
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Garganese | + 8596 word(s) | 8596 | 2021-08-25 04:42:19 | | | |
| 2 | Rita Xu | -3710 word(s) | 4886 | 2021-11-03 09:49:03 | | | | |
| 3 | Rita Xu | -3710 word(s) | 4886 | 2021-11-03 09:52:41 | | | | |
| 4 | Catherine Yang | Meta information modification | 4886 | 2021-11-11 03:07:11 | | |
Video Upload Options
The Philaenus spumarius L. (Hemiptera Aphrophoridae) is a xylem-sap feeder vector that acquires Xylella fastidiosa subsp. pauca ST53 during feeding on infected plants. The bacterium is the plant pathogen responsible for olive quick decline syndrome that has decimated olive trees in Southern Italy. Damage originates mainly from the insect vector attitude that multiplies the pathogen potentialities propagating Xf in time and space. The principal action to manage insect borne pathogens and to contain the disease spread consists in vector and transmission control. The analysis of an innovative and sustainable integrated pest management quantitative strategy that targets the vector and the infection by combining chemical and physical control means demonstrates that it is possible to stop the Xylella invasion. This entry updates the available topics addressing vectors' identification, bionomics, infection management, and induced disease by Xylella invasion to discuss major available tools to mitigate the damage consequent to the disease.
1. The Insect-Borne Plant Pathogen
Xylella fastidiosa Wells et al., 1987 (Xf) [1] is a xylem-restricted “fastidious” bacterium that lives in plant xylem and foregut vector lumina [2][3][4] of some xylem-feeders auchenorrhynchan [5]. Some phytopathological characteristics related to Xf (e.g., diversified range of plant hosts) are due to the ability of the bacterium to acquire DNA from the environment through horizontal transfer [6]. Xf is able to infect more than 300 different host species including crops, ornamental and spontaneous plants [7][8]. The olive strain found in 2013 in the Gallipoli area (Apulia, South Italy) that cause the Olive Quick Decline Syndrome (OQDS or CoDiRO, in Italian) is Xylella fastidiosa subsp. pauca ST53 (Xfp53) [9][10]. Xylella fastidiosa is in the quarantine organism list [11][12] and it is known as a biological weapon for the damage it can infer to a country’s crop production system [13][14][15][16][17][18]. Xylella moves from plant to plant and invades the territory [19][20] mainly by insect vectors. Some Aphrophoridae can also acquire the pathogen from infected plants to transmit it to other plants [21][22]. Aphrophoridae-borne plant pathogen transmission could be interspecific or intraspecific. Xylella fastidiosa may spread solely by vectors, and Xf invasion is vector-mediated, eventually. Vector–host–pathogen interactions determine whether or not an incursion or isolated pathogen outbreak will lead to settlement, persistence, and resulting epidemic development [23].
2. Non-Vector/Vector Pest Damage
The interest in vector-borne pathogen and vector control management rises because the interaction among the actors—vector, pathogen, and crop—causes relevant damage. We consider first the proportionality of the damage inflicted by the insect pest alone, then discuss the damage due to the vector–pathogen interaction. The damage is more relevant in a vector–pathogen interplay than the damage eventually due to the Aphrophoridae or the Xf alone.
3. Non-Vector Pest Damage
Usually, pest damage is directly proportional to single insect actions, where every single action is not restricted to a particular place or a limited period. Overall, the damage is limited because space and time hinder the pest population as its dispersal ability and lifespan. Usually, a single insect can feed on a specific part of its host/food plant (e.g., Bactrocera oleae (Rossi, 1790) or Prays oleae Bernard, 1788) [24][25] or cause damage by laying the eggs into a plant organ (e.g., Cicadidae) [26]. In non-vector pest, the probing (=an unconcluded feeding attempt) is not genuinely damaging or not at all. A single pest individual is hardly lethal for the plant it targets, eventually ruining a particular organ. Often, a single pest individual may damage a part of the total production for a perennial horticultural crop in a particularly productive year [27][28][29][30]. Most of the pest damage appears as inflicted at the end of a limited interval from the pest action. The discussion may consider if the crop is annual or perennial to quantify the damage amount properly. However, the pest number/damage proportionality allows using such proper techniques as pest trapping and similar approaches for thresholds evaluation [31]. In control actions, the damage forecast should promptly compare the damage value with the control cost [32][33]. The pest control issue consists of managing the pest population to get acceptable damage without suppressing all the pest individuals.
4. Vector Pest Damage
A vector sums its proper direct damaging ability over the infested plant with the additive damage inflicted by the borne pathogen. The damage due to the pathogen transmission is connected with direct pest behaviors, e.g., feeding or egg laying, and indirect conduct, such as plant probing [34]. The vector could spread the pathogen among susceptible plants in both cases [35]. Generally, vector–pathogen transmission can be non-persistent, semi-persistent or persistent [35]. Non-persistent pathogen transmission occurs within minutes from pathogen acquisition, and retention occurs on insect stylets [36]. The vector can rapidly lose all the borne pathogens in this transmission path, and multiple encounters with infected host plants are required for the vectors to remain viruliferous [36]. Semi-persistent pathogen retention can last for days, and the pathogen thrives in the insects’ alimentary canal [37]. Pathogen semi-persistent transmission occurs after hours or days of feeding to get the microorganism. Finally, vectors are infectious until death after a single encounter with an infected plant by persistent transmission. It takes hours or days for vectors to acquire the persistent pathogen. In Xf, the acquisition consists of one event, as fast as the non-persistent modality, but with persistent propagative, non-circulative modality. Adults Aphrophoridae have a persistent relationship with Xf [21][38]. Vector pest damage is greater than the sum of single insect actions, because every single inoculation projects the borne pathogen and the consequent damage in space and time. An infection on a plant organ can propagate to the entire plant [39], depending on the infected plant size. In time, the pathogen can continue to inflict damage after the vector death for years [40]. Therefore, it is necessary to consider the plant habit and damage severity. A lethal infection and consequent incidence of disease in trees orchards will substantially causes the annihilation of all future production, struggling the agricultural production system [41]. Vector-bearing poses a severe risk of preventing any other plant production for the crop if subjected to the pathogen spreading. Vector damage can be lethal by itself to the plant if the pathogen is lethal, making damage essential and not allowing the use of techniques for threshold assessment. Conventional approaches do not have functional timing or resolving power to avoid the first transmission of the pathogen (damaging event) [42][43] preventing the infection and pathogen-driven escalation.
