
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gunes Ozhan | + 2589 word(s) | 2589 | 2021-11-03 09:08:22 | | | |
| 2 | Beatrix Zheng | + 199 word(s) | 2788 | 2021-11-03 10:59:35 | | |
Video Upload Options
Wnt signaling pathways constitute a group of signal transduction pathways that direct many physiological processes, such as development, growth, and differentiation. Dysregulation of these pathways is thus associated with many pathological processes, including neurodegenerative diseases, metabolic disorders, and cancer. At the same time, alterations are observed in plasma membrane compositions, lipid organizations, and ordered membrane domains in brain and metabolic diseases that are associated with Wnt signaling pathway activation.
1. Introduction
Wnt signaling pathways are highly conserved in the animal kingdom, based on their components and functional roles in the regulation of development, tissue homeostasis, and regeneration [1][2][3][4][5][6][7][8]. Thus, it is not surprising that changes in Wnt pathway components and modulators—including loss or gain of function—play a role in many pathologies associated with growth, development, and cancer. Although major pathway components have been characterized in detail, misregulation of Wnt signaling within the context of human diseases is extremely complex, and remains only partially understood. Understanding of this underlying complexity will enable the identification of novel therapeutic targets for many diseases associated with the Wnt pathway [9][10][11].
The plasma membrane plays a fundamental role in the regulation of cell signaling. Regulation occurs through the surface receptors, modulators, and associated lipids that actively control the transmission of molecular signals from the outside to the inside and activate downstream signaling events. The plasma membrane consists of nanodomains—the so-called ordered membrane domains or lipid rafts that are defined as dynamic assemblies of various saturated lipids, sterols, glycosphingolipids, and glycosyl-phosphatidylinositol (GPI)-anchored proteins [12][13][14]. These domains influence membrane fluidity and receptor trafficking, thereby playing a key role in the functioning of receptors, protein sorting, and regulation of receptor-mediated signaling [15][16][17][18]. These nanodomains have been revealed to be altered in various diseases, including cancer, neurological and neurodegenerative diseases, and metabolic diseases [19][20][21]. Changes in the composition and organization of membrane proteins and lipids also play an important role in Wnt pathway activation and, thus, in the pathology of pathway-associated diseases [22][23]. Considering that the membrane proteins account for over 60% of the targets of all FDA-approved small-molecule drugs, it is critical to characterize Wnt pathway components that act across the plasma membrane as potential therapeutic targets [9][22][24][25]. Here, we review the abnormal regulation of the Wnt signaling pathway in brain and metabolic disorders. In particular, we address how plasma membrane components of Wnt pathways and membrane domain organization are affected in Alzheimer’s disease (AD), Parkinson’s disease (PD), Schizophrenia (SZ), diabetes, obesity, nonalcoholic fatty liver disease (NAFLD), and nonalcoholic steatohepatitis (NASH).
