
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Heechul Yoon | + 1772 word(s) | 1772 | 2021-10-18 08:17:42 | | | |
| 2 | Dean Liu | Meta information modification | 1772 | 2021-11-03 07:17:31 | | |
Video Upload Options
Laser-activated perfluorocarbon nanodroplets (PFCnDs) are emerging phase-change contrast agents that showed promising potential in ultrasound and photoacoustic (US/PA) imaging. Unlike monophase gaseous microbubbles, PFCnDs shift their state from liquid to gas via optical activation and can provide high US/PA contrast on demand. Depending on the choice of perfluorocarbon core, the vaporization and condensation dynamics of the PFCnDs are controllable.
1. Introduction
Over the past decade, phase-change contrast agents were rigorously studied and showed promising outcomes in diagnostic ultrasound imaging and therapeutic applications [1][2][3][4][5][6]. These contrast agents consisting of a liquid perfluorocarbon (PFC) core, referred to as perfluorocarbon nanodroplets (PFCnDs), undergo a phase transition from liquid to gaseous state in response to an external trigger [7][8]. Ultrasound energy (or optical energy for laser activation of PFCnDs with a photo-absorber) can be applied to induce vaporization of PFCnDs, capable of providing on-demand controllable contrast. Unlike micrometer-sized gaseous bubbles, these liquid PFCnDs can be stably generated in a broad range of sizes down to a few hundred nanometers and remain stable in blood circulation [9][10]. Thus, applications of the PFCnDs are not necessarily limited to the vascular space [11][12]. Before activation, the administered PFCnDs could be small enough to extravasate through the leaky cancerous neovasculature for extravascular cancer imaging.
2. Activation and Deactivation of Laser-Activated PFCnDs
| Perfluorocarbon Name (Chemical Formular) | Boiling Point (°C) |
|---|---|
| Perfluorooctylbromide (C8F17Br) | 143 |
| Perfluorooctane (C8F18) | 99–106 |
| Perfluorohexane (C6F14) | 58–60 |
| Perfluoropentane (C5F12) | 28–30 |
| Perfluorobutane (C4F10) | −1.7 |

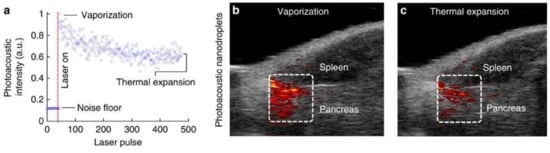
3. Ultrasound and Photoacoustic Imaging of Laser-Activated PFCnDs



References
- Sheeran, P.S.; Dayton, P.A. Phase-Change Contrast Agents for Imaging and Therapy. Curr. Pharm. Des. 2012, 18, 2152–2165.
- Dayton, P.A.; Zhao, S.; Bloch, S.H.; Schumann, P.; Penrose, K.; Matsunaga, T.O.; Zutshi, R.; Doinikov, A.; Ferrara, K.W. Application of Ultrasound to Selectively Localize Nanodroplets for Targeted Imaging and Therapy. Mol. Imaging 2006, 5, 160–174.
- Zullino, S.; Argenziano, M.; Stura, I.; Guiot, C.; Cavalli, R. From Micro- to Nano-Multifunctional Theranostic Platform: Effective Ultrasound Imaging Is Not Just a Matter of Scale. Mol. Imaging 2018, 17, 1536012118778216.
- Loskutova, K.; Grishenkov, D.; Ghorbani, M. Review on Acoustic Droplet Vaporization in Ultrasound Diagnostics and Therapeutics. BioMed Res. Int. 2019, 2019, 9480193.
- Lin, S.; Shah, A.; Hernández-Gil, J.; Stanziola, A.; Harriss, B.I.; Matsunaga, T.O.; Long, N.; Bamber, J.; Tang, M.-X. Optically and Acoustically Triggerable Sub-Micron Phase-Change Contrast Agents for Enhanced Photoacoustic and Ultrasound Imaging. Photoacoustics 2017, 6, 26–36.
- Rapoport, N. Phase-Shift, Stimuli-Responsive Perfluorocarbon Nanodroplets for Drug Delivery to Cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 492–510.
- Strohm, E.; Rui, M.; Gorelikov, I.; Matsuura, N.; Kolios, M. Vaporization of Perfluorocarbon Droplets Using Optical Irradiation. Biomed. Opt. Express 2011, 2, 1432–1442.
- Kripfgans, O.D.; Fabiilli, M.L.; Carson, P.L.; Fowlkes, J.B. On the Acoustic Vaporization of Micrometer-Sized Droplets. J. Acoust. Soc. Am. 2004, 116, 272–281.