5. Vector Species Identification
The relationship between the Xf and the vectors is persistent and propagative [44][45] but restricted only in the Aphrophoridae adult stage, where Xf behave as a non-mutualistic ectosymbiont. Italian vectors are all Aphrophoridae, and the most apparent Mediterranean vectors shall pertain to the same family. The primary vector is Philaenus spumarius (L., 1758, the meadow spittlebug), while less efficient vectors are Neophilaenus campestris (Fallén, 1805) and Philaenus italosignus (Drosopoulos and Remane, 2000) [46]. P. spumarius (Ps) is the most diffused and common spittlebugs species in the Palearctic area [47][48] with continental climate, ranging from Portugal to Primorye Russian Territories (Πpимopcкий кpaй = Primorskij Kraj). Furthermore, Ps was introduced and acclimated in the Nearctic area with subtropical, tropical, temperate, and arctic climate, where it can transmit Xf on grape [49].
Apart from Ps, the other Philaenus species have a Central-South Mediterranean distribution [50]. Only P. signatus goes Northern enough to approach European continental areas (Balkan peninsula). However, with plenty of olive and citrus orchards, the East Adriatic shores belong to the Csa group (hot summer Mediterranean climate) and the continental Balkan peninsula areas to Csb (warm summer Mediterranean climate) and to Dfb (warm summer humid continental climate) according to the Köppen-Geiger system [51]. Available data from the male’ genitalia study [51] suggest speciation occurred (or is ongoing) [52] from a plesiomorphic ancestor of the actual Ps originating apomorphic taxa in new territories and shifting on monocot host plants. The last short-glaciation—the Younger Dryas (from 12,900 to 11,700 years ago)—and the subsequent warming [52] provoked the consequent sea level rise and fall that interplayed with territories, flora, and species dispersion to originate actual Philaenus complex biogeography.
This biogeographic [53] interpretation also includes the host plant shift from continental central European cold-intermediate to hot-dry Mediterranean environments. South Aprhophoridae’s host range includes various herbs and spontaneous plants usually ignored by Ps pre-imaginal instars as the genus Asphodelus [54] and Eryngium [55] or trees and shrubs [56] during the adult stage.
6. Morphology and Identification
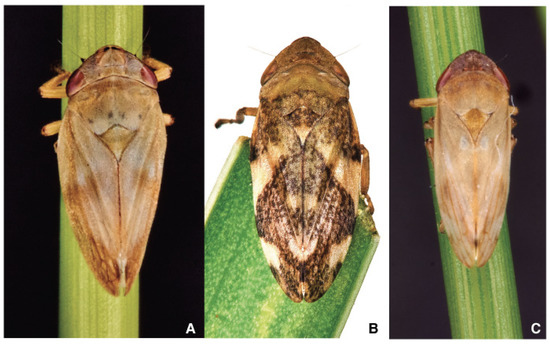
Philaenus spumarius (Figure 1A) adult head is broad, short, and equal in width to the rounded pronotum [57]. The vertex is angular and bluntly and twice as wide as it is long. Eyes are prominent on the side of the head. Ocelli are as far apart from each other as they are from the eyes. The antennae insert the genae between the eyes [58][59]. In Ps, the antennae are in the transition zone between the fronto-clypeus and the compound eyes. The antennae are inserted almost perpendicularly on the cuticular wall through an articulated socket, resulting in antennomere oriented towards the sides of the insect’s body [60]. The antenna is about 820 mm long in both male and female, with three segments: a short cone-shaped scape (length about 140 mm) connecting the antenna with the head capsule, a cylindrical pedicel (length about 120 mm) and a long thread-like flagellum (length about 750 mm) [60]. The general body shape is squat and stout, not much pubescent, giving the insect a frog-like appearance because they expose the posterior legs. Philaenus italosignus (Figure 1B) is distinguishable from other species by studying slide-mounted genitalia [48]. In the case of genus Philaenus, the size of adults is not so affordable character status to identify individuals from a previously not studied population. The suggestion is to identify male slide-mounted genitalia to identify Philaenus species. Neophilaenus campestris (Figure 1C) differs at first sight from Ps because the costal margins of tegmina are straight at rest.
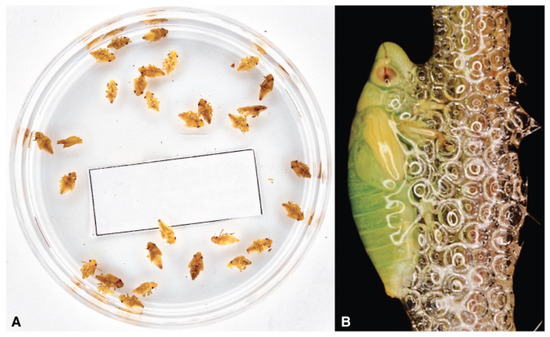
Juvenile development consists of five instars: three naiades plus two nymphs. Newborn Ps naiades are orange with a sclerotized prosoma (head and thorax) changing through yellow to green nymphs by aging. N. campestris juveniles have almost black prosoma (head + abdomen) and yellow abdomen; the color scheme does not change with the growth. Today, discriminating between naiades and nymphs is almost negligible in heteropteran juvenile description, even if morphological differences are often noticeable. The presence of nymphs in the field population of spittlebugs alerts for the timing vector control actions [61]. For this, we suggest discriminating naiades from the nymphs on the base of the two pairs of prominent wing buds (Figure 2A) and last nymph because of apparent venation in the buds (Figure 2B). Wing buds are evident in field observations and alert the operator to execute the control actions versus juveniles timely [42].
7. Vector Bionomics
The life history of Ps is based on a relatively long egg overwintering, a short win-ter-early spring pre-imaginal development, and a relatively long adult life interval from spring to winter [50][62][63][64][65]. The life cycle ends with the female dies after laying eggs [63]. Ps feeds on a vast repertoire of host plants in the field [66], either herbs, or bushes or trees. Philaenus spumarius participates to that 10% of phytophagous insects that feed on more than three different plant families [67]. Ps prefers Asteraceae, Fa-baceae, Apiaceae, and Lamiaceae as food plant, both in juvenile and adult instars [7]. The egg is the resistance stage for the insect [51]. Most of the eggs are laid near the ground in slits or similar receptacles, offering two facing surfaces [68]. Urged to lay eggs, in rearing, the female accepts plastic foils, cardboards, and similar substrates to oviposit as the internal wall surfaces or cotton wool of plastic rearing boxes. The temperature modulates the hatching [69] that needs exposure to a long period below 5°C to break the eggs diapause. In Southern Italy (Apulia region), first, newborns appeared vagrant in middle February [70], but the peak in hatching is un-clear, maybe because the weather varies considerably. Hatching lasts about one month, and different first instar naiades may coexist in the field for an extended period be-cause of a long hatching interval.
