [4]. In particular, some BIM from cruciferous plants inhibit breast cancer cell proliferation by activation of multiple pathways, suggesting a potential role in the clinical treatment of ER-negative breast cancer [5]. The methods of BIM synthesis involve both one step as well as multistep strategies, based on the condensation of indoles and carbonyl compounds, using protic acids and Lewis acids as catalysts [6]. Recently, Grosso et al. reported the use of ChCl-based DES as green solvents to carry out a bis-hetero-Diels–Alder addition of nitroso- and azoalkenes with indoles [7]. The obtained BIM furnished, after hydrolysis, the corresponding carbonyl-BIM. Among the numerous reactions proposed, the best yields (50–80%) were obtained in the synthesis of hydrazonomethyl-BIM in H2O/[ChCl/glycerol (2:1)] (3:1) starting from alkylhydrazones (Scheme 2).
Scheme 2. Condition reactions: H2O/[ChCl/glycerol (2:1)] (3:1), Na2CO3, 10–60 min, r.t.
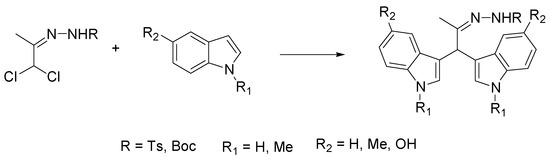
Chiral 1,3-dicarbonyl compounds bearing an α-amine moiety represent an important structural motif present in natural compounds, most of them having biological and pharmaceutical activity [8]. Among the methods to gain access to this motif, significant developments have been reached in the asymmetric organocatalyzed amination of prochiral carbonyl compounds employing diazocarboxylates as nitrogen source [9]. Nigues et al. have improved the sustainability of this reaction by using ChCl/glycerol and ChCl/urea as the solvents and 2-aminobenzimidazole derivatives as catalysts [10]. As depicted in Scheme 3, the reaction of ethyl 2-oxocyclopentane-1-carboxylate with di-t-butyl-azodicarboxylate (DBAB) with a chiral 2-aminobenzimidazole derivative as catalyst under the optimized conditions gave the product of amination in excellent yield (94%) and good enantioselectivity (73% e.e.).
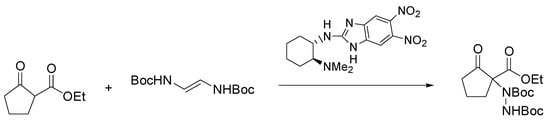
Scheme 3. Condition reactions: ChCl/glycerol (1:2), 1 h, 25 °C.
Propargylamines are alkyne coupled amine compounds used as skeletal structures in heterocyclic chemistry and drug design [11]. For example, rasagiline is a monoamine oxidase (MAO)-B inhibitor with neuroprotective and antiapoptotic effects, used in Parkinson’s disease and other neurodegenerative conditions [12]. Because of its versatility and wide use in organic synthesis, a number of methodologies have been developed for propargylamine derivatives, in particular three-component reactions of aldehydes, amines, and alkynes [13]. This method has been recently improved by substituting the classical solvents with environmentally friendly ChCl/urea [14]. First, the reaction between benzaldehyde, morpholine, and phenylacetylene in the presence of CuCl (5 mol%) as a catalyst was selected as a model to set the best conditions (solvent, catalyst amount, temperature, and time). Then, various aldehydes were used, and the best results were obtained with aromatic aldehydes with electron donor groups (38–90%) (Scheme 4).
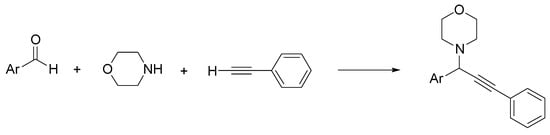
Scheme 4. Condition reactions: ChCl/urea (1:2), CuCl (5 mol%), 15 h, 60 °C (Ar = aryl).