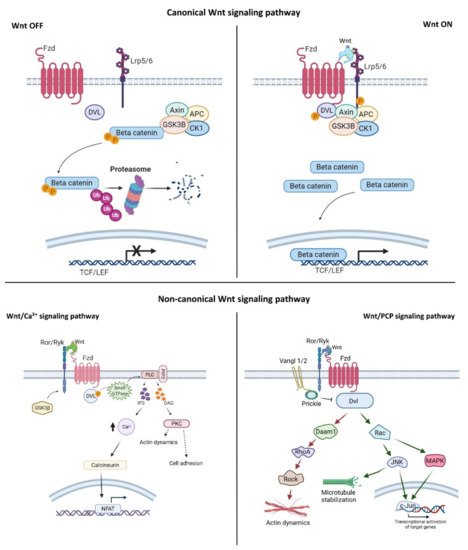
2. Wnt Signaling Pathways
Wnt signaling is an evolutionarily conserved signaling pathway that controls a wide range of biological responses, including proliferation, differentiation, preservation of the stem cell pool, control of lineage-specific tissue differentiation during embryogenesis, and maintenance of adult tissue homeostasis [3][4][5]. The Wnt pathway is divided into two main groups—i.e., β-catenin-dependent (canonical) and β-catenin-independent (non-canonical)—which can be further divided into the planar cell polarity (PCP) and the Wnt/Ca 2+ pathways ( Figure 1 ). The canonical Wnt cascade is inactive in the absence of Wnt ligands, and this leads to phosphorylation of β-catenin by a cytoplasmic multiprotein complex that contains the kinases glycogen synthase kinase 3β (Gsk3β) and casein kinase 1a (Ck1a), the scaffold protein Axin, and adenomatous polyposis coli (Apc) [26][27]. This phosphorylation targets cytoplasmic β-catenin for degradation by the ubiquitin–proteasome system. Canonical Wnt signaling is activated by binding of Wnt ligands to the membrane receptor Frizzled (Fzd) and the co-receptor low-density lipoprotein-receptor-related protein (Lrp) 5/6. Formation of the Wnt–receptor complex leads to the recruitment of the core components of the destruction complex to the cell surface, phosphorylation of the cytoplasmic tail of Lrp6 by Gsk3β and Ck1α, and stabilization of β-catenin in the cytoplasm and its nuclear translocation. In the nucleus, β-catenin interacts with the T-cell factor/lymphoid enhancer factor (Tcf/Lef) family of transcription factors, and regulates the expression of target genes [28][29]. The PCP pathway was originally described in the fruit fly Drosophila melanogaster , and controls coordinated, uniformly polarized cellular behavior in a wide variety of cells [30]. In mammals, PCP regulates key developmental processes ranging from neural tube closure to determination of left– right (L–R) asymmetry, and demonstrates essential roles in vertebrate development [31]. In the PCP pathway, the non-canonical Wnt ligands interact with the receptor Fzd and co-receptors (receptor tyrosine kinase-like orphan receptor (Ror)/receptor tyrosine kinase-related tyrosine kinase (Ryk)/protein tyrosine kinase 7 (Ptk7)). These interactions regulate the small GTPase molecules Rho, Rac, and Cdc42, and activate the kinases c-Jun N-terminal kinase (Jnk), the mitogen-activated protein kinase (MAPK) pathways, and Rho/Rho-associated coiled-coil-containing protein kinase (Rock) to control cell polarization and migration [32][33][34]. In the Wnt/Ca 2+ pathway, intracellular Ca 2+ is activated by the binding of Wnt to Fzd and coupling between Fzds and G proteins. This further activates protein kinase C (PKC), calcium/calmodulin-dependent protein kinase type II (CaMKII), and nuclear factor of activated T cells (NFAT), and regulates cell movement, cell fate, and cell migration as well as suppressing the canonical Wnt pathway ( Figure 1 ) [33][34][35].
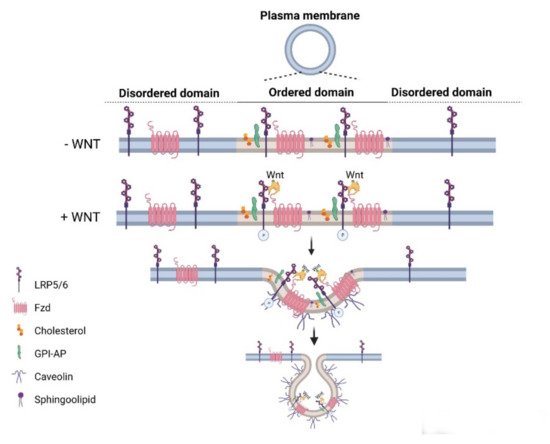
Wnt pathways are fine-tuned by a number of positive and negative regulators that can affect the ligand–receptor complex interactions at the plasma membrane, cytoplasmic events, or nuclear control of transcription [22][36][37][38][39][40][41][42][43]. The plasma membrane plays key roles in protection of the cell from its surroundings, providing a stable environment inside the cell, management of molecular transport, and cell–cell communication. Embodying numerous receptors and lipids that take part in cell signaling, the plasma membrane is critical for the reception of signals and their transmission through a series of molecular switches to internal signaling pathways. The activity of the canonical Wnt signaling pathway is also dependent on the membrane components that tightly regulate the interaction of ligands with their (co)receptors in the specialized membrane nanodomains, i.e., the ordered membrane domains or lipid rafts ( Figure 2 ). The ordered domains are necessary not only for the proper interaction of the canonical Wnt ligand with its (co)receptors, phosphorylation of Lrp6, endocytosis of receptor complexes, and downstream canonical signaling activity, but also for the regulation of non-canonical Wnt signaling activity [15][42][44][45]. The roles of the ordered membrane domains in the activation of Wnt pathways have been reviewed in detail previously [22]. Here, we focus on the involvement of Wnt–receptor complex components and ordered membrane domains or lipid rafts in certain brain disorders and metabolic diseases.
