Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carmine Rocca | + 1351 word(s) | 1351 | 2021-10-26 10:25:17 | | | |
| 2 | Camila Xu | Meta information modification | 1351 | 2021-11-05 01:40:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rocca, C. Chemistry and Biosynthesis of Apigenin. Encyclopedia. Available online: https://encyclopedia.pub/entry/15670 (accessed on 08 February 2026).
Rocca C. Chemistry and Biosynthesis of Apigenin. Encyclopedia. Available at: https://encyclopedia.pub/entry/15670. Accessed February 08, 2026.
Rocca, Carmine. "Chemistry and Biosynthesis of Apigenin" Encyclopedia, https://encyclopedia.pub/entry/15670 (accessed February 08, 2026).
Rocca, C. (2021, November 03). Chemistry and Biosynthesis of Apigenin. In Encyclopedia. https://encyclopedia.pub/entry/15670
Rocca, Carmine. "Chemistry and Biosynthesis of Apigenin." Encyclopedia. Web. 03 November, 2021.
Copy Citation
Apigenin, a natural bioactive flavonoid widely present in medicinal plants, functional foods, vegetables and fruits, exerts protective effects in models of neurological disorders and cardiovascular diseases and most of these effects are attributed to its antioxidant action.
apigenin
flavonoids
1. Introduction
Public-health-related chronic diseases, such as diabetes, cancer, depression, hypertension, stroke, Alzheimer’s disease and metabolic syndrome are prevalent worldwide. Both healthy diet and physical activities can combat or delay these diseases [1][2][3]. A major contribution to the healthy diet is provided by fruits and vegetables, which are rich in natural bioactive chemical compounds. Phytochemicals are a vital part of a healthy diet and have been shown to play a predominant role in combating these chronic diseases. There are numerous phytochemical compounds, such as polyphenols, alkaloids, tannins and glycosides, which have been described as having no toxicity but high therapeutic potential in several disease settings. Polyphenols are the largest contributing group within natural product compounds consisting of subgroups such as flavonoids, flavanones, isoflavonoids, flavones and flavanols [1][4][5][6][7].
Flavonoids are the secondary metabolites which have widespread metabolic functions in plants. They are widely distributed in fruits, flowers, seeds, vegetables and in both legumes and non-legume plants. More than 6000 flavonoid compounds have been isolated and investigated for different biological purposes and the number is constantly increasing. They are known to have diverse pharmacological activities, acting as anticancer, antioxidant and anti-inflammatory agents, and also inhibiting platelet aggregation and viral replication [6][7][8].
Moreover, several studies have indicated that the consumption of dietary flavonoids can be associated with a reduced risk of metabolic syndrome. Metabolic syndrome is considered to be a worldwide epidemic complex disorder that is defined by a cluster of interconnected factors that are able to significantly augment the risk of several cardiovascular diseases (CVD), including coronary heart disease (CHD), atherosclerosis and diabetes mellitus type 2 [9]. Thanks to their antioxidative and anti-inflammatory effects, flavonoids have been widely studied for the prevention and/or amelioration of metabolic syndrome and its related disorders. However, the beneficial effects of flavonoids need to be corroborated, also contextualizing their effects with the different individual risk factors associated with metabolic syndrome, as well as the different effects of flavonoids and their metabolites on the body [10].
Among the flavonoids, apigenin is one of the most investigated and isolated compounds in human health. It is abundant in plants such as parsley, onion, oranges, chamomile, celery, spices and honey and some plant-origin beverages such as wine, tea and beer. Diverse biological effects of apigenin have been reported in both in vitro and in vivo studies and several studies revealed that apigenin could display anti-inflammatory, antioxidant and anti-obesity actions, as well as antiproliferative and anticancer activities [11][12][13]. On the other hand, emerging evidence established a potential interaction between apigenin and human gut microbiota, which contains enzymes that could degrade apigenin; this is of particular interest in the context of metabolic disorders, since, as revealed by several studies conducted on humans and animal models, gut microbiota may exert a strong influence in the pathogenesis of metabolic syndrome. Accordingly, an imbalance between gut microbes and the host’s immune system could induce “metabolic endotoxemia”, leading to systemic inflammation and insulin resistance [14]. The intense investigation carried out over the last twenty years regarding the possibility of manipulating gut microbiota through dietary polyphenols for preventing metabolic disorders suggests that apigenin can also play a beneficial role. To date, although the potential activity of dietary apigenin for the modulation of the colonic microbial population composition or activity is still limited, some studies demonstrated that apigenin can affect both the growth and gene expression of selective gut bacteria strains, paving the way for further investigation [15][16].