Benzoxazines are compounds where an oxazine ring is condensed to a benzene ring, used in polymers, resins, and in the construction of interesting drug candidates as antilipidemics [15]. In this field, interesting compounds are the cardanol-derived benzoxazines. Cardanol is a natural oil with a phenolic structure, extracted from cashew or from Gingko biloba, extensively studied for its possible applications in industry and in pharmaceutical chemistry [16]. Behalo et al. developed a green Mannich-like condensation of a cardanol with its unsaturated derivative, aniline, and formaldehyde, in ChCl/urea as DES with good yields (81–88%) (Scheme 5). They also demonstrated the use of cardanol-based benzoxazines as scaffolds for the preparation of vesicular nanosystems [17].
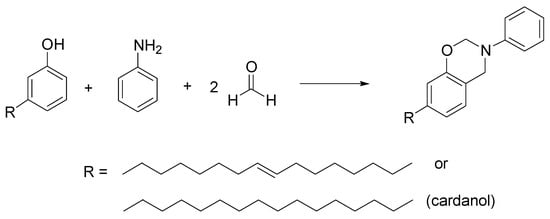
Scheme 5. Condition reactions: ChCl/urea (1:2), 4 h, 70 °C.
Nicotinamide is an important naturally occurring heterocycle, which belongs to group B vitamins. Quaternary derivatives of nicotinamide have attracted much attention because of their antimicrobial, fungicidal, and cytotoxic properties [18]. The most common strategy for quaternization of nicotinamide is the nucleophilic substitution between pyridine and substituted 2-bromoacetophenones, called the Menschutkin reaction [19]. Busic et al. investigated the use of three ChCl-based DES in the quaternization of nicotinamide with 2-bromoacetophenones, performed by a conventional approach, microwaves (MW), and ultrasonic irradiation (blue). The highest yields were obtained by MW, and independently from the adopted solvent (Scheme 6) [20].
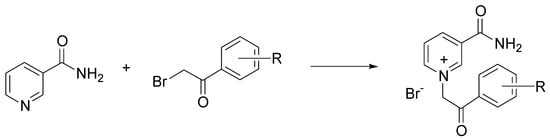
Scheme 6. Condition reactions: ChCl/urea, ossalic acid, or levulinic acid, MW, 20 min, 80 °C.
2. Choline-Based DES as Solvents/Catalysts
As described above, DES had great potential as a green alternative to classical organic solvents; in addition, in the last years, they have emerged as catalysts for various organic reactions, because they can be applied both as reaction media and as catalytic active species, providing homogeneous solutions. Although there are numerous examples of the catalytic effect of DES, this role has not been fully defined yet. To date, some mechanisms of catalysis have been proposed, mostly based on the formation of hydrogen bonds between the reactants and DES, thus facilitating the breaking of the chemical bonds involved in the reaction [21]. In this section, some representative examples of reactions carried out in ChCl-based DES working both as solvents and catalysts will be described. In reacting substrates, the function involved is always a carbonyl group, which is activated by DES thanks to its ability to establish strong hydrogen bonds, thus facilitating the subsequent electrophilic attack in reactions such as Michael additions, transesterifications, and condensations (Figure 1).
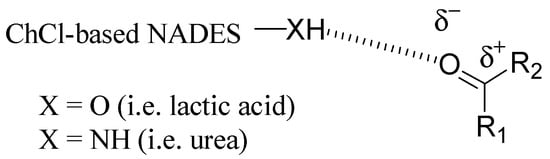
Figure 1. Activation of a carbonyl group by a ChCl-based DES/NADES as catalyst.
Aurones, (Z)-2-benzylidenebenzofuran-3(2H)-ones, are a subclass of flavonoids that provide the golden-yellow color in many ornamental flowers. They exhibit a broad spectrum of activities including anticancer, antioxidant, antiparasitic, and antibacterial properties [22]. The simplest chemical synthesis of aurones is based on the condensation of a coumaranone with an aldehyde, under acidic, or basic conditions. Hawkins and Handy discovered a neutral condition for this condensation, based on the use of ChCl/urea, although with not excellent yields (max 73%) [23]. The reaction proceeds simply and cleanly by mixing the reactants in DES, and the desired aurones were recovered by extractive workup (Scheme 7).