3. Wnt Signaling Pathway in Metabolic Diseases
3.1. Diabetes and Obesity
3.2. Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
4. Conclusions
References
- Aman, A.J.; Fulbright, A.N.; Parichy, D.M. Wnt/β-catenin regulates an ancient signaling network during zebrafish scale development. eLife 2018, 7, e37001.
- Behari, J. The Wnt/β-catenin signaling pathway in liver biology and disease. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 745–756.
- Grainger, S.; Willert, K. Mechanisms of Wnt signaling and control. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1422.
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999.
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589.
- Demirci, Y.; Cucun, G.; Poyraz, Y.K.; Mohammed, S.; Heger, G.; Papatheodorou, I.; Ozhan, G. Comparative Transcriptome Analysis of the Regenerating Zebrafish Telencephalon Unravels a Resource with Key Pathways During Two Early Stages and Activation of Wnt/β-Catenin Signaling at the Early Wound Healing Stage. Front. Cell Dev. Biol. 2020, 8, 584604.
- Ozhan, G.; Weidinger, G. Wnt/β-catenin signaling in heart regeneration. Cell Regen. 2015, 4, 3.
- Zhan, G.; Weidinger, G. Restoring Tissue Homeostasis: Wnt Signaling in Tissue Regeneration after Acute Injury. In Wnt signaling in Development and Disease: Molecular Mechanisms and Biological Functions; Hoppler, S.P., Moon, R.T., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014.
- Bonnet, C.; Brahmbhatt, A.; Deng, S.X.; Zheng, J.J. Wnt signaling activation: Targets and therapeutic opportunities for stem cell therapy and regenerative medicine. RSC Chem. Biol. 2021, 2, 1144–1157.
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480.
- Luo, J.; Chen, J.; Deng, Z.-L.; Luo, X.; Song, W.-X.; A Sharff, K.; Tang, N.; Haydon, R.C.; Luu, H.H.; He, T.-C. Wnt signaling and human diseases: What are the therapeutic implications? Lab. Investig. 2007, 87, 97–103.
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39.
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020, 30, 341–353.
- Sviridov, D.; Miller, Y.I. Biology of Lipid Rafts: Introduction to the Thematic Review Series. J. Lipid Res. 2020, 61, 598–600.
- Sezgin, E.; Azbazdar, Y.; Ng, X.W.; Teh, C.; Simons, K.; Weidinger, G.; Wohland, T.; Eggeling, C.; Ozhan, G. Binding of canonical Wnt ligands to their receptor complexes occurs in ordered plasma membrane environments. FEBS J. 2017, 284, 2513–2526.
- Sezgin, E.; Gutmann, T.; Buhl, T.; Dirkx, R.; Grzybek, M.; Coskun, Ü.; Solimena, M.; Simons, K.; Levental, I.; Schwille, P. Adaptive Lipid Packing and Bioactivity in Membrane Domains. PLoS ONE 2015, 10, e0123930.
- Staubach, S.; Hanisch, F.-G. Lipid rafts: Signaling and sorting platforms of cells and their roles in cancer. Expert Rev. Proteom. 2011, 8, 263–277.
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408–413.