After hatching, the first naiades must make their way from abandoned choria to a suitable host plant. Vagrant first naiades are perhaps the most delicate since nymphs’ subsequent steps have froth to shelter helping survivorship [51][63]. Therefore, the first naiades are likely found on plants with basal leaves rosette or offering closely ap-posed leaf and stem surfaces [63][71]. Most olive groves host a relevant spontaneous herb layer during egg hatching time, and the young naiades may aggregate on preferred plants. The juveniles will crawl up to find a sheltered site on a suitable succulent plant to insert the stylets [64][65]. Feeding elicit the secretion/excretion of mucus and faeces, and a small liquid pond expands centered on the first instar. Despite the efforts, the very young insects cannot produce true spittle but a mix of liquid and some scattered bubbles, even if several individuals aggregate [72]. Foamy mass does not adequately shape the fluid prevalence that stays apart by gravity, and the insect bodies are too small to maintain around the bubbles. The second instar produces more fluid and feces and the insect starts bubbling into the liquid to form the spittle [51][73][74], the sequence repeats each molt. The juveniles are spittle-protected from drying, T °C peaks, sun, and other environmental stresses [63][71][75][76][77][78][79][80]. The spittle is a truly liquid niche for spittlebug juveniles that catch occasional visitors. The spittle could be involved in juveniles Aphrophoridae breathing [81]. Naiades’ and nymphs’ ensembles are ordinary in field condition and represent adaptive behavior [72] and several juvenile individuals on the same host plant can thrive in shared bubble masses [51]. Older naiades and nymphs set on the plants lonely, preferably head down and abdomen up. Later, mucus and bubbles slip on the body and in between the plant and body space.
The insect rises the anus out of the spittle and retracts the abdomen bubbling the air into the fluid, several times per minute. The secretion of the Batelli glands [73][74][75] seems to ameliorate the bubbling in the anal fluid sheltering under a foamy cover. The naiades (the first three juvenile instars) and nymphs (the last two instars) produce 1–3 cm long spittle masses on a high number of annuals or perennials herbs, shrubs, and trees host plants [76][77]. The juveniles pierce the plant tissue with their stylets and suck the xylem plant sap they feed [78]. The plant xylem sap exceeds in water, the excess fluid passes through the alimentary canal, and the anus eliminates it [65][72]. Juveniles last from 35 to 58 days in the field [63]. This period is degree-day triggered so that the juveniles’ lifespan may last 30 days only or extend to 110 days, manipulating the temperature [79][80]. Juveniles are abundant in spittle on herbs in either natural, or agricultural habitats [81] or urban environments [44] but are not as significant vectors because they cannot move over distances. Naiades and nymphs spend their life feeding and managing their position into the herb layer. They are not immotile, but they change the feeding place on the same plant or change the host plant with an adjacent one [77]. Most of the position changes consist of moving near to the plant tip and in a more exposed position as the younger ages. After about seven weeks from the hatching, the eclosion occurs on the herbs also. The last nymph stops bubbling, and the fluid within the foam drops from the spittle, drying it in the ending the pre-imaginal life [65]. A chamber opens in the foam around the nymph that molts to adult [72]. A neat cavity stays in the spittle shortly after Ps eclosion and its abandonment.
The individuals will become infective as adults when they move from herbs to the olive trees (or other reservoir plants) during the spring–summer and after acquiring Xf from infected plants [82]. Adults are free-living insects and can fly, but they prefer crawling or jumping [16][60][83][84]; in fact, flying seems more a gliding than an active fly. Adults feed on any available food plant and excrete liquid feces, projecting droplets around with any bubble inclusion. The adults usually remain in the area until the food plants stay available and before herbs dry out [85]. As available food plants run out the adults mass-move to another available food source around, even to gymnosperms [86][87][88] as Pinus spp., Cupressus spp., or Tuja spp. Adults mate continuously throughout the summer, and eggs appear in ovaries in August, but females refrain from oviposition because of extended daylight and the high temperature that induces a parapause [89][90]. Oviposition starts in September, and eggs undergo overwintering diapause [51][88]. Egg-laying continues until the death of the last females in December [91]. Males start to die before the females; all insects die once exhausted they role and field population declines quickly in synchrony by climate, like other animal populations [92][93].
8. Damage
In OQDS species of Aphrophoridae, juveniles are not harmful but adults inflict their most serious damage [44]. However, the attitude to damage plants is contextual and not intrinsic to insect action. Spittlebugs originate essential damage to olive plants and orchards because of their ability to transmit Xfp53. Alternative damage by host plant or food plant feeding is due because Ps can ingest xylem sap 150–200 times the body weight in a day (more than 10 times the ratio typical of the phloem‐feeding pests) [93][94]. Adult belonging to few species of Aphrophoridae can transmit Xfp53 [95] and further strains of the bacterium on their food plants. P. spumarius, P. italosignus, and N. campestris demonstrated to inject Xfp53 into the xylem vessels through Apulian olive orchards, inducing extensive OQDS and damaging olive trees to death because of sudden and massive dieback [96] with different symptoms intensity on plant and in the time.
9. Damage by Xylella fastidiosa pauca ST53 Infection
Xf damage to infected host plants varies from leaf scorch to partial desiccation to plant death. Once the bacterium entered the xylem-vessels plant system, it tries to invade all the lumina, mostly adhering to vessel wall surfaces and floating in the sap stream. Bacteria reach a high concentration in xylem vessels [97], eliciting a quorum sensing: a communicating system among bacteria that reply to increased density by producing signal molecules [98][99][100] inducing a biofilm production. Xf reacts to quorum sensing producing a great mass of biofilm [51][101][102][103][104][105] that embeds the bacteria and plugs the vessel. The occlusion unpair the xylem-vessel water transport performances. Plant organs distal to the plug can no longer receive the water they need if intense and sudden evapotranspiration stress occurs [106]. During intense and sudden evapotranspiration stressing event for hot-dry summer or cold-dry winter, the plant’s organs distal to the occlusion wilt and wither because they do not get water enough to survive [106][107][108][109]. The plant’s organs suffer abrupt desiccation due to environmental and unpredictable events; for this reason, "Disseccamento Rapido" and "Quick Decline" terms conjoin into the description of Xylella-associated lethal damage. Sub-lethal damage takes place in the form of recoverable wilting, stunted stub-born-like twigs growing, miss flowering or any flowering, and other changes related to water stress that hyperspectral image analysis can perceive [110].