2. Chemistry and Biosynthesis of Apigenin
Apigenin is a natural bioactive flavonoid. Chemically, it is 4′,5,7-trihydroxyflavone (Figure 1) and is basically related to flavone, a subclass of flavonoids [17]. Apigenin is widely distributed in the plant kingdom, and is found in vegetables such as onions and parsley, fruits such as oranges and grape fruits and plant-derived beverages such as wine and tea. Apigenin is richly found in the plant species belonging to family Achillea, Sideritis, Fabaceae, Asteraceae, Teucrium, Genista, Artemisia, Matricaria and Lamiaceae [18][19][20][21][22].
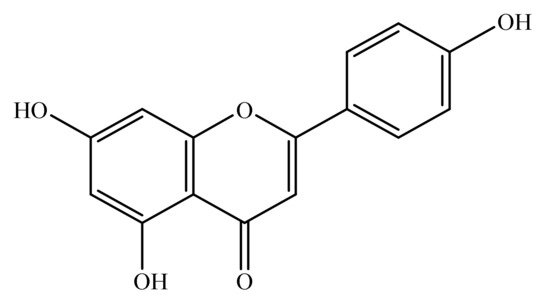
Figure 1. Chemical structure of apigenin (4′,5,7-trihydroxyflavone).
Apigenin is synthesized biogenetically by the phenylpropanoid pathway. It is synthesized from tyrosine and phenylalanine precursors. Tyrosine is directly deaminated to p-coumaric acid while phenylalanine is converted into cinnamic acid via non-oxidative deamination which is further oxidized at C-4 and then converted into p-coumaric acid. After the synthesis of p-coumaric acid by both pathways, the synthesized p-coumaric acid is further activated with Coenzyme-A. Three molecules of malonyl-CoA are then condensed with the p-coumarate. The compound is then aromatized to chalcone by chalcone synthase. Chalcone isomerase then isomerizes the chalcone and converts it into naringenin. In the final step, naringenin is oxidized into apigenin by flavanone synthase. The biosynthesis of apigenin is summarized in Figure 2 [1][23][24][25].
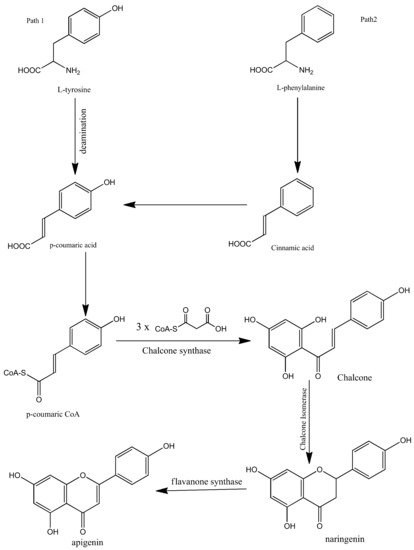
Figure 2. Biosynthesis of apigenin.
3. Pharmacokinetics of Apigenin
Apigenin is characterized by poor systemic availability, due to its low lipid and water solubility; it is either excreted unabsorbed in the urine or faeces or rapidly metabolized after absorption [16].