- Drolle, E.; Turnbull, S.; Mei, N.; Filice, C.; Lee, B.Y.; Robinson, M.; Pavlov, E.; Finot, E.; Leonenko, Z. Changes in Lipid Membrane May Trigger Amyloid Toxicity in Alzheimer’s Disease. Biophys. J. 2019, 116, 427a.
- Fabiani, C.; Antollini, S.S. Alzheimer’s Disease as a Membrane Disorder: Spatial Cross-Talk Among Beta-Amyloid Peptides, Nicotinic Acetylcholine Receptors and Lipid Rafts. Front. Cell. Neurosci. 2019, 13, 309.
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810.
- Azbazdar, Y.; Karabicici, M.; Erdal, E.; Ozhan, G. Regulation of Wnt Signaling Pathways at the Plasma Membrane and Their Misregulation in Cancer. Front. Cell Dev. Biol. 2021, 9, 631623.
- Driehuis, E.; Clevers, H. WNT signalling events near the cell membrane and their pharmacological targeting for the treatment of cancer. Br. J. Pharmacol. 2017, 174, 4547–4563.
- Liu, C.; Takada, K.; Zhu, D. Medicine in Drug Discovery Targeting Wnt/β-Catenin Pathway for Drug Therapy. Med. Drug Discov. 2020, 8, 100066.
- Karabicici, M.; Azbazdar, Y.; Ozhan, G.; Senturk, S.; Karagonlar, Z.F.; Erdal, E. Changes in Wnt and TGF-β Signaling Mediate the Development of Regorafenib Resistance in Hepatocellular Carcinoma Cell Line HuH. Front. Cell Dev. Biol. 2021, 9, 639779.
- Kimelman, D.; Xu, W. β-Catenin destruction complex: Insights and questions from a structural perspective. Oncogene 2006, 25, 7482–7491.
- Macdonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26.
- LeCarpentier, Y.; Schussler, O.; Hébert, J.-L.; Vallée, A. Multiple Targets of the Canonical WNT/β-Catenin Signaling in Cancers. Front. Oncol. 2019, 9, 1248.
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165.
- Maung, S.M.T.W.; Jenny, A. Planar cell polarity in Drosophila. Organogenesis 2011, 7, 165–179.
- Gray, R.; Roszko, I.; Solnica-Krezel, L. Planar Cell Polarity: Coordinating Morphogenetic Cell Behaviors with Embryonic Polarity. Dev. Cell 2011, 21, 120–133.
- Flores-Hernández, E.; Velázquez, D.M.; Castañeda-Patlán, M.C.; Fuentes-García, G.; Fonseca-Camarillo, G.; Yamamoto-Furusho, J.K.; Romero-Avila, M.T.; García-Sáinz, J.A.; Robles-Flores, M. Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells. Cell. Signal. 2020, 72, 109636.
- Wook-Jin, C.; Bothwell, A.L.M. Canonical and Non-Canonical Wnt Signaling in Immune Cells Wook-Jin. Physiol. Behav. 2016, 176, 100–106.
- Xiao, Q.; Chen, Z.; Jin, X.; Mao, R.; Chen, Z. The many postures of noncanonical Wnt signaling in development and diseases. Biomed. Pharmacother. 2017, 93, 359–369.
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2019, 6, 170.
- Cruciat, C.-M.; Niehrs, C. Secreted and Transmembrane Wnt Inhibitors and Activators. Cold Spring Harb. Perspect. Biol. 2012, 5, a015081.
- Torres, V.I.; Godoy, J.A.; Inestrosa, N.C. Modulating Wnt signaling at the root: Porcupine and Wnt acylation. Pharmacol. Ther. 2019, 198, 34–45.
- Zhang, X.; MacDonald, B.T.; Gao, H.; Shamashkin, M.; Coyle, A.J.; Martinez, R.V.; He, X. Characterization of Tiki, a New Family of Wnt-specific Metalloproteases. J. Biol. Chem. 2016, 291, 2435–2443.