10. Vector–Pathogen: Rationale Control
we share the rationale to counteract the ability of vector adults to infect their food plants with Xfp53. Based on the impact of an insect-borne pathogen [111][112] vector control is the only available approach. The infectious efficiency of spittlebug vectors, the high percentage of virulent adults [113], and their mobility [18] concerning the rapid induced decline of olive suggest that Xylella-vectors should be considered the new key-pest of olive trees [114].
11. Control Strategy
The semi-abandoned olive orchards apparently host the highest population of Aphrophoridae, compared with the corresponding cultivated one. Moreover, the organic olive orchards are more diversified in Aphrophoridae population size compared to the other management systems. Weed control provide a significant Xf-vector population impact among olive orchards. However, insecticide applications against primary olive pests do not affect Aphrophoridae abundance. The overall management strategy consists of an integrated pest management (IPM) decision support system (DSS) [43][44][115][116][117] based on quantitative sampling and vector survivor analysis with preventive and protective intents. The model [43][44] includes three control steps, each composed of one or a few actions, targeting to reduce the vectors population size and the number of adult infecting Xylella-free plants. The first control step targets eggs, while the second decimates the juvenile vectors on the orchard spontaneous plants/herbs [118], and the third limits the infections up to one maximum per adult vector. The first control step relies on physical control action(s), the second on a combination of physical plus synthetics or not chemical formulates distribution, and the third on the chemical distribution of a synthetic insecticide capable of bi-directional translocation.
Control Step Sequences
Current management measures focus on the mechanical and chemical control of eggs and juveniles on weeds [114][119] in early spring, and later on the insecticidal application against the mass-spreading adults. The effective and fast control of adult vectors is crucial to prevent or at least mitigate the bacteria acquisition [120], avoiding the subsequent spreading of the infection and eventual damage. Modern non-conventional neonicotinoids tested [119] on several vector stages and field trials effectively controlled P. spumarius. This efficacy also originates from a quick feeding cessation followed by vector mortality resulting in low bacterial infection [43][44], higher yield and a healthier plantation in comparison to the untreated.
12. First Step: Details
The first control step acts versus the population at its peak that corresponds to the overwintering eggs. Unfortunately, eggs are uncountable in the field [114] and cold-resistant [51][63][68]. The control action(s) in the first step consist(s) of one or a few winter light-tillage(s) to disrupt the sites of egg-laying, putting the naiades in trouble at born. Generally, winter tillage is a well-integrated and suggested practice necessary for water preservation [121]. It is critical to choose the tillage timing to counteract vector newborns. Tillage impact is nihil to moderate and strongly suggested in olive farming.
13. Second Step: Details
Second control step acts versus the juveniles survived at the winter light-tillage that lives on spontaneous herbs array in olive orchards. Juvenile’s peak at the time of first/second nymphs corresponds to fourth and fifth juvenile instars [74]. At that time, mid-April in Southern Italy, sampling reveals that the juvenile population comprises instars from the second to the fifth [74]. However, the relevant datum is the maximum absolute number of countable individuals per unit area. Ex-ante and ex-post quantitative sampling also measure the control action efficacy versus juveniles, giving the estimation of surviving adults [74]. The impact of the quantitative sampling is null to moderate, being strongly suggested in olive farming to manage spontaneous herbs in olive farming. Physical vector control targets nymph fourth and fifth, revealing feasible and effective [44].
14. Third Step: Details
It is impossible to kill all vector adult from a wide area consistently managed with olive orchards. Vectors damage does not consist in feeding acts but in the number of transmissions that each vector inflicts on the same or different target plants. The attitude to spread a pathogen among different plants [122] is the main "multiplier", causing extensive damage and pathogen invasion. The pathogen will trigger the final damage aggressively, much more than the sole vector activity. The first transmission to a plant is commonly called "infection", and it is discriminating from the "infective process" occurring with the subsequent plant tissues pathogen multiplication [43][44]. The infection only changes the status of the plant from healthy to infect. The third control step kills the adults who survived the previous control actions. However, the third step could act unfruitfully as "vector control" or effectively as "infection control", depending on the timing of the chemical control action.
In case the insecticide distribution precedes the vectors eclosion, two opportunities occur: 1) the vector feeds on a treated olive tree and dies independently on the plant status (infected/not infected); 2) the vector feeds on the non-treated infected olive tree and acquires Xfp53.
The third control step acts versus adult to impede them from feeding on the olive trees more than once [43][44], thus limiting the number of infections at the number of first feeding events. The third control step tailors the IPM strategy as:
- Preventive, to impede all the future infections from the vector because the insect dies on the tree during Xfp53 acquisition;
- Protective, to limit the vector action to one infection; vector dies on the just in-fected twig and impeding the infection of other olive trees or the repeated trans-missions on the same plant.
The infection control steps may use chemical or biological control actions [43][44]. One or a sequence of few distributions of synthetic insecticide by tree injection will contain the infections, depending on the size of the adult target population. The infection control action could also consist of vector biocontrol via antagonists’ inundation, e.g., Reduviidae [44][114]. Delaying the adults’ control allows multiple infections per vector and demonstrates the difference between ineffective and effective infection control.
15. Quantitative Control Approach
Accepting that the critical event in the pathogen invasion is the infection, we consider the key parameter the ratio between the number of possible infections and the number of susceptible plants. As in Fierro [43] and Bucci [123] discussion, any control actions proposed must concern the management of several vectors because capable of inflicting many infections. The quantitative approach is the only available (= effective) to plan, execute, harmonize, and verify a proper IPM strategy [124].
16. Symptomatic Plants Uprooting
The uprooting and extirpation of OQDS infected-asymptomatic or symptomatic plants is not an effective action because those plants have been acting as bacterium reservoirs for one or more years (3–4) at least [125]. The limit of 50–100 m ray for up-rooting/extirpating [104] around an infected tree is ineffective because the active vector dispersion per year exceeds some hundred meters [18]. The vectors acquire the pathogen from the infected plant and transmit it to other plants in the ray of 200–400 m before that focal plant revealed to be infected. The idea to uproot/extirpate diseased or asymptomatic plants only helps to mitigate the pathogen invasion.
17. Vector Census
The juvenile-vector population size knowledge is crucial in establishing an effective control strategy whereby choosing action thresholds, tuning control action intensity, and estimating control efficacy [74]. The first experience on spittle quantitative sampling, by direct in-field scrutiny, revealed an expensive approach in time and workforce [113]. Moreover, the population size gathered data are not congruous among pre-imaginal instar. Divergence in observation could be due to the small size of the early juvenile instar, the spittle inconsistency, and the inherent difficulty collecting all from a growing herb volume (0.5–0.8 m3/m2). Quantitative sampling can forecast the timing and tune the control actions intensity [74]. Several spittlebugs share the behavior and the common name. They can infest uncultured fields, amassing an exceedingly high population and difficult to manage because of their protected lifestyle. Ps control was marginal until the demonstration of its ability to transmit the Xfp53. Moreover, the percentage of Ps [81][114] over the total foamy-mass number is almost unpredictable and the missing of a selective control means, forced to control the entire spittlebugs population either as juveniles or adults.