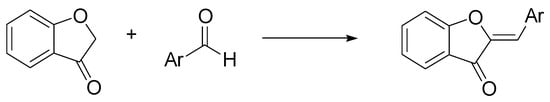
Scheme 7. Condition reactions: ChCl/urea (1:2), 12-48 h, 80 °C (Ar = aryl).
Novobiocin is a naturally occurring compound containing the aminocoumarin scaffold, with hopeful anticancer activity. Besides, other dihydrocoumarin and aminocoumarin derivatives possess significant bioactivities such as cytotoxic, anti-inflammatory, and antibiotic [24]. Despite the role as therapeutic agents, the available synthetic methods require toxic solvents or metal catalysts, with adverse effects on the environment. A new tandem approach to prepare the 3-aminohexahydroxcoumarins directly from aryl aldehydes, ippuric acid, acetic anhydride, and 1,3-dicarbonyl compounds, with ChCl/urea as a biorenewable solvent was developed by Zamani and Khosropour [25]. After preliminary studies in the bath, the reactions were conducted in a continuous micro-flow reactor, including two tubing glass (Q1 for aryl aldehydes, hippuric acid, and acetic anhydride, and Q2 for 1,3-dicarbonyl compounds) giving faster and quicker results. In Q1 an azalactone intermediate is formed, and then in Q2 the Michael addition of 1,3-dicarbonyl compounds gives rise to the target products (Scheme 8).
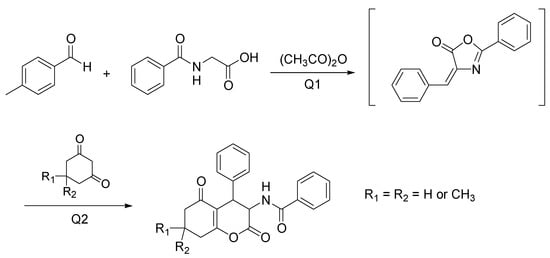
Scheme 8. Optimized condition reactions: ChCl/urea (1:2), 120 °C, 40 min (Q1) and 10 min (Q2).
The cyclopentenone moiety is a fundamental structural motif present in molecules such as prostaglandins, clavulones, and cephalotaxine esters, with significant anticancer activity [26]. Most of the reported synthetic strategies for the preparation of cyclopentenone require expensive catalysts, toxic solvents, and proceed with low yields. Therefore, there is an ongoing interest to develop new methodologies; in particular, erbium (III) chloride has found a good catalyst acting in green solvents, such as ethyl lactate [27]. Recently, Di Gioia et al. developed a simple and rapid method for the conversion of furfural into bifunctionalized cyclopentenones under green conditions by using the ChCl:urea, without any product purification [28]. They observed that reaction of furfural with secondary amines furnished, after 5 min, the bifuntionalized cyclopentenone A, that was totally converted to the structural isomer B after 4 h. These two conditions were applied for the synthesis of a series of derivatives, as shown in Scheme 9.
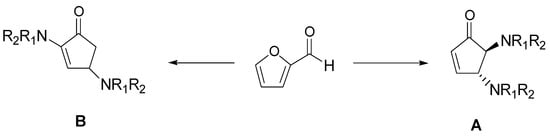
Scheme 9. Optimized reaction conditions: ChCl/urea (1:2), 60 °C, 5 min (A) and 4 h (B).
The merger of pyrazolo[3,4-b]pyridine and spirooxindole backbones into a hybrid structure (pyrazolo[3,4-b]pyridine spirooxindole) may result in interesting compounds with potential medicinal applications, since both scaffolds have remarkable pharmacological properties [29]. Of note is the anti-breast-cancer activity of some of these hybrid derivatives [30]. Zangh et al. proposed a sustainable protocol for the synthesis of pyrazolo[3,4-b]pyridines spirooxindoles with a variety of substituents through a one-pot three-component assembly between 1,3-aromatic-1H-pyrazol-5-amines, substituted isatins, and enolizable 1,3 dicarbonyl compounds. Excellent yields (82–95%) were obtained under microwave (MW) irradiation in ChCl:lactic acid as NADES (Scheme 10) [31].