- Sheeran, P.S.; Wong, V.P.; Luois, S.; McFarland, R.J.; Ross, W.D.; Feingold, S.; Matsunaga, T.O.; Dayton, P.A. Decafluorobutane as a Phase-Change Contrast Agent for Low-Energy Extravascular Ultrasonic Imaging. Ultrasound Med. Biol. 2011, 37, 1518–1530.
- Wilson, K.; Homan, K.; Emelianov, S. Biomedical Photoacoustics beyond Thermal Expansion Using Triggered Nanodroplet Vaporization for Contrast-Enhanced Imaging. Nat. Commun. 2012, 3, 618.
- Matsunaga, T.O.; Sheeran, P.S.; Luois, S.; Streeter, J.E.; Mullin, L.B.; Banerjee, B.; Dayton, P.A. Phase-Change Nanoparticles Using Highly Volatile Perfluorocarbons: Toward a Platform for Extravascular Ultrasound Imaging. Theranostics 2012, 2, 1185–1198.
- Yarmoska, S.K.; Yoon, H.; Emelianov, S.Y. Lipid Shell Composition Plays a Critical Role in the Stable Size Reduction of Perfluorocarbon Nanodroplets. Ultrasound Med. Biol. 2019, 45, 1489–1499.
- Yu, J.; Chen, X.; Villanueva, F.S.; Kim, K. Vaporization and Recondensation Dynamics of Indocyanine Green-Loaded Perfluoropentane Droplets Irradiated by a Short Pulse Laser. Appl. Phys. Lett. 2016, 109, 243701.
- Luke, G.P.; Hannah, A.S.; Emelianov, S.Y. Super-Resolution Ultrasound Imaging in Vivo with Transient Laser-Activated Nanodroplets. Nano Lett. 2016, 16, 2556–2559.
- Yoon, H.; Hallam, K.A.; Yoon, C.; Emelianov, S.Y. Super-Resolution Imaging With Ultrafast Ultrasound Imaging of Optically Triggered Perfluorohexane Nanodroplets. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 2277–2285.
- Shpak, O.; Verweij, M.; Vos, H.J.; de Jong, N.; Lohse, D.; Versluis, M. Acoustic Droplet Vaporization Is Initiated by Superharmonic Focusing. Proc. Natl. Acad. Sci. USA 2014, 111, 1697.
- Wu, Q.; Mannaris, C.; May, J.P.; Bau, L.; Polydorou, A.; Ferri, S.; Carugo, D.; Evans, N.D.; Stride, E. Investigation of the Acoustic Vaporization Threshold of Lipid-Coated Perfluorobutane Nanodroplets Using Both High-Speed Optical Imaging and Acoustic Methods. Ultrasound Med. Biol. 2021, 47, 1826–1843.
- Rojas, J.D.; Dayton, P.A. Optimizing Acoustic Activation of Phase Change Contrast Agents With the Activation Pressure Matching Method: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 264–272.
- Aliabouzar, M.; Kumar, K.N.; Sarkar, K. Effects of Droplet Size and Perfluorocarbon Boiling Point on the Frequency Dependence of Acoustic Vaporization Threshold. J. Acoust. Soc. Am. 2019, 145, 1105–1116.
- Arnal, B.; Perez, C.; Wei, C.-W.; Xia, J.; Lombardo, M.; Pelivanov, I.; Matula, T.J.; Pozzo, L.D.; O’Donnell, M. Sono-Photoacoustic Imaging of Gold Nanoemulsions: Part I. Exposure Thresholds. Photoacoustics 2015, 3, 3–10.
- Arnal, B.; Wei, C.-W.; Perez, C.; Nguyen, T.-M.; Lombardo, M.; Pelivanov, I.; Pozzo, L.D.; O’Donnell, M. Sono-Photoacoustic Imaging of Gold Nanoemulsions: Part II. Real Time Imaging. Photoacoustics 2015, 3, 11–19.
- Liu, W.-W.; Huang, S.-H.; Li, P.-C. Synchronized Optical and Acoustic Droplet Vaporization for Effective Sonoporation. Pharmaceutics 2019, 11, 279.
- Li, D.S.; Jeng, G.-S.; Pitre, J.J.; Kim, M.; Pozzo, L.D.; O’Donnell, M. Spatially Localized Sono-Photoacoustic Activation of Phase-Change Contrast Agents. Photoacoustics 2020, 20, 100202.