AquaSamPling (ASP) is an appropriate area-wide quantitative sampling technique purposely tailored for spittlebugs juvenile census [74][126][127]. The technique is based on the (micro) habitat plant-unit removal [128] and following in-liquid insect extraction to get complete census counting [74]. The juveniles sampling technique used in EFSA accounts [116] and sweeping net [129][130] are not considered because phenetic and non-quantitative. They return a count of individual neither actual nor valuable for the vector control. Furthermore, Kretzschmar [131] and Pedigo [132] consider the sweeping an underestimating sampling method for immatures. The need for a quantitative evaluation of the target population (Aphrophoridae, until now) exists because the control targets are the Aphrophoridae as vectors and not as conventionally damaging pests [74]. In the case of conventionally dangerous pest [63][133][134], even a non-quantitative pest population census method works because the need is to correlate the number of individuals (e.g., per trap) with an (economic/action) threshold. Such a correlation considers the damage rather than population census. In vectors, the main parameter is the ratio between the number of vectors and the number of susceptible hosts [118]. The vector control aims to minimize the absolute number below a threshold during juvenile instars to protect the susceptible host plants and prevent them from infections by the survived adults [43][44]. The actual population census by ASP shows the need to manage a population estimate to range from one to one hundred million vectors per hectare [74] to gain a possible coexistence with Xfp53 in Italy.
18. Actual Engagement
Several xylem sap-feeders Hemipteran are or will be candidate key-pests for the Mediterranean inasmuch vectors of Xfp53 in Apulia. Nonspecific vectors could transmit Xf; local xylem-feeders may also transmit the bacterium when this one enters a new biogeographical region [119]. Therefore, the study’s main aim in not-yet-invaded countries is to assess the guild of possible local Xf vectors to improve the surveillance and build an effective DSS IPM control strategy in time [124]. OQDS affects millions of olive trees, threatening three-quarters of the world’s olive oil production [135]. Recent estimates quantified the desiccation of more than 6,500,000 olive trees [136], and the infected area in Southern Italy continues to expand. OQDS imposes a reduction in the supply of ecosystem services derived from olive orchards of 30–34% and a decrease in associated biodiversity of 28%, in addition to the impacts on productivity and the entire olive oil supply chain [137]. The current economic impact is conspicuous, and future projections do not promise an excellent productive scenario. The economic benefit derived from reducing the spread in Xylella-free areas and implementing mitigation measures in affected areas could guarantee a reduction of the disease impact ranged from 41% to 91% [42].
References
- Wells, J.M.; Raju, B.C.; Hung, H.Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov.: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Sys. Evol. Microbiol. 1987, 37, 136–143.
- Brlansky, R.H.; Timmer, L.W.; French, W.J.; McCoy, R.E. Colonization of the sharpshooter vector, Oncometopia nigricans and Homalodisca coagulata by xylem-limited bacteria. Phytopathology 1983, 73, 530–535.
- Hill, B.L.; Purcell, A.H. Populations of Xylella fastidiosa in plants required for transmission by an efficient vector. Phytopathol-ogy 1997, 87, 1197–1201.
- Newman, K.L.; Almeida, R.P.; Purcell, A.H.; Lindow, S.E. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Aca. Sci. USA 2004, 101, 1737–1742.
- Lambais, M.R.; Goldman, M.H.; Camargo, L.E.; Goldman, G.H. A genomic approach to the understanding of Xylella fastidiosa pathogenicity. Curr. Opin. Microbiol. 2000, 3, 459–462.
- Firrao, G.; Scortichini, M.; Pagliari, L. Orthology-Based Estimate of the Contribution of Horizontal Gene Transfer from Dis-tantly Related Bacteria to the Intraspecific Diversity and Differentiation of Xylella fastidiosa. Pathogens 2021, 10, 46.
- EFSA. Update of the Xylella spp. host plant database. EFSA J. 2018, 16, e05408.
- EFSA. Update of the Xylella spp. host plant database–systematic literature search up to 30 June 2019. EFSA J. 2020, 18, e06114.
- Su, C.C.; Deng, W.L.; Jan, F.J.; Chang, C.J.; Huang, H.; Shih, H.T.; Chen, J. Xylella taiwanensis sp. nov., causing pear leaf scorch disease. Int. J. Sys. Evol. Microbiol. 2016, 66, 4766–4771.
- Loconsole, G.; Saponari, M.; Boscia, D.; D’Attoma, G.; Morelli, M.; Martelli, G.P.; Almeida, R.P.P. Intercepted isolates of Xylella fastidiosa in Europe reveal novel genetic diversity. Eur. J. Plant. Pathol. 2016, 146, 85–94.
- Coletta-Filho, H.D.; Francisco, C.S.; Lopes, J.R.S.; De Oliveira, A.F.; de Oliveira Da Silva, L.F. First report of olive leaf scorch in Brazil, associated with Xylella fastidiosa subsp. pauca. Phytopathol. Mediterr. 2016, 55, 130–135.
- Gomila, M.; Moralejo, E.; Busquets, A.; Segui, G.; Olmo, D.; Nieto, A.; Juan, A.; Lalucat, J. Draft genome resources of two strains of Xylella fastidiosa XYL1732/17 and XYL2055/17 isolated from Mallorca vineyards. Phytopathology 2019, 109, 222–224.
- EPPO. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed on 2nd August 2021).
- Casagrande, R. Biological terrorism targeted at agriculture: The threat to US national security. Nonproliferation Rev. 2000, 7, 92–105.
- Budowle, B.; Murch, R.; Chakraborty, R. Microbial forensics: The next forensic challenge. Int. J. Leg. Med. 2005, 119, 317–330.
- Mark, J.A.; Green, L.D.; Deshpande, A.; White, P.S. System integration and development for biological warfare agent surveillance. Opt. Photonics Glob. Homel. Secur. 2007, 6540, 65401D.
- Nutter, F.W.; Madden, L.V. Plant Pathogens as Biological Weapons Against Agriculture. In Beyond Anthrax; Lutwick, L.I., Lutwick, S.M., Eds.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2008.