3.1. Absorption
Absorption of apigenin takes place throughout the gastrointestinal (GI) tract from stomach (deglycosylated) to colon (non-deglycosylated). Apigenin is transported via active carrier transport and passive diffusion in the duodenum and jejunum, while in the ileum and colon it is transported only by passive diffusion [26][27]. It has been established by Chen et al. that systemically absorbed apigenin was in conjugated form [28]. Li et al. found that topically administered apigenin was absorbed into local skin tissues despite transdermal blood circulation [29].
3.2. Distribution
The presence of apigenin was not found in the kidneys and GI lumen after 12 h of administration of apigenin glycosides (flavonoid extract). In the liver it was detected in 1.5 and 12 h [27]. Orally administered single-dose radiolabelled apigenin was detected after 10 days with radioactivity of 0.4% in kidneys, 1.2% in liver, 9.4% in intestine, 12% in faeces and 51% in urine [30]. Apigenin was also detected in human red blood cells [31].
3.3. Metabolism
Due to the poor bioavailability of apigenin, complete metabolism of apigenin occurs. The metabolism of apigenin occurs through phase II conjugation (sulfation and glucuronidation) reaction. Phase II biotransformation of apigenin involves both enteric and enterohepatic cycling [34]. Gradollato et al. found, in the rat liver, that the metabolism of apigenin follows phase I process via cytochrome P450 and nicotinamide adenine dinucleotide phosphate (NADPH). This phase I was followed by phase II process (glucuronidation and sulfation). One metabolite from sulfation and 3 β-monoglucuronides were seen after glucuronidation reaction [30]. The metabolites of both reactions were detected in plasma. Some other studies reported that sulfation of apigenin was less as compared to glucuronidation in both in vitro and in vivo assays [35].
Apigenin is metabolized by conjugation reactions. Prior to absorption of apigenin into blood and liver, the extensive conjugation of apigenin takes place in the gastrointestinal tract [36]. Some studies have reported that apigenin is also metabolized by hydrolysis in the liver and intestine. Deglycosylation of apigenin glucosides occurs in the epithelial β-glucosidases. Liver microsomes also metabolize apigenin [37].
3.4. Excretion
Apigenin is mostly excreted in urine and faeces. In particular, after a single oral dose of apigenin, 51.0% and 12.0% of apigenin were detected in urine and faeces. Most of the administered apigenin is excreted in unabsorbed form [30].
3.5. Bioavailability
Apigenin has been categorized as a class II drug with high intestinal membrane permeability and poor solubility according to the Biopharmaceutics Classification System [38]. Apigenin is poorly soluble in non-polar solvents (0.001–1.63 mg/mL), while it is soluble as more than 100 mg/mL (freely) in dimethylsulfoxide. The solubility in the phosphate buffer was 2.16 μg/mL at pH 7.5. Innovative techniques are used to enhance the bioavailability of apigenin [26][39]. For instance, carbon nanopowders with apigenin system was used and it was found that dissolution of apigenin was improved about 275% as compared to pure apigenin in 60 min, resulting in an increase of apigenin bioavailability of 183% [40]. In vitro assays reported that apigenin nanocrystals have faster dissolution velocity than coarse powder. These are prepared by a supercritical antisolvent process [41].
References
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305.
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539.
- Spence, J.D. Nutrition and Risk of Stroke. Nutrients 2019, 11, 647.
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392.
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S151–S183.
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222.
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22.
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370.
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48.
- Galleano, M.; Calabro, V.; Prince, P.D.; Litterio, M.C.; Piotrkowski, B.; Vazquez-Prieto, M.A.; Miatello, R.M.; Oteiza, P.I.; Fraga, C.G. Flavonoids and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012, 1259, 87–94.
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406.
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435.
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet 2005, 366, 1059–1062.
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094.
- Wang, M.; Firrman, J.; Zhang, L.; Arango-Argoty, G.; Tomasula, P.; Liu, L.; Xiao, W.; Yam, K. Apigenin Impacts the Growth of the Gut Microbiota and Alters the Gene Expression of Enterococcus. Molecules 2017, 22, 1292.
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed Res. Int. 2019, 2019, 7010467.
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978.