- Van Namen, A.; Jandhyala, S.; Jordan, T.; Luke, G. Repeated Acoustic Vaporization of Perfluorohexane Nanodroplets for Contrast-Enhanced Ultrasound Imaging; IEEE: Manhattan, NY, USA, 2021.
- Zhu, Y.I.; Yoon, H.; Zhao, A.X.; Emelianov, S.Y. Leveraging the Imaging Transmit Pulse to Manipulate Phase-Change Nanodroplets for Contrast-Enhanced Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 692–700.
- Yoon, H.; Yarmoska, S.K.; Hannah, A.S.; Yoon, C.; Hallam, K.A.; Emelianov, S.Y. Contrast-Enhanced Ultrasound Imaging in Vivo with Laser-Activated Nanodroplets. Med. Phys. 2017, 44, 3444–3449.
- Hannah, A.; Luke, G.; Wilson, K.; Homan, K.; Emelianov, S. Indocyanine Green-Loaded Photoacoustic Nanodroplets: Dual Contrast Nanoconstructs for Enhanced Photoacoustic and Ultrasound Imaging. ACS Nano 2014, 8, 250–259.
- Yang, L.; Cheng, J.; Chen, Y.; Yu, S.; Liu, F.; Sun, Y.; Chen, Y.; Ran, H. Phase-Transition Nanodroplets for Real-Time Photoacoustic/Ultrasound Dual-Modality Imaging and Photothermal Therapy of Sentinel Lymph Node in Breast Cancer. Sci. Rep. 2017, 7, 45213.
- Tang, W.; Yang, Z.; Wang, S.; Wang, Z.; Song, J.; Yu, G.; Fan, W.; Dai, Y.; Wang, J.; Shan, L.; et al. Organic Semiconducting Photoacoustic Nanodroplets for Laser-Activatable Ultrasound Imaging and Combinational Cancer Therapy. ACS Nano 2018, 12, 2610–2622.
- Yang, C.; Zhang, Y.; Luo, Y.; Qiao, B.; Wang, X.; Zhang, L.; Chen, Q.; Cao, Y.; Wang, Z.; Ran, H. Dual Ultrasound-Activatable Nanodroplets for Highly-Penetrative and Efficient Ovarian Cancer Theranostics. J. Mater. Chem. B 2020, 8, 380–390.
- Hannah, A.S.; VanderLaan, D.; Chen, Y.-S.; Emelianov, S.Y. Photoacoustic and Ultrasound Imaging Using Dual Contrast Perfluorocarbon Nanodroplets Triggered by Laser Pulses at 1064 nm. Biomed. Opt. Express 2014, 5, 3042–3052.
- Hannah, A.S.; Luke, G.P.; Emelianov, S.Y. Blinking Phase-Change Nanocapsules Enable Background-Free Ultrasound Imaging. Theranostics 2016, 6, 1866–1876.
- Yoon, H.; Emelianov, S.Y. Combined Multiwavelength Photoacoustic and Plane-Wave Ultrasound Imaging for Probing Dynamic Phase-Change Contrast Agents. IEEE Trans. Biomed. Eng. 2019, 66, 595–598.
- Jeng, G.-S.; Li, M.-L.; Kim, M.; Yoon, S.J.; Pitre, J.J.; Li, D.S.; Pelivanov, I.; O’Donnell, M. Real-Time Interleaved Spectroscopic Photoacoustic and Ultrasound (PAUS) Scanning with Simultaneous Fluence Compensation and Motion Correction. Nat. Commun. 2021, 12, 716.
- Rojas, J.D.; Dayton, P.A. In Vivo Molecular Imaging Using Low-Boiling-Point Phase-Change Contrast Agents: A Proof of Concept Study. Ultrasound Med. Biol. 2019, 45, 177–191.
- Li, D.S.; Schneewind, S.; Bruce, M.; Khaing, Z.; O’Donnell, M.; Pozzo, L. Spontaneous Nucleation of Stable Perfluorocarbon Emulsions for Ultrasound Contrast Agents. Nano Lett. 2019, 19, 173–181.
- Paproski, R.J.; Forbrich, A.; Huynh, E.; Chen, J.; Lewis, J.D.; Zheng, G.; Zemp, R.J. Porphyrin Nanodroplets: Sub-Micrometer Ultrasound and Photoacoustic Contrast Imaging Agents. Small 2016, 12, 371–380.
- Li, D.S.; Yoon, S.J.; Pelivanov, I.; Frenz, M.; O’Donnell, M.; Pozzo, L.D. Polypyrrole-Coated Perfluorocarbon Nanoemulsions as a Sono-Photoacoustic Contrast Agent. Nano Lett. 2017, 17, 6184–6194.