- Russmann, H.; Richardt, A. Biological Warfare Agents. In Decontamination of Warfare Agents; Richardt, A., Blum, M.M., Eds. Wiley-VCH Verl GmbH Co.: Weinheim, Germany, 2008.
- Janse, J.D.; Obradovic, A. Xylella fastidiosa: Its biology, diagnosis, control and risks. J. Plant. Pathol. 2010, 92, S35–S48.
- Almeida, R.P.; Nunney, L. How do plant diseases caused by Xylella fastidiosa emerge? Plant. Dis. 2015, 99, 1457–1467.
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Simonetto, A.; Saladini, M.A.; Plazio, E.; Gilioli, G.; Molinatto, G.; Saponari, M.; Bosco, D. Dispersal of Philaenus spumarius (Hemiptera: Aphrophoridae), a Vector of Xylella fastidiosa, in Olive Grove and Meadow Agroecosystems. Environ. Entomol. 2021, 50, 267–279.
- Purcell, A.H.; Finlay, A.H.; McLean, D.L. Pierce’s disease bacterium: Mechanism of transmission by leafhopper vectors. Science 1979, 206, 839–841.
- Killiny, N.; Almeida, R.P. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl. Environ. Microbiol. 2009, 75, 521–528.
- Sanderlin, R.S.; Melanson, R.A. Transmission of Xylella fastidiosa through pecan rootstock. HortScience 2006, 41, 1455–1456.
- Burckhardt, D. Biology, ecology, and evolution of gall-inducing psyllids (Hemiptera: Psylloidea). In Biology, Ecology and Evolution of Gall-Inducing Arthropods; Raman, A., Schaefer, C.W., Withers, T.M., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 2, pp. 143–157.
- Burckhardt, D.; Ouvrard, D.; Queiroz, D.; Percy, D. Psyllid host-plants (Hemiptera: Psylloidea): Resolving a semantic problem. Fla. Entomol. 2014, 97, 242–246.
- Flory, S.L.; Mattingly, W.B. Response of host plants to periodical cicada oviposition damage. Oecologia 2008, 156, 649–656.
- Baser, N.; Broutou, O.; Lamaj, F.; Verrastro, V.; Porcelli, F. First finding of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in Apulia, Italy, and its population dynamics throughout the year. Fruits 2015, 70, 225–230.
- Baser, N.; Broutou, O.; Verrastro, V.; Porcelli, F.; Ioriatti, C.; Anfora, G.; Mazzoni, V.; Rossi Stacconi, M.V. Susceptibility of table grape varieties grown in south‐eastern Italy to Drosophila suzukii. J. Appl. Entomol. 2018, 142, 465–472.
- Salerno, M.; Mazzeo, G.; Suma, P.; Russo, A.; Diana, L.; Pellizzari, G.; Porcelli, F. Aspidiella hartii (Cockerell 1895) (Hemiptera: Diaspididae) on yam (Dioscorea spp.) tubers: A new pest regularly entering the European part of the EPPO region. EPPO Bull. 2018, 48, 287–292.
- Bouslama, T.; Chaieb, I.; Jerbi-Elayed, M.; Laarif, A. Observations of some biological characteristics of Helicoverpa armigera reared under controlled conditions. Tunis. J. Plant. Prot. 2019, 14, 17–27.
- Arbogast, R.T.; Kendra, P.E.; Mankin, R.W.; McGovern, J.E. Monitoring insect pests in retail stores by trapping and spatial analysis. J. Econ. Entomol. 2000, 93, 1531–1542.
- Sardaro, R.; Grittani, R.; Scrascia, M.; Pazzani, C.; Russo, V.; Garganese, F.; Porfido, C.; Diana, L.; Porcelli, F. The Red Palm Weevil in the City of Bari: A First Damage Assessment. Forests 2018, 9, 452.
- Sardaro, R.; Roselli, L.; Grittani, R.; Scrascia, M.; Pazzani, C.; Russo, V.; Garganese, F.; Porfido, C.; Diana, L.; Porcelli, F. Community preferences for the preservation of Canary Palm from Red Palm Weevil in the city of Bari. Arab. J. Plant. Prot. 2019, 37, 206–211.
- Murdock, C.C.; Luckhart, S.; Cator, L.J. Immunity, host physiology, and behaviour in infected vectors. Curr. Opin. Insect. Sci. 2017, 20, 28–33.
- Perilla-Henao, L.M.; Casteel, C.L. Vector-borne bacterial plant pathogens: Interactions with hemipteran insects and plants. Front. Plant. Sci. 2016, 7, 1163.
- Ng, J.C.K.; Falk, B.W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212.
- Ng, J.C.K.; Zhou, J.S. Insect vector-plant virus interactions associated with non-circulative, semi-persistent transmission: Current perspectives and future challenges. Curr. Opin. Virol. 2015, 15, 48–55.
- Hill, B.L.; Purcell, A.H. Acquisition and retention of Xylella fastidiosa by an efficient vector, Graphocephala atropunctata. Phytopathology 1995, 85, 209–212.
- Bruening, G.; Kirkpatrick, B.; Esser, T.; Webster, R. Managing newly established pests: Cooperative efforts contained spread of Pierce’s disease and found genetic resistance. Calif. Agric. 2014, 68, 134–141.
- Almeida, R.P. Ecology of emerging vector-borne plant diseases. In Vector-Borne Diseases: Understanding the Environmental, Human Health, and Ecological Connections; Lemon, S.M., Sparling, P.F., Hamburg, M.A., Relman, D.A.; Choffnes, E.R., Rappor-teurs, A.M., Eds.; National Academies Press: Washington, DC, USA, 2008; pp. 70–77.
- Schneider, K.; Van der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Lansink, A.O. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259.
- Fierro, A.; Liccardo, A.; Porcelli, F. A lattice model to manage the vector and the infection of the Xylella fastidiosa on olive trees. Sci. Rep. 2019, 9, 1–14.
- Liccardo, A.; Fierro, A.; Garganese, F.; Picciotti, U.; Porcelli, F. A biological control model to manage the vector and the in-fection of Xylella fastidiosa on olive trees. PLoS ONE 2020, 15, e0232363.
- Butter, N.S. Insect Vectors and Plant Pathogens. CRC Press: Boca Raton, FL, USA, 2018; pp. 496.
- Purcell, A.H.; Finlay, A.H. Evidence for noncirculative transmission of Pierce’s disease bacterium by sharpshooter leafhop-pers. Phytopathology 1979, 69, 393–395.
