
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Micaela Gliozzi | + 2077 word(s) | 2077 | 2021-11-03 07:43:29 | | | |
| 2 | Amina Yu | + 10 word(s) | 2087 | 2021-11-04 03:35:16 | | |
Video Upload Options
Natural compounds with anticancer properties are capable of killing transformed or cancerous cells without being toxic to healthy cells. Most fruits and vegetables consumed with food are made up of bioactive molecules belonging to the family of polyphenols, a group of natural compounds widely distributed in the plant kingdom; this group is varied, and to date, more than 8000 phenolic structures are known. Polyphenols are classified according to chemical structure, and their subdivision is represented in.
1. Introduction
To date, it is well-known that cancer is one of the leading causes of death globally. The report entitled Global Cancer Statistics 2020, produced in collaboration with the American Cancer Society (ACS) and the International Agency for Research on Cancer (IARC), confirmed that in 2020 about 17 million people were affected by this disease, comprising 36 types of cancer in 185 countries around the world [1][2]. As a result, the annual global health expenditure is extremely high. For this reason, projections of cancer incidence and mortality are crucial to understanding the evolving scenario of cancer risk. Within a population, the number of individuals who are diagnosed or die of cancer is largely influenced by age, environment, and lifestyle. A very large study considered the incidence and mortality of 26 types of cancer and highlighted their evolution from 1993 to 2014, and made projections from 2014 to 2035. Obviously, the projections do not include assumptions about changes in risk factors [3]. Figure 1 shows 6 of the 26 types of cancer reported in this study.
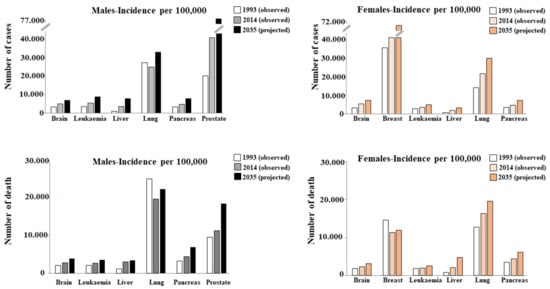
Cancer is a disease in which some cells grow uncontrollably and can spread to other parts of the body. In fact, it is important to stress that not all tumors are cancerous: benign tumors are characterized by cells that do not show signs of transformation and remain confined to the site of origin. On the contrary, the main characteristic of malignant tumors (cancer) is the ability of the cells that constitute them to migrate from the original site to a secondary location and metastasize to adjacent tissues, organs, and/or different parts of the body through lymphatic or hematogenic diffusion [4]. Cancer has a multifactorial origin, and its causes are found in genetic mutations, infection or inflammation, unhealthy eating habits, exposure to radiation, work stress, and/or intake of toxins [5]. Before achieving the aggressiveness necessary to become life-threatening, a tumor must be able to: (a) replicate limitlessly ; (b) move; (c) evade apoptosis; (d) produce growth signals that are self-sufficient; (e) be insensitive to anti-growth signals; (f) degrade the extracellular matrix ; (g) survive in the blood; (h) share in the environment of a new tissue [6]. Normal cells can transform into cancerous cells, but before this happens, they must undergo the phenomena of abnormal changes known as hyperplasia and dysplasia. In hyperplasia, there is a considerable increase in the number of cells that maintain normal characteristics. In contrast, cells assume abnormal phenotypic characteristics in dysplasia. It is important, however, to point out that hyperplasia and dysplasia do not necessarily cause cancer [7]. In general, early detection of cancer and proper treatment increase the chances of survival and healing. The type of cancer and the stage suggest the most suitable treatment to use; treatment options may be chemotherapy, surgery, radiotherapy, hormonal therapy, targeted therapy, etc. Today, it is particularly appropriate to use a combination of treatment methods to ensure the maximum effectiveness and optimal results [8]. However, each treatment has its side effects on the patient, and the oncologist should choose the most appropriate treatment, considering the risk–benefit ratio [9]. Chemotherapy is generally accepted as the standard therapy and remains one of the main strategies in the treatment of primary tumors, although it is well-known to cause DNA damage and affect both cancerous and non-cancerous cells. In addition, cardiotoxicity is a complication of this treatment; the severity of cardiotoxicity is