- Binnerts, M.E.; Kim, K.-A.; Bright, J.M.; Patel, S.M.; Tran, K.; Zhou, M.; Leung, J.M.; Liu, Y.; Lomas, W.E.; Dixon, M.; et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP. Proc. Natl. Acad. Sci. USA 2007, 104, 14700–14705.
- Vonica, A.; Bhat, N.; Phan, K.; Guo, J.; Iancu, L.; Weber, J.A.; Karger, A.; Cain, J.W.; Wang, E.C.; DeStefano, G.M.; et al. Apcdd1 is a dual BMP/Wnt inhibitor in the developing nervous system and skin. Dev. Biol. 2020, 464, 71–87.
- Kagermeier-Schenk, B.; Wehner, D.; Ozhan-Kizil, G.; Yamamoto, H.; Li, J.; Kirchner, K.; Hoffmann, C.; Stern, P.; Kikuchi, A.; Schambony, A.; et al. Waif1/5T4 inhibits Wnt/beta-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev. Cell 2011, 21, 1129–1143.
- Özhan, G.; Sezgin, E.; Wehner, D.; Pfister, A.S.; Kühl, S.J.; Kagermeier-Schenk, B.; Kühl, M.; Schwille, P.; Weidinger, G. Lypd6 Enhances Wnt/β-Catenin Signaling by Promoting Lrp6 Phosphorylation in Raft Plasma Membrane Domains. Dev. Cell 2013, 26, 331–345.
- Ozalp, O.; Cark, O.; Azbazdar, Y.; Haykir, B.; Cucun, G.; Kucukaylak, I.; Alkan-Yesilyurt, G.; Sezgin, E.; Ozhan, G. Nradd Acts as a Negative Feedback Regulator of Wnt/β-Catenin Signaling and Promotes Apoptosis. Biomolecules 2021, 11, 100.
- Sakane, H.; Yamamoto, H.; Kikuchi, A. LRP6 is internalized by Dkk1 to suppress its phosphorylation in the lipid raft and is recycled for reuse. J. Cell Sci. 2010, 123, 360–368.
- Yamamoto, H.; Sakane, H.; Michiue, T.; Kikuchi, A. Wnt3a and Dkk1 Regulate Distinct Internalization Pathways of LRP6 to Tune the Activation of β-Catenin Signaling. Dev. Cell 2008, 15, 37–48.
- Fujino, T.; Asaba, H.; Kang, M.-J.; Ikeda, Y.; Sone, H.; Takada, S.; Kim, D.-H.; Ioka, R.X.; Ono, M.; Tomoyori, H.; et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc. Natl. Acad. Sci. USA 2002, 100, 229–234.
- Liu, Z.; Habener, J.F. Wnt signaling in pancreatic islets. In The Islets of Langerhans; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 391–419.
- Rulifson, I.C.; Karnik, S.K.; Heiser, P.; Berge, D.T.; Chen, H.; Gu, X.; Taketo, M.M.; Nusse, R.; Hebrok, M.; Kim, S.K. Wnt signaling regulates pancreatic beta cell proliferation. Proc. Natl. Acad. Sci. USA 2007, 104, 6247–6252.
- Xu, W.; Geng, H.; Liu, X.; Wang, X.; Li, R.; Lv, Q.; Liu, Y.; Wang, J.; Yang, M.; Jones, P.M.; et al. Wingless-type MMTV integration site family member 5a: A novel biomarker regulated in type 2 diabetes mellitus and diabetic kidney disease. J. Diabetes Metab. Disord. 2019, 18, 525–532.
- Azar, F.A.; Lim, G.E. Metabolic Contributions of Wnt Signaling: More than Controlling Flight. Front. Cell Dev. Biol. 2021, 9, 709823.
- Müller-Wieland, M.D. Definition, Klassifikation und Diagnostik des Diabetes mellitus; Definition, Classification and Diagnosis of Diabetes Mellitus. Der Diabetol. 2019, 15, 128–134.