- Panzavolta, T.; Bracalini, M.; Croci, F.; Ghelardini, L.; Luti, S.; Campigli, S.; Goti, E.; Marchi, R.; Tiberi, R.; Marchi, G. Phi-laenus italosignus a potential vector of Xylella fastidiosa: Occurrence of the spittlebug on olive trees in Tuscany. Bull. Insectol. 2019, 72, 317–320.
- Stewart, A.J.; Lees, D.R. The colour/pattern polymorphism of Philaenus spumarius (L.) (Homoptera: Cercopidae) in England and Wales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 69–89.
- Drosopoulos, S.; Remane, R. Biogeographic studies on the spittlebug Philaenus signatus Melichar, 1896 species group (Hemiptera: Aphrophoridae) with the description of two new allopatric species. Ann. Soc. Entomol. Fr. 2000, 36, 269–277.
- Stewart, A.J.A.; Lees, D.R. Genetic control of colour/pattern polymorphism in British populations of the spittlebug Philaenus spumarius (L.) (Homoptera: Aphrophoridae). Biol. J. Linn. Soc. Lond. 1988, 34, 57–79.
- Yurtsever, S. On the polymorphic meadow spittlebug, Philaenus spumarius (L.) (Homoptera: Cercopidae). Turk. Zool. Derg. 2000, 24, 447–460.
- Lahbib, N.; Boukhris-Bouhachem, S.; Cavalieri, V.; Rebha, S.; Porcelli, F. A survey of the possible insect vectors of the bacterium Xylella fastidiosa in seven regions of Tunisia. In Poster Section, Proceedings of the II European Conference on Xylella fastidiosa: How research can support solutions, Ajaccio, France, 29–30 October 2019.
- Boukhris-Bouhachem, S.; Souissi, R.; Porcelli, F. Taxonomy and re-description of Philaenus Mediterranean species. In Poster Section, Proceedings of the II European Conference on Xylella fastidiosa: How research can support solutions, Ajaccio, France, 29–30 October 2019.
- Remane, R.; Drosopoulos, S. Philaenus tarifa nov. sp.—an additional spittlebug from Southern Spain (Homoptera—Cercopidae). Mitt. Mus. Nat. Berl. Dtsch. Entomol. Z. 2001, 48, 277–279.
- Kapantaidaki, D.E.; Antonatos, S.; Evangelou, V.; Papachristos, D.P.; Milonas, P. Genetic and endosymbiotic diversity of Greek populations of Philaenus spumarius, Philaenus signatus and Neophilaenus campestris, vectors of Xylella fastidiosa. Sci. Rep. 2021, 11, 1–17.
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644.
- Broecker, W.S.; Denton, G.H.; Edwards, R.L.; Cheng, H.; Alley, R.B.; Putnam, A.E. Putting the Younger Dryas cold event into context. Quat. Sci. Rev. 2010, 29, 1078–1081.
- Craw, R.C.; Grehan, J.R.; Heads, M.J. Panbiogeography Tracking the History of Life; Oxford University Press: New York, NY, USA, 1999.
- Maryańska-Nadachowska, A.; Kuznetsova; V.G.; Lachowska, D.; Drosopoulos, S. Mediterranean species of the spittlebug genus Philaenus: Modes of chromosome evolution. J. Insect Sci. 2012, 12, 54.
- Rodrigues, A.S. Evolutionary History of Philaenus spumarius (Hemiptera, Aphrophoridae) and the Adaptive Significance and Genetic Basis of its Dorsal Colour Polymorphism. Ph.D. Thesis, Lisboa University, Lisboa, Portugal, 2017.
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Part 2: The families Cicadidae, Cer-copidae, Membracidae, and Cicadellidae (excl. Deltocephalinae). Fauna Entomol. Scand. 1981, 7, 223–593.
- Ranieri, E.; Ruschioni, S.; Riolo, P.; Isidoro, N.; Romani, R. Fine structure of antennal sensilla of the spittlebug Philaenus spumarius L. (Insecta: Hemiptera: Aphrophoridae). I. Chemoreceptors and thermos-hygroreceptors. Arthropod Struct. Dev. 2016, 45, 432–439.
- Weaver, C.R.; King, D.R. Meadow spittlebug, Philaenus leucophthalmus (L.). Res. Bull. Ohio Agric. Exp. Stn. 1954, 741, 1–99.
- Mundinger, F.G. The control of spittle insects in strawberry plantings. J. Econ. Entomol. 1946, 39, 299–305.
- Wiegert, R.G. Population Energetics of Meadow Spittlebugs (Philaenus spumarius L.) as Affected by Migration and Habitat. Ecol. Monogr. 1964, 34, 217–241.
- Stöckmann, M.; Biedermann, R.; Nickel, H.; Niedringhaus, R. The Nymphs of the Planthoppers and Leafhoppers of Germany; WABW Fründ: Bremen, Germany, 2013.
- Nickel, H.; Remane, R. Check list of the planthoppers and leafhoppers of Germany, with notes on food plants, diet width, life cycles, geographic range and conservation status (Hemiptera, Fulgoromorpha and Cicadomorpha). Beiträge Zur Zik-adenkunde 2002, 5, 27–64.
- Halkka, A.; Halkka, L.; Halkka, O.; Roukka, K.; Pokki, J. Lagged effects of North Atlantic Oscillation on spittlebug Philaenus spumarius (Homoptera) abundance and survival. Glob. Chang. Biol. 2006, 12, 2250–2262.
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Simonetto, A.; Fumarola, G.; Di Carolo, M.; Porcelli, F.; et al. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 1–17.
- Wood, Z.M.; Jones, P.L. The Effects of Host Plant Species and Plant Quality on Growth and Development in the Meadow Spittlebug (Philaenus spumarius) on Kent Island in the Bay of Fundy. Northeast. Nat. 2020, 27, 168–185.
- Bernays, E.A.; Graham, M. On the evolution of host specificity in phytophagous arthropods. Ecology 1988, 69, 886–892.
- Barber, G.; Ellis, W.O. Eggs of three Cercopidae. Psyche 1922, 29, 1–3.
- Medler, J.T. Method of predicting the hatching date of the meadow spittlebug. J. Econ. Entomol. 1955, 48, 204–205.
- Picciotti, U.; D’Accolti, A.; Garganese, F.; Gammino, R.P.; Tucci, V.; Russo, V.; Diana, F.; Salerno, M.; Diana, L.; Porfido, C.; et al. Aphrophoridae (Hemiptera) vectors of Xylella fastidiosa pauca OQDS juvenile quantitative sampling. In Proceedings of the XI European Congress of Entomology, Naples, Italy, 2–6 July 2018; pp. 162–163.