dependent on cumulative dose, the type and combination of drugs used, and the presence of co-existing pathologies such as diabetes mellitus, cardiac diseases, and other risk factors [10][11]. Radiation therapy also has side effects, such as neurological deficits caused by vascular damage and fibrosis of neuronal structures [12][13]. Hormone therapy can be used to manage hormone-dependent malignant tumors, to manipulate the endocrine system, and to interfere with hormonal production or the activity of their receptors. In general, hormone therapy involves the administration of exogenous hormones such as corticosteroids, selective estrogen receptor modulators, somatostatin analogs, progestins, gonadotropin-releasing hormone agonists and antagonists, aromatase inhibitors, and antiandrogens. Some of these have antiproliferative and pro-apoptotic effects. Unfortunately, this treatment can cause a wide range of complications including liver steatosis, thrombosis, endometrial and osteoporosis hypertrophy, intestinal perforation, pulmonary embolism, vascular necrosis, and breast and endometrial cancers [14]. Surgical resection is still widely used in cancer treatment as it effectively relieves the patient’s symptoms. However, much scientific evidence has shown that cancer recurs in many patients after a short time, owing to the stress induced by surgery, which at the systemic level, stimulates inflammation, increased release of cytokines, and the risk of cancer recurrence [15]. In addition, surgical resection potentially enhances metastatic seeding of tumor cells, spreading cancer cells in the vascular and lymphatic systems and favoring their migration into distant organs [16].
2. Natural Compounds and Cancer
The use of natural compounds with anticancer effects has increased thanks to their low toxicity and lower side effects, which allow their use in the treatment or adjuvant therapy of cancer. Apoptosis is programmed cellular death, finely regulated at the gene level, resulting in efficient removal of damaged cells. Induction of apoptosis is crucial in precancerous lesions since harmful cells are eliminated by preventing uncontrolled cell proliferation and cancer progression. Deregulation of apoptosis is considered one of the characteristics of cancer progression, and transformed cells are able to circumvent this process, although the mechanisms involved are not sufficiently known. For this reason, therapeutic strategies aimed at restoring the sensitivity of cancer cells to apoptosis are increasingly tested [17][18][19]. Citrus fruits represent major sources of flavonoids. Several experimental studies have strongly indicated that bergamot and its extracts can exert antitumor effects thanks to the ability of flavonoids to interfere with the main stages of carcinogenicity: the onset, promotion, and progression of cancer [20]. The anticancerous action of BEO has been adequately highlighted in several in vitro works. In particular, a reduction in cell proliferation was triggered by the shutdown of the cell cycle in phase G0–G1. In addition, intense pro-oxidant activity and cellular DNA damage have been appreciated [21][20][22]. A very comprehensive work [23] conducted in vitro on human cancerous cells of the nervous system (SH-SY5Y, PC12), prostate (PC3), and breast (MDA-MB-231) showed that treatment with BJ at different concentrations (1–5%) arrested cancer progression. In addition, BJ demonstrated its ability to reduce the growth rate of various cancer cell lines with mechanisms dependent on the type of cancer [24][25]. Finally, it has been shown in human colon cancer cells that low concentrations of BJ can induce inhibition of the mitogen-activated protein kinase (MAPK)-dependent pathways, and cause cell cycle arrest and alteration of apoptosis, while high concentrations produce oxidative stress, causing DNA damage [26]. BPF has attracted scientific attention for its peculiar composition and high content of flavonoids, such as naringin, hesperidin, and neoeriocitrin [27]. Although few studies on the correlation between BPF and cancer are available, multiple papers indirectly involving BPF are known. In fact, cholesterol-lowering drugs are able to reduce cancer incidence and cancer-related mortality [28]. To date, it is known that BPF possesses several hypolipidizing properties against many metabolic dysfunctions [29][30][31][32].