- Ornano, L.; Venditti, A.; Donno, Y.; Sanna, C.; Ballero, M.; Bianco, A. Phytochemical analysis of non-volatile fraction of Artemisia caerulescens subsp. densiflora (Viv.)(Asteraceae), an endemic species of La Maddalena Archipelago (Sardinia–Italy). Nat. Prod. Res. 2016, 30, 920–925.
- Venditti, A.; Maggi, F.; Vittori, S.; Papa, F.; Serrilli, A.M.; Di Cecco, M.; Ciaschetti, G.; Mandrone, M.; Poli, F.; Bianco, A. Volatile compounds from Achillea tenorii (Grande) growing in the Majella National Park (Italy). Nat. Prod. Res. 2014, 28, 1699–1704.
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica All. from Lipari Island (Aeolian Islands). Nat. Prod. Res. 2016, 30, 912–919.
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; Del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88.
- Venditti, A.; Frezza, C.; Guarcini, L.; Foddai, S.; Serafini, M.; Bianco, A. Phytochemical Study of a Species with Ethnopharmacological Interest: Sideritis romana L. Eur. J. Med. Plants 2016, 12, 1–9.
- Forkmann, G. Flavonoids as flower pigments: The formation of the natural spectrum and its extension by genetic engineering. Plant Breed. 1991, 106, 1–26.
- Herrmann, K.M. The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol. 1995, 107, 7–12.
- Martens, S.; Forkmann, G.; Matern, U.; Lukacin, R. Cloning of parsley flavone synthase I. Phytochemistry 2001, 58, 43–46.
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317.
- Pforte, H.; Hempel, J.; Jacobasch, G. Distribution pattern of a flavonoid extract in the gastrointestinal lumen and wall of rats. Nahrung 1999, 43, 205–208.
- Chen, J.; Lin, H.; Hu, M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J. Pharmacol. Exp. Ther. 2003, 304, 1228–1235.
- Li, B.; Birt, D.F. In vivo and in vitro percutaneous absorption of cancer preventive flavonoid apigenin in different vehicles in mouse skin. Pharm. Res. 1996, 13, 1710–1715.
- Gradolatto, A.; Basly, J.P.; Berges, R.; Teyssier, C.; Chagnon, M.C.; Siess, M.H.; Canivenc-Lavier, M.C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab. Dispos. 2005, 33, 49–54.
- Bader, N.; Bosy-Westphal, A.; Koch, A.; Mueller, M.J. Influence of Vitamin C and E Supplementation on Oxidative Stress Induced by Hyperbaric Oxygen in Healthy Men. Ann. Nutr. Metab. 2006, 50, 173–176.
- Zhang, X.; Han, R.; Sun, X.; Li, G.; Yang, Q.; Li, Q.; Gai, W.; Zhang, M.; Chen, L.; Yang, G.; et al. The Effect of the Skeleton Structure of Flavanone and Flavonoid on Interaction with Transferrin. Bioorg. Med. Chem. Lett. 2013, 23, 6677–6681.
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477.
- Tang, L.; Zhou, J.; Yang, C.H.; Xia, B.J.; Hu, M.; Liu, Z.Q. Systematic studies of sulfation and glucuronidation of 12 flavonoids in the mouse liver S9 fraction reveal both unique and shared positional preferences. J. Agric. Food Chem. 2012, 60, 3223–3233.
- Griffiths, L.A.; Smith, G.E. Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem. J. 1972, 128, 901–911.
- Galijatovic, A.; Otake, Y.; Walle, U.K.; Walle, T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica 1999, 29, 1241–1256.
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998, 436, 71–75.
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330.
- Li, B.; Robinson, D.H.; Birt, D.F. Evaluation of properties of apigenin and apigenin and analytic method development. J Pharm Sci. 1997, 86, 721–725.
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327–2333.
- Zhang, J.; Huang, Y.; Liu, D.; Gao, Y.; Qian, S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013, 48, 740–747.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
05 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