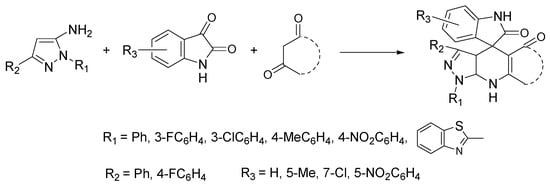
Scheme 10. Reaction conditions: ChCl/lactic acid (1:2), MW, 60 °C, 15–30 min.
Pyrazolylphosphonates are biologically interesting compounds containing phosphonate and pyrazole. They have received considerable attention due to their remarkable bioactivity profiles, with promising antioxidant and anticancer activity [32]. This interest has prompted the development of various synthetic approaches, including one-pot multicomponent reactions. Of interest is the condensation of pyrazolones with aldehydes and trialkyl phosphites, based on the in situ formation of benzylidene pyrazolone and subsequent reaction with a phosphorus nucleophile. Kalla and Kim have used as a catalyst for this one-pot synthesis a ChCl-urea mixture, with a series of advantages such as bio-friendly profile, mild conditions at r.t., and the avoidance of chromatographic purification [33]. The authors first developed a model reaction between 5-methylpyrazolone, benzaldehyde, and triethyl phosphite in mild conditions (Scheme 11). Then, the scope reaction was investigated using various aromatic aldehydes, pyrazolones, and trialkyl phosphites, allowing us to assess a clear improvement in product yields (90–96%) when using DES with respect to the other catalysts previously reported.
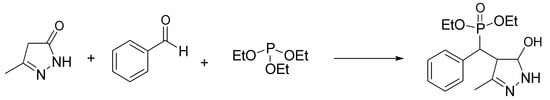
Scheme 11. Condition reactions: ChCl/urea (1:2), 25 °C, 30 min.
From a synthetic chemistry point of view, the above illustrated 2-imino-4-thiazolidinones are very interesting scaffolds, due to the presence of electrophilic centers and activated exocyclic double bonds, and therefore they can behave like dipolarophiles in 1,3-dipolar cycloaddition reactions. In this context, Singh et al. have used 2-iminothiazolidin-4-ones as a core system for the construction of hybrids with spiro-oxindole-pyrrolidines as novel druglike molecules [34]. The preparation of hybrids was carried out with excellent yields (50–92%) and high regio- and stereo-selectivity by means of the multicomponent 1,3-dipolar cycloaddition of (2Z)-methyl-2-[(Z)-4-oxo-3-aryl-2-(arylimino) thiazolidin-5-ylidene] acetate as dipolarophile with substituted isatins and sarcosine, using ChCl/urea as the green solvent (Scheme 12).
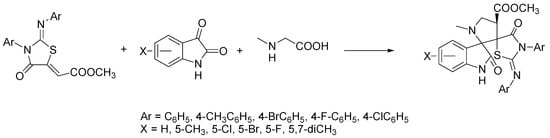
Scheme 12. Condition reactions: ChCl/urea (1:2), 40–100 °C, 2–5 h.
3. Choline-Based DES as Chemical Donors
In the last years, biocompatible materials for development of drug delivery systems have been synthesized by using NADES as sustainable reaction media [35]. An interesting study conducted by Pradeepkumar et al. described the preparation of a ε-caprolactone-citric acid micellar nanocarrier for controlled release of camptothecin [36]. In this procedure, the NADES acted both as a solvent and as a citric acid donor for the functionalization of caprolactone (Scheme 13). The structure of encapsulated micelles was analyzed by FT-IR and UV spectrometry, and biological studies were performed, showing a controlled release of camptothecin and time-dependent apoptosis. These results suggest that these micellar nanocarriers could represent a potent system for cancer therapy.