Scientific literature has amply demonstrated that bergamot fruit has a robust antioxidant property, and for this reason, its consumption is encouraged as health-promoting. For example, BJ has been shown in vitro to possess a significant antiradical property against superoxide and nitric oxide, O 2• scavenging activity, and lipid peroxidation inhibition. Parallel studies conducted in vivo on subjects fed hearts of mice with BJ or vehicle for three months showed statistically significant antioxidant responses [33]. Naringenin, a polyphenol belonging to the class of flavanones and widely distributed in citrus fruits, is one of the major components of BPF [34]. In fact, this compound has been shown to induce cytotoxic and apoptotic effects and prevent cell proliferation in different types of cancer cells [35][36][37]. Unfortunately, its practical use in vivo is reduced owing to its hydrophobic nature, short half-life, and poor absorption. For this reason, the use of nanomaterials was suggested to improve its bioavailability [38].
The protective effects of oleuropein against inflammation are multiple: in vivo preliminary studies demonstrated a significant anti-inflammatory effect generated by oleuropein during lipopolysaccharide-induced sepsis (LPS) in mice. To study an induced inflammatory effect, LPS has been widely used in both in vitro and in vivo scientific work [39][40]. In fact, pretreatment with oleuropein ameliorated LPS-induced liver and kidney histological changes, mitigated the increased levels of malondialdehyde, and reduced the levels of reduced glutathione and the number of inflammatory biomarkers (TNF-α, IL-1β, and IL-6) [41]. Scientific works already published have highlighted the protective role of oleuropein in several cancer cell lines, including leukemia, breast, pancreatic, prostate, and colorectal [42][43][44]. It is important to point out that oleuropein proved capable of discriminating between cancer and normal cells, inhibiting proliferation and inducing apoptosis only in cancer cells [45][46][47]. Oleuropein’s mechanism is downregulation of proinflammatory enzymes IL-6 and interleukin 1β [48][49].
Epigenetic alterations often occur in the early stages of cancer development and cancer cells; therefore, may inadequately activate oncogenes or inactivate tumor suppressors. For this reason, being able to prevent epigenetic alterations can reduce the proliferation of cancer cells, the severity of cancer growth, and metastases [50][51].
3. Discussion
Although cancer is one of the world’s leading causes of death, a more optimistic view for the future stems from the awareness that there have been many improvements in diagnosis and treatment approaches. In particular, early detection can address the disease with more satisfactory results, and less invasive treatments aim to increase tolerability in patients [52]. The ultimate goal is to reduce the mortality rate of cancer patients by increasing the expectation of quality of life. A total of 90% of cancers are attributable to modifiable risk factors, including a non-optimal diet, environmental pollution, excessive body weight, consumption of alcohol or tobacco smoke, physical inactivity, and infectious agents [53]. The diet is an essential element for maintaining health, and it has been estimated that bad eating habits are responsible for 5–10% of total cancer cases [54]. Several studies in the scientific literature reported that the healthiest diet is the Mediterranean diet, which is based on the consumption of high quantities of fruit, vegetables, dried fruits, legumes, cereals, fish, and extra virgin olive oil, a moderate amount of wine and small amounts of red meat, eggs, and dairy products [55]. Numerous clinical and epidemiological studies have shown that the Mediterranean diet is protective against the onset of many diseases such as diabetes, obesity, cardiovascular diseases, and cancer [56]. Most foods of plant origin belong to the class of polyphenols, the largest group of phytochemicals, that are proven to play an important role in the prevention of various diseases, including cancer, cardiovascular diseases, diabetes, and degenerative neurodegenerative diseases [57].
In this entry, the anticancer properties of four of these compounds were explored: bergamot, oleuropein, curcumin, and quercetin. As reported in the literature, the natural compounds considered have numerous protective effects and tend to reduce altered physiological conditions, respecting cellular homeostasis. Conversely, in the case of cancer, the main purpose is to use substances that may be harmful to cancer cells by exerting an antiproliferative and pro-apoptotic effect to block their growth [58][59]. A suitable anticancer drug is a molecule able to distinguish specifically between healthy and transformed cells, so as to be harmless to the first and harmful to the second. Since a molecule with these characteristics is not yet available, the scientific community is investigating the action of natural compounds, which generally produce fewer side effects than conventional drugs. Due to concomitant events possibly amplifying tumor transformation and growth, such as the inflammatory process and oxidant activity, it is necessary to find a molecule with antiproliferative action and at the same time anti-inflammatory and antioxidant properties [60][61]. The compounds considered and explored in this review (bergamot, oleuropein, curcumin and quercetin) have demonstrated exactly this behavior in vitro and in vivo. In particular, cell growth was reduced, triggering antiproliferative and/or death pathways. At the same time, antioxidant and anti-inflammatory effects were noted, reflecting the chemical structure of these natural compounds and preventing the addition of characteristics to the tumor that would exacerbate it. It was also interesting to note that some mechanisms of these natural compounds acted selectively on cancer cells but not on their healthy counterparts.