- Elhourch, S.; Arrouchi, H.; Mekkaoui, N.; Allou, Y.; Ghrifi, F.; Allam, L.; Elhafidi, N.; Belyamani, L.; Ibrahimi, A.; Elomri, N.; et al. Significant Association of Polymorphisms in the TCF7L2 Gene with a Higher Risk of Type 2 Diabetes in a Moroccan Population. J. Pers. Med. 2021, 11, 461.
- Muendlein, A.; Saely, C.H.; Geller-Rhomberg, S.; Sonderegger, G.; Rein, P.; Winder, T.; Beer, S.; Vonbank, A.; Drexel, H. Single Nucleotide Polymorphisms of TCF7L2 Are Linked to Diabetic Coronary Atherosclerosis. PLoS ONE 2011, 6, e17978.
- Sanghera, D.K.; Nath, S.K.; Ortega, L.; Gambarelli, M.; Kim-Howard, X.; Singh, J.R.; Ralhan, S.K.; Wander, G.S.; Mehra, N.K.; Mulvihill, J.J.; et al. TCF7L2 Polymorphisms are Associated with Type 2 Diabetes in Khatri Sikhs from North India: Genetic Variation Affects Lipid Levels. Ann. Hum. Genet. 2008, 72, 499–509.
- He, Q.J.; Wang, P.; Liu, Q.Q.; Wu, Q.G.; Li, Y.F.; Wang, J.; Lee, S.C. Secreted Wnt6 mediates diabetes-associated centrosome amplification via its receptor FZD. Am. J. Physiol. Physiol. 2020, 318, C48–C62.
- Chen, Y.; Hu, Y.; Zhou, T.; Zhou, K.K.; Mott, R.; Wu, M.; Boulton, M.; Lyons, T.J.; Gao, G.; Ma, J.-X. Activation of the Wnt Pathway Plays a Pathogenic Role in Diabetic Retinopathy in Humans and Animal Models. Am. J. Pathol. 2009, 175, 2676–2685.
- Toomes, C.; Bottomley, H.M.; Jackson, R.M.; Towns, K.V.; Scott, S.; Mackey, D.A.; Craig, J.E.; Jiang, L.; Yang, Z.; Trembath, R.; et al. Mutations in LRP5 or FZD4 Underlie the Common Familial Exudative Vitreoretinopathy Locus on Chromosome 11q. Am. J. Hum. Genet. 2004, 74, 721–730.
- Gaudio, A.; Privitera, F.; Battaglia, K.; Torrisi, V.; Sidoti, M.H.; Pulvirenti, I.; Canzonieri, E.; Tringali, G.; Fiore, C.E. Sclerostin Levels Associated with Inhibition of the Wnt/β-Catenin Signaling and Reduced Bone Turnover in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, 3744–3750.
- Semënov, M.; Tamai, K.; He, X. SOST Is a Ligand for LRP5/LRP6 and a Wnt Signaling Inhibitor. J. Biol. Chem. 2005, 280, 26770–26775.
- Wang, X.; Zhang, X.; Li, F.; Ji, Q. MiR-128-3p accelerates cardiovascular calcification and insulin resistance through ISL1-dependent Wnt pathway in type 2 diabetes mellitus rats. J. Cell. Physiol. 2018, 234, 4997–5010.
- Shi, M.; Tian, P.; Liu, Z.; Zhang, F.; Zhang, Y.; Qu, L.; Liu, X.; Wang, Y.; Zhou, X.; Xiao, Y.; et al. MicroRNA-27a targets Sfrp1 to induce renal fibrosis in diabetic nephropathy by activating Wnt/β-Catenin signalling. Biosci. Rep. 2020, 40, BSR20192794.
- Bagchi, D.P.; Nishii, A.; Li, Z.; DelProposto, J.B.; Corsa, C.A.; Mori, H.; Hardij, J.; Learman, B.S.; Lumeng, C.N.; MacDougald, O.A. Wnt/β-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol. Metab. 2020, 42, 101078.
- Chen, N.; Wang, J. Wnt/β-Catenin Signaling and Obesity. Front. Physiol. 2018, 9, 792.