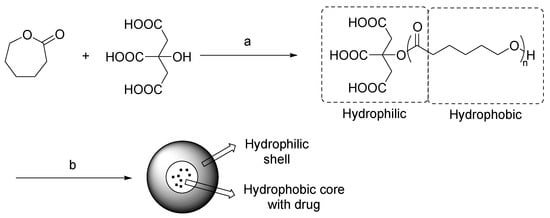
Scheme 13. Condition reactions: (a) ChCl/citric acid (1:2), 120 °C, 24 h; (b) self-assembly, campthotecin.
Pyrrole is a five membered aromatic heterocycle present in complex macromolecules including porphyrins of heme and chlorophyll and in natural products. Pyrrole derivatives can serve as promising drug candidates with antimicrobial, antiviral, antimalarial, antitubercular, anti-inflammatory, and enzyme inhibiting activities [37]. Hu et al. reported the condensation reaction between tricarbonyl compounds and ammonia generated in situ from the eutectic mixture of ChCl/urea [38]. Triketones were completely transformed into pyrrole products with high yields at an optimum temperature of 100 °C, and the urea present in the DES was transferred to the final product of the reaction following its thermal decomposition (Scheme 14).
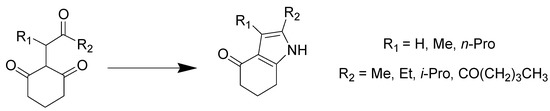
Scheme 14. Condition reactions: ChCl/urea (1:2), 100 °C, 8 h.
Caffeic acid is a natural hydroxycinnamic acid phenolic derivative (3,4-dihydroxycinnamic acid), with antioxidant, anti-inflammatory, antimicrobial, and antineoplastic activities [39]. However, its unsatisfactory solubilities in both hydrophobic and hydrophilic systems limit its application in chemical industries. Considering that caffeoyl lipophilic alkyl esters show superior biological and pharmacological properties with respect to their phenolic acid counterparts, much research has focused on their preparation in chemical, biochemical, or enzymatic ways [40]. In this field, Wang et al. recently described a novel method for octodecyl caffeate production by using a NADES consisting of ChCl and caffeic acid as the caffeoyl donor, with cation-exchange resins as green catalysts and fatty alcohols (ROH) as caffeoyl acceptors (yield 91%) (Scheme 15) [41]. The proposed esterification mechanism involves the H+ released from the cation-exchange resin, that attacks the carboxyl carbon of caffeic acid to form a carbocation. Then, the OH of stearyl alcohol reacts with the carbocation, giving a tetrahedral intermediate, that releases H2O and H+ to form octodecyl caffeate.
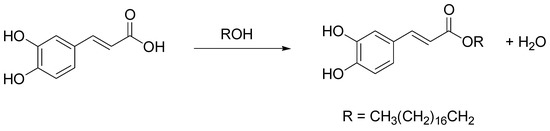
Scheme 15. Optimized condition reactions: ChCl/caffeic acid, cation-exchange resin A-35 load 5%, 85 °C, 24 h.
References
- Nirwan, S.; Chahal, V.; Kakkar, R. Thiazolidinones: Synthesis, reactivity, and their biological applications. J. Heterocycl. Chem. 2019, 56, 1239–1253.
- Ashraf, S.; Saeed, A.; Moon, S.-H.; Flörke, U.; Kim, S.H.; Ashraf, Z.; Yaseen, M.; Muhammad, L. Design, synthesis and biological evaluation of 2-(naphthoyl) iminothiazolidin-4-ones as potential anticancer agents. ChemistrySelect 2020, 5, 3965–3970.
- Mobinikhaledi, A.; Amiri, A.K. Natural eutectic salts catalyzed one-pot synthesis of 5-arylidene-2-imino-4-thiazolidinones. Res. Chem. Intermed. 2013, 39, 1491–1498.
- Shiri, M.; Zolfigol, M.A.; Kruger, H.G.; Tanbakouchian, Z. Bis- and trisindolylmethanes (BIMs and TIMs). Chem. Rev. 2010, 110, 2250–2293.
- Vanderlaag, K.; Su, Y.; Frankel, A.E.; Grage, H.; Smith, R., 3rd; Khan, S.; Safe, S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit proliferation of estrogen receptor-negative breast cancer cells by activation of multiple pathways. Breast Cancer Res. Treat. 2008, 109, 273–283.