References
- Schiller, J.T.; Lowy, D.R. An Introduction to Virus Infections and Human Cancer. Recent Results Cancer Res. 2021, 217, 1–11.
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459.
- Smittenaar, C.R.; Petersen, K.A.; Stewart, K.; Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer 2016, 115, 1147–1155.
- Patel, A. Benign vs. Malignant Tumors. JAMA Oncol. 2020, 6, 1488.
- Wang, J.J.; Lei, K.F.; Han, F. Tumor microenvironment: Recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864.
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68.
- Kabir, K.M.M.; Donald, W.A. Cancer breath testing: A patent review. Expert Opin. Ther. Pat. 2018, 28, 227–239.
- Colli, L.M.; Machiela, M.J.; Zhang, H.; Myers, T.A.; Jessop, L.; Delattre, O.; Yu, K.; Chanock, S.J. Landscape of Combination Immunotherapy and Targeted Therapy to Improve Cancer Management. Cancer Res. 2017, 77, 3666–3671.
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419.
- Grimaldi, M.; Bo, V.D.; Ferrari, B.; Roda, E.; De Luca, F.; Veneroni, P.; Barni, S.; Verri, M.; De Pascali, S.A.; Fanizzi, F.P.; et al. Long-term effects after treatment with platinum compounds, cisplatin and : Autophagy activation in rat B50 neuroblastoma cells. Toxicol. Appl. Pharmacol. 2019, 364, 1–11.
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. The Effects of Bergamot Polyphenolic Fraction, Cynara cardunculus, and Olea europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients 2021, 13, 2158.
- Pich, O.; Muiños, F.; Lolkema, M.P.; Steeghs, N.; Gonzalez-Perez, A.; Lopez-Bigas, N. The mutational footprints of cancer therapies. Nat. Genet. 2019, 51, 1732–1740.
- Barazzuol, L.; Coppes, R.P.; van Luijk, P. Prevention and treatment of radiotherapy-induced side effects. Mol. Oncol. 2020, 14, 1538–1554.
- Fairchild, A.; Tirumani, S.H.; Rosenthal, M.H.; Krajewski, K.M.; Nishino, M.; Shinagare, A.B.; Jagannathan, J.P.; Ramaiya, N.H. Hormonal therapy in oncology: A primer for the radiologist. AJR Am. J. Roentgenol. 2015, 204, W620–W630.
- Chen, Z.; Zhang, P.; Xu1, Y.; Yan, J.; Liu, Z.; Bond Lau, W.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132.
- Mohme, M.; Riethdorf, S.; Pantel, K. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 2017, 14, 155–167.
- Parveen, N.; Shadab, G.G. The dual clastogenic and anti-clastogenic properties of quercetin is dose dependent. Front. Biosci. 2017, 9, 139–153.
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619.
- Wu, D.; Wei, C.; Li, Y.; Yang, X.; Zhou, S. Pyroptosis, a New Breakthrough in Cancer Treatment. Front. Oncol. 2021, 11, 698811.
- Chota, A.; George, B.P.; Abrahamse, H. Interactions of multidomain pro-apoptotic and anti-apoptotic proteins in cancer cell death. Oncotarget 2021, 12, 1615–1626.
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Wo’zniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11.
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer potential of citrus juices and their extracts: A systematic review of both preclinical and clinical studies. Front. Pharmacol. 2017, 8, 420.