- Akoumianakis, I.; Sanna, F.; Margaritis, M.; Badi, I.; Akawi, N.; Herdman, L.; Coutinho, P.; Fagan, H.; Antonopoulos, A.S.; Oikonomou, E.K.; et al. Adipose tissue–derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci. Transl. Med. 2019, 11, eaav5055.
- Wang, J.; Liu, R.; Wang, F.; Hong, J.; Li, X.; Chen, M.; Ke, Y.; Zhang, X.; Ma, Q.; Wang, R.; et al. Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nature 2013, 15, 1455–1463.
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2011, 8, 1031–1063.
- Giannessi, D.; Maltinti, M.; Del Ry, S. Adiponectin circulating levels: A new emerging biomarker of cardiovascular risk. Pharmacol. Res. 2007, 56, 459–467.
- Salinas, M.L.; Fuentes, N.; Choate, R.; Wright, R.C.; McMurray, D.N.; Chapkin, R.S. AdipoRon Attenuates Wnt Signaling by Reducing Cholesterol-Dependent Plasma Membrane Rigidity. Biophys. J. 2019, 118, 885–897.
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197.
- Tian, Y.; Mok, M.T.; Yang, P.; Cheng, A.S. Epigenetic Activation of Wnt/β-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis. Cancers 2016, 8, 76.
- Bhala, N.; Angulo, P.; Van Der Poorten, D.; Lee, E.; Hui, J.; Saracco, G.M.; Adams, L.A.; Charatcharoenwitthaya, P.; Topping, J.H.; Bugianesi, E.; et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: An international collaborative study. Hepatology 2011, 54, 1208–1216.
- Wang, S.; Song, K.; Srivastava, R.; Dong, C.; Go, G.-W.; Li, N.; Iwakiri, Y.; Mani, A. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 2015, 29, 3436–3445.
- Li, C.-P.; Li, H.-J.; Nie, J.; Chen, X.; Zhou, X. Mutation of miR-21 targets endogenous lipoprotein receptor-related protein 6 and nonalcoholic fatty liver disease. Am. J. Transl. Res. 2017, 9, 715–721.
- Bacle, A.; Kadri, L.; Khoury, S.; Ferru-Clément, R.; Faivre, J.-F.; Cognard, C.; Bescond, J.; Krzesiak, A.; Contzler, H.; Delpech, N.; et al. A comprehensive study of phospholipid fatty acid rearrangements in the metabolic syndrome: Correlations to organ dysfunction. Dis. Model. Mech. 2020, 13, dmm043927.
- Imamura, F.; Sharp, S.J.; Koulman, A.; Schulze, M.B.; Kröger, J.; Griffin, J.L.; Huerta, J.M.; Guevara, M.; Sluijs, I.; Agudo, A.; et al. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study. PLoS Med. 2017, 14, e1002409.
- Ma, D.; Arendt, B.M.; Hillyer, L.M.; Fung, S.K.; McGilvray, I.; Guindi, M.; Allard, J.P. Plasma phospholipids and fatty acid composition differ between liver biopsy-proven nonalcoholic fatty liver disease and healthy subjects. Nutr. Diabetes 2016, 6, e220.
- Perona, J.S. Membrane lipid alterations in the metabolic syndrome and the role of dietary oils. Biochim. et Biophys. Acta (BBA) Biomembr. 2017, 1859, 1690–1703.
- Imran, M.; Sergent, O.; Tête, A.; Gallais, I.; Chevanne, M.; Lagadic-Gossmann, D.; Podechard, N. Membrane Remodeling as a Key Player of the Hepatotoxicity Induced by Co-Exposure to Benzopyrene and Ethanol of Obese Zebrafish Larvae. Biomolecules 2018, 8, 26.
- Zhao, L.; Zhang, C.; Luo, X.; Wang, P.; Zhou, W.; Zhong, S.; Xie, Y.; Jiang, Y.; Yang, P.; Tang, R.; et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J. Hepatol. 2018, 69, 705–717.
- Moon, J.S.; Karunakaran, U.; Suma, E.; Chung, S.M.; Won, K.C. The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond. Diabetes Metab. J. 2020, 44, 222–233.