- Singh, A.; Kaur, G.; Bubun Banerjee, B. Recent developments on the synthesis of biologically significant bis/tris(indolyl)methanes under various reaction conditions: A review. Curr. Org. Chem. 2020, 24, 583–621.
- Grosso, C.; Brigas, A.; de los Santos, J.M.; Palacios, F.; Lemos, A.; Pinho e Melo, T.M.V.D. Natural deep eutectic solvents in the hetero-Diels–Alder approach to bis(indolyl)methanes. Bioorg. Med. Chem. 2017, 25, 1122–1131.
- Broggini, G.; Borsini, E.; Piarulli, U. Science of Synthesis, Cross Coupling and Heck-Type Reactions; Molander, G.A., Wolfe, J.P., Larhed, M., Eds.; Thieme: Stuttgart, Germany, 2013; Volume 3, pp. 521–583.
- Khose, V.N.; John, M.E.; Pandey, A.D.; Karnik, A.V. Chiral benzimidazoles and their applications in stereodiscrimination processes. Tetrahedron Asymmetry 2017, 28, 1233–1289.
- Ñíguez, D.R.; Khazaeli, P.; Alonso, D.A.; Guillena, G. Deep eutectic mixtures as reaction media for the enantioselective organocatalyzed-amination of 1,3-dicarbonyl compounds. Catalysts 2018, 8, 217.
- Xu, Y.-X.; Wang, H.; Li, X.-K.; Dong, S.-N.; Liu, W.-W.; Gong, Q.; Wang, T.-D.-Y.; Tang, Y.; Zhu, J.; Li, J.; et al. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinylthiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 143, 33–47.
- Yogev-Falach, M.; Amit, T.; Bar-Am, O.; Youdim, M.B.H. The importance of propargylamine moiety in the anti-Parkinson drug rasagiline and its derivatives in MAPK-dependent amyloid precursor protein processing. FASEB J. 2003, 17, 2325–2327.
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 2017, 117, 14091–14200.
- Abtahi, B.; Tavakol, H. Choline chloride-urea deep eutectic solvent as an efficient media for the synthesis of propargylamines via organocuprate intermediate. Appl. Organomet. Chem. 2020, 34, e5895.
- Matralis, A.N.; Katselou, M.G.; Nikitakis, A.; Kourounakis, A.P. Novel benzoxazine and benzothiazine derivatives as multifunctional antihyperlipidemic agents. J. Med. Chem. 2011, 54, 5583–5591.
- Andrea Minigher, A.; Elena Benedetti, E.; De Giacomo, O.; Campaner, P.; Aroulmoji, V. Synthesis and characterization of novel cardanol based benzoxazines. Nat. Prod. Commun. 2009, 4, 521–528.
- Behalo, M.S.; Bloise, E.; Mele, G.; Salomone, A.; Messa, F.; Carbone, L.; Mazzetto, S.E.; Lomonaco, D. Bio-based benzoxazines synthesized in a deep eutectic solvent: A greener approach toward vesicular nanosystems. J. Heterocycl. Chem. 2020, 57, 768–773.
- Günther, A.; Pełech, R. Bio-active pyridinium salts: A mini-review on properties and selected reactions. Mini-Rev. Org. Chem. 2019, 16, 610–616.
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry; Wiley: Hoboken, NJ, USA, 2001.
- Bušić, V.; Roca, S.; Vikić-Topić, D.; Vrandečić, K.; Ćosić, J.; Molnar, M.; Gašo-Sokač, D. Eco-friendly quaternization of nicotinamide and 2-bromoacetophenones in deep eutectic solvents. Antifungal activity of the products. Environ. Chem. Lett. 2020, 18, 889–894.
- Ünlü, A.E.; Arıkaya, A.; Takaç, S. Use of deep eutectic solvents as catalyst: A mini-review. Green Process Synth. 2019, 8, 355–372.