- Navarra, M.; Ferlazzo, N.; Cirmi, S.; Trapasso, E.; Bramanti, P.; Lombardo, G.E.; Minciullo, P.L.; Calapai, G.; Gangemi, S. Effects of bergamot essential oil and its extractive fractions on SH-SY5Y human neuroblastoma cell growth. J. Pharm. Pharmacol. 2015, 67, 1042–1053.
- Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Russo, C.; Gangemi, S.; Calapai, G.; Cirmi, S.; Navarra, M. Bergamottin and 5-Geranyloxy-7-methoxycoumarin Cooperate in the Cytotoxic Effect of Citrus bergamia (Bergamot) Essential Oil in Human Neuroblastoma SH-SY5Y Cell Line. Toxins 2021, 13, 275.
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus Bergamia juice. PLoS ONE 2013, 8, e61484.
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment With a Flavonoid-Rich Fraction of Bergamot Juice Improved Lipopolysaccharide-Induced Periodontitis in Rats. Front. Pharmacol. 2019, 9, 1563.
- Ming, D.S.; Hillhouse, B.J.; Guns, E.S.; Eberding, A.; Xie, S.; Vimalanathan, S.; Towers, G.N. Bioactive compounds from Rhodiola rosea (Crassulaceae). Phyther. Res. An. Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 740–743.
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apcam1137). Eur. J. Nutr. 2020, 59, 885–894.
- Visalli, G.; Ferlazzo, N.; Cirmi, S.; Campiglia, P.; Gangemi, S.; Di Pietro, A.; Calapai, G.; Navarra, M. Bergamot juice extract inhibits proliferation by inducing apoptosis in human colon cancer cells. Anticancer Agents Med. Chem. 2014, 14, 1402–1413.
- Fernández, L.P.; Merino, M.; Colmenarejo, G.; Moreno-Rubio, J.; Sánchez-Martínez, R.; Quijada-Freire, A.; Gómez de Cedrón, M.; Reglero, G.; Casado, E.; Sereno, M.; et al. Metabolic enzyme ACSL3 is a prognostic biomarker and correlates with anticancer effectiveness of statins in non-small cell lung cancer. Mol. Oncol. 2020, 14, 3135–3152.
- Cappello, A.R.; Dolce, V.; Iacopetta, D.; Martello, M.; Fiorillo, M.; Curcio, R.; Muto, L.; Dhanyalayam, D. Bergamot (Citrus bergamia Risso) Flavonoids and Their Potential Benefits in Human Hyperlipidemia and Atherosclerosis: An Overview. Mini Rev. Med. Chem. 2016, 16, 619–629.
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504.
- Lu, Y.; Shan, S.; Li, H.; Shi, J.; Zhang, X.; Li, Z. Reversal effects of bound polyphenol from foxtail millet bran on multidrug resistance in human HCT-8/Fu colorectal cancer cell. J. Agric. Food Chem. 2018, 66, 5190–5199.
- Tan, J.; de Bruijn, W.J.C.; van Zadelhoff, A.; Lin, Z.; Vincken, J.P. Browning of Epicatechin (EC) and Epigallocatechin (EGC) by Auto-Oxidation. J. Agric. Food Chem. 2020, 68, 13879–13887.
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure-Activity Relationships. Mol. Nutr. Food Res. 2018, 11, e1701073.
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, Pharmacodynamic and Formulations Aspects of Naringenin: An Update. Life Sci. 2018, 215, 43–56.
- Noori, S.; Rezaei Tavirani, M.; Deravi, N.; Mahboobi Rabbani, M.I.; Zarghi, A. Naringenin Enhances the Anti-Cancer Effect of Cyclophosphamide against MDA-MB-231 Breast Cancer Cells Via Targeting the STAT3 Signaling Pathway. Iran. J. Pharm. Res. 2020, 19, 122–133.
- Lee, J.; Kim, D.-H.; Kim, J.H. Combined Administration of Naringenin and Hesperetin with Optimal Ratio Maximizes the Anti-cancer Effect in Human PanCreatic Cancer via down Regulation of FAK and p38 Signaling Pathway. Phytomedicine 2019, 58, 152762.