- Miura, K. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 7381–7391.
- Roh, Y.S.; Seki, E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 2013, 28, 38–42.
- Dattaroy, D.; Seth, R.; Das, S.; Alhasson, F.; Chandrashekaran, V.; Michelotti, G.; Fan, D.; Nagarkatti, M.; Nagarkatti, P.; Diehl, A.M.; et al. Sparstolonin B attenuates early liver inflammation in experimental NASH by modulating TLR4 trafficking in lipid rafts via NADPH oxidase activation. Am. J. Physiol. Liver Physiol. 2016, 310, G510–G525.
- Di Liddo, R.; Bertalot, T.; Schuster, A.; Schrenk, S.; Tasso, A.; Zanusso, I.; Conconi, M.T.; Schäfer, K.H. Anti-inflammatory activity of Wnt signaling in enteric nervous system: In vitro preliminary evidences in rat primary cultures. J. Neuroinflammation 2015, 12, 23.
- Yin, Y.; Li, F.; Li, S.; Cai, J.; Shi, J.; Jiang, Y. TLR4 Influences Hepatitis B Virus Related Hepatocellular Carcinoma by Regulating the Wnt/β-Catenin Pathway. Cell. Physiol. Biochem. 2017, 42, 469–479.
- Kim, M.-H.; Lee, M.-K. The Incretins and Pancreatic β-Cells: Use of Glucagon-Like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide to Cure Type 2 Diabetes Mellitus. Korean Diabetes J. 2010, 34, 2–9.
- Yokomori, H.; Ando, W. Spatial expression of glucagon-like peptide 1 receptor and caveolin-1 in hepatocytes with macrovesicular steatosis in non-alcoholic steatohepatitis. BMJ Open Gastroenterol. 2020, 7, e000370.
- Gustafson, B.; Smith, U. WNT signalling is both an inducer and effector of glucagon-like peptide. Diabetologia 2008, 51, 1768–1770.
- Huang, L.; Luo, R.; Li, J.; Wang, D.; Zhang, Y.; Liu, L.; Zhang, N.; Xu, X.; Lu, B.; Zhao, K. β-catenin promotes NLRP3 inflammasome activation via increasing the association between NLRP3 and ASC. Mol. Immunol. 2020, 121, 186–194.
- Chen, Y.; He, X.; Yuan, X.; Hong, J.; Bhat, O.; Li, G.; Li, P.-L.; Guo, J. NLRP3 Inflammasome Formation and Activation in Nonalcoholic Steatohepatitis: Therapeutic Target for Antimetabolic Syndrome Remedy FTZ. Oxidative Med. Cell. Longev. 2018, 2018, 2901871.
- Ioannou, G.N. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol. Metab. 2015, 27, 84–95.
- Van Rooyen, D.M.; Larter, C.Z.; Haigh, W.G.; Yeh, M.M.; Ioannou, G.; Kuver, R.; Lee, S.P.; Teoh, N.C.; Farrell, G.C. Hepatic Free Cholesterol Accumulates in Obese, Diabetic Mice and Causes Nonalcoholic Steatohepatitis. Gastroenterology 2011, 141, 1393–1403.e5.
- Ho, C.-M.; Ho, S.-L.; Jeng, Y.-M.; Lai, Y.-S.; Chen, Y.-H.; Lu, S.-C.; Chen, H.-L.; Chang, P.-Y.; Hu, R.-H.; Lee, P.-H. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J. Inflamm. 2019, 16, 7.
- Kang, Q.; Chen, A. Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor. Lab. Investig. 2009, 89, 1275–1290.
- Scott, C.C.; Vossio, S.; Vacca, F.; Snijder, B.; Larios, J.; Schaad, O.; Guex, N.; Kuznetsov, D.; Martin, O.; Chambon, M.; et al. Wnt directs the endosomal flux of LDL -derived cholesterol and lipid droplet homeostasis. EMBO Rep. 2015, 16, 741–752.