- Popova, A.; Bondarenko, S.P.; Frasinyuk, M.S. Aurones: Synthesis and properties. Chem. Heterocycl. Comp. 2019, 55, 285–299.
- Hawkins, I.; Handy, S.T. Synthesis of aurones under neutral conditions using a deep eutectic solvent. Tetrahedron 2013, 69, 9200–9204.
- Dlugosz, A.; Janecka, A. Novobiocin analogs as potential anticancer agents. Mini Rev. Med. Chem. 2017, 17, 728–733.
- Zamani, P.; Khosropour, A.R. A combination of natural deep eutectic solvents and microflow technology: A sustainable innovation for the tandem synthesis of 3-aminohexahydrocoumarins. Green Chem. 2016, 18, 6450–6455.
- Conti, M. Cyclopentenone: A special moiety for anticancer drug design. Anticancer Drugs 2006, 17, 1017–1022.
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) chloride in ethyl lactate as a smart ecofriendly system for efficient and rapid stereoselective synthesis of trans-4,5-diaminocyclopent-2-enones. ACS Sustain. Chem. Eng. 2013, 1, 541–544.
- Di Gioia, M.L.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable Deep Eutectic Solvent for selective and scalable conversion of furfural into cyclopentenone derivatives. Molecules 2018, 23, 1891.
- Bora, D.; Kaushal, A.; Shankaraiah, N. Anticancer potential of spirocompounds in medicinal chemistry: A pentennial expedition. Eur. J. Med. Chem. 2021, 215, 113263.
- Eldehna, W.M.; EL-Naggar, D.H.; Hamed, A.R.; Ibrahim, H.S.; Ghabbour, H.A.; Abdel-Aziz, H.A. One-pot three-component synthesis of novel spirooxindoles with potential cytotoxic activity against triple-negative breast cancer MDA-MB-231 cells. J. Enzyme Inhib. Med. Chem. 2018, 33, 309–318.
- Zhang, W.-H.; Chen, M.-N.; Hao, Y.; Jiang, X.; Zhou, X.-L.; Zhang, Z.-H. Choline chloride and lactic acid: A natural deep eutectic solvent for onepot rapid construction of spiropyridines]. J. Mol. Liq. 2019, 278, 124–129.
- Lin, R.; Chiu, G.; Yu, Y.; Connolly, P.J.; Li, S.; Lu, Y.; Adams, M.; Fuentes-Pesquera, A.R.; Emanuel, S.L.; Greenberger, L.M. Design, synthesis, and evaluation of 3,4-disubstituted pyrazole analogues as anti-tumor CDK inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4557–4561.
- Kalla, R.M.N.; Kim, I. Highly efficient synthesis of pyrazolylphosphonate derivatives in biocompatible deep eutectic solvent. Mol. Catal. 2019, 473, 110396.
- Singh, R.; Saini, M.R.; Bhardwaj, D.; Singh, A. An expedient synthesis of new iminothiazolidinone grafted dispiro-pyrrolidineoxindole/indeno hybrids via a multicomponent cycloaddition reaction in a deep eutectic solvent. New J. Chem. 2020, 44, 7923–7931.
- del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. ChemSusChem 2014, 7, 999–1009.
- Pradeepkumar, P.; Elgorban, A.M.; Bahkali, A.H.; Rajan, M. Natural solvent-assisted synthesis of amphiphilic co-polymeric nanomicelles for prolonged release of camptothecin delivery. New J. Chem. 2018, 42, 10366–10375.
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31.
- Hu, L.; Luo, J.; Lu, D.; Tang, Q. Urea decomposition: Efficient synthesis of pyrroles using the deep eutectic solvent choline chloride/urea. Tetrahedron Lett. 2018, 59, 1698–1701.
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246.
- Sørensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant properties and efficacies of synthesized alkyl caffeates, ferulates, and coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562.
- Wang, X.; Sun, S.; Hou, X. Synthesis of lipophilic caffeoyl alkyl ester using a novel natural deep eutectic solvent. ACS Omega 2020, 5, 11131–11137.
More
Information
Subjects:
Chemistry, Medicinal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
04 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