- Currò, M.; Risitano, R.; Ferlazzo, N.; Cirmi, S.; Gangemi, C.; Caccamo, D.; Ientile, R.; Navarra, M. Citrus bergamia Juice Extract Attenuates beta-Amyloid-Induced Pro-Inflammatory Activation of THP-1 Cells Through MAPK and AP-1 Pathways. Sci. Rep. 2016, 6, 20809.
- Cheng, N.; Liang, Y.; Du, X.; Ye, R.D. Serum amyloid A promotes LPS clearance and suppresses LPS-induced inflammation and tissue injury. EMBO Rep. 2018, 19, e45517.
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Macri, R.; Oppedisano, F.; Scarano, F.; Zito, M.C.; et al. The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. Int. J. Mol. Sci. 2021, 22, 7910.
- Alsharif, K.F.; Al-Amer, O.; Mufti, A.H.; Theyab, A.; Lokman, M.S.; Ramadan, S.S.; Almeer, R.S.; Hafez, M.M.; Kassab, R.B.; Abdel Moneim, A.E. Oleuropein protects against lipopolysaccharide-induced sepsis and alleviates inflammatory responses in mice. IUBMB Life 2020, 72, 2121–2132.
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical Properties and Anti-Proliferative Activity of Olea europaea L. Leaf Extracts against Pancreatic Cancer Cells. Molecules 2015, 20, 12992–13004.
- Samet, I.; Han, J.; Jlaiel, L.; Sayadi, S.; Isoda, H. Olive (Olea europaea) Leaf Extract Induces Apoptosis and Monocyte/Macrophage Differentiation in Human Chronic Myelogenous Leukemia K562 Cells: Insight into the Underlying Mechanism. Oxid. Med. Cell. Longev. 2014, 2014, 927619.
- Quirantes-Piné, R.; Zurek, G.; Barrajón-Catalán, E.; Bäßmann, C.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A metabolite-profiling approach to assess the uptake and metabolism of phenolic compounds from olive leaves in SKBR3 cells by HPLC-ESI-QTOF-MS. J. Pharm. Biomed. Anal. 2013, 72, 121–126.
- Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G.; Vanella, L. Antiproliferative effect of oleuropein in prostate cell lines. Int. J. Oncol. 2012, 41, 31–38.
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food Chem. Toxicol. 2013, 53, 310–316.
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; Alarcón de la Lastra, C. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156.
- Lamy, S.; Ben Saad, A.; Zgheib, A.; Annabi, B. Olive oil compounds inhibit the paracrine regulation of TNF-α-induced endothelial cell migration through reduced glioblastoma cell cyclooxygenase-2 expression. J. Nutr. Biochem. 2015, 27, 136–145.
- López-Camarillo, C.; Gallardo-Rincón, D.; Álvarez-Sánchez, M.E.; Marchat, L.A. Pharmaco-epigenomics: On the Road of Translation Medicine. Adv. Exp. Med. Biol. 2019, 1168, 31–42.
- Davalos, V.; Martinez-Cardus, A.; Esteller, M. The Epigenomic Revolution in Breast Cancer: From Single-Gene to Genome-Wide Next-Generation Approaches. Am. J. Pathol. 2017, 187, 2163–2174.
- Xiong, S.; Hong, Z.; Huang, L.S.; Tsukasaki, Y.; Nepal, S.; Zhong, M.; Wu, W.; Ye, Z.; Gao, X.; Rao, G.N.; et al. IL-1beta suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J. Clin. Investig. 2020, 130, 3684–3698.
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabrof, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics. CA A Cancer J. Clin. 2019, 69, 363–385.
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54.
- Zhang, F.F.; Cudhea, F.; Shan, Z.; Michaud, D.S.; Imamura, F.; Eom, H.; Ruan, M.; Rehm, C.D.; Liu, J.; Du, M.; et al. Preventable Cancer Burden Associated With Poor Diet in the United States. JNCI Cancer Spectr. 2019, 3, pkz34.
- Menotti, A.; Puddu, P.E. How the seven countries study contributed to the defnition and development of the mediterranean diet concept: A 50-year journey. Nutri. Metab. Cardiovas Dis. 2015, 25, 245–252.
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidemiol. 2018, 33, 909–931.
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528.
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397.
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757.
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552.