- Grant, J.F.; Lambdin, P.L.; Follum, R.A. Infestation levels and seasonal incidence of the meadow spittlebug (Homoptera: Cercopidae) on musk thistle in Tennessee. J. Agric. Entomol. 1998, 15, 83–91.
- Wise, M.J.; Kieffer, D.L.; Abrahamson, W.G. Costs and benefits of gregarious feeding in the meadow spittlebug, Philaenus spumarius. Ecol. Entomol. 2006, 31, 548–555.
- Guilbeau, B.H. The origin and formation of the froth in spittle-insects. Am. Nat. 1908, 42, 783–798.
- Malykh, Y.N.; Krisch, B.; Gerardy-Schahn, R.; Lapina, E.B.; Shaw, L.; Schauer, R. The presence of N-acetylneuraminic acid in Malpighian tubules of larvae of the cicada Philaenus spumarius. Glycoconj. J. 1999, 16, 731–739.
- Rakitov, R.A. Structure and function of the Malpighian tubules, and related behaviors in juvenile cicadas: Evidence of ho-mology with spittlebugs (Hemiptera: Cicadoidea & Cercopoidea). Zool. Anz. 2002, 241, 117–130.
- Tonelli, M.; Gomes, G.; Silva, W.D.; Magri, N.T.C.; Vieira, D.M.; Aguiar, C.L. Spittlebugs produce foam as a thermoregula-tory adaptation. Sci. Rep. 2018, 8, 4729.
- Beckett, K.I.; Robertson, A.B.; Matthews, P.G. Studies on gas exchange in the meadow spittlebug, Philaenus spumarius: The metabolic cost of feeding on, and living in, xylem sap. J. Exp. Biol. 2019, 222, 1–9.
- Berlese, A. Gli Insetti, Loro Organizzanzione, Sviluppo, Abitudini e Rapporti Coll’uomo; Società Editrice Libraria: Milan, Italy, 1909; Volume 1, pp. 539–540.
- Šulc, K. Uber Respiration, Tracheen system, Und Schaumproduction der Schaumcikaden Larven. Z. F. Wiss. Zool. 1911, 99, 147–188.
- Marshall, A.T. Batelli glands of cercopoid nymphs (Homoptera). Nature 1965, 205, 925–925.
- Dongiovanni, C.; Altamura, G.; Di Carolo, M.; Fumarola, G.; Saponari, M.; Cavalieri, V. Evaluation of efficacy of different insecticides against Philaenus spumarius L., vector of Xylella fastidiosa in olive orchards in Southern Italy, 2015–2017. Arthropod Manag. Tests 2018, 43, tsy034.
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauro, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean olive groves: Host-plant exploitation throughout the year. Insects 2020, 11, 130.
- Malone, M.; Watson, R.; Pritchard, J. The spittlebug Philaenus spumarius feeds from mature xylem at the full hydraulic tension of the transpiration stream. New Phytol. 1999, 143, 261–271.
- Chmiel, S.M.; Wilson, M.C. Estimation of the lower and upper developmental threshold temperatures and duration of the nymphal stages of the meadow spittlebug, Philaenus spumarius. Environ. Entomol. 1979, 8, 682–685.
- West, J.; Lees, D.R. Temperature and egg development in the spittlebug Philaenus spumarius (L.) (Homoptera: Aphrophoridae). Entomologist 1988, 13, 46–51.
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest. Sci. 2019, 92, 1101–1109.
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, D.; Di Carolo, M.; Fumarola, G.; Altamura, G.; Lasorella, C.; Bosco, D. Plant selection and population trend of spittlebug immatures (Hemiptera: Aphrophoridae) in olive groves of the Apulia region of Italy. J. Econ. Entomol. 2019, 112, 67–74.
- Halkka, O.; Raatikainen, M.; Halkka, L.; Lokki, J. Factors determining the size and composition of island populations of Philaenus spumarius (L.) (Homoptera). Acta Entomol. Fenn. 1971, 28, 83–100.
- Burrows, M. Jumping performance of froghopper insects. J. Exp. Biol. 2006, 209, 4607–4621.
- Press, M.C.; Whittaker, J.B. Exploitation of the xylem stream by parasitic organisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1993, 341, 101–111.
- Cornara, D.; Panzarino, O.; Santoiemma, G.; Bodino, N.; Loverre, P.; Mastronardi, M.G.; Mattia, C.; de Lillo, E.; Addante, R. Natural areas as reservoir of candidate vectors of Xylella fastidiosa. Bull. Insectology 2021, 74, (Accepted).
- Gargani, E.; Benvenuti, C.; Marianelli, L.; Roversi, P.F.; Ricciolini, M.; Scarpelli, I.; Sacchetti, P.; Nencioni, A.; Rizzo, D.; Strangi, A.; et al. A five-year survey in Tuscany (Italy) and detection of Xylella fastidiosa subspecies multiplex in potential in-sect vectors, collected in Monte Argentario. J. Zool. 2021, 104, 75–88.
- Witsack, W. Dormanzformen mitteleuropäischer Zikaden. In Zikaden Leafhoppers, Planthoppers and Cicadas (Insecta: Hemiptera: Auchenorrhyncha); Holzinger, W., Ed.; Oberösterreichische Landesmuseen: Linz, Austria, 2002; pp. 471–482.
- Witsack, W. Synchronisation der Entwicklung durch Dormanz und Umwelt an Beispielen von Zikaden (Homoptera Auchenorrhyncha). Mitt. Dtsch. Ges. Allg. Angew. Ent. 1993, 8, 563–567.
- Weaver, C.R. The Seasonal Behavior of Meadow Spittlebug and Its Relation to a Control Method. J. Econ. Entomol. 1951, 44, 350–353.
- Ranta, E.; Veijo, K.; Lindströom, J. Spatially autocorrelated disturbances and patterns in population synchrony. Proc. R. Soc. Lond. Biol. 1999, 266, 1851–1856.
- Post, E.; Forchhammer, M.C. Synchronization of animal population dynamics by large-scale climate. Nature 2002, 420, 168–171.
- Raven, J.A. Phytophages of xylem and phloem: A comparison of animal and plant sap-feeders. Adv. Ecol. Res. 1983, 13, 135–234.
- Bragard, C.; Dehnen‐Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Mag-nusson, C.S.; Milonas, P.; et al. Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17, 5665.
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology 2019, 109, 175–186.
- Ionescu, M.; Zaini, P.A.; Baccari, C.; Tran, S.; da Silva, A.M.; Lindow, S.E. Xylella fastidiosa outer membrane vesicles modulate plant colonization by blocking attachment to surfaces. Proc. Natl. Acad. Sci. USA 2014, 111, E3910–E3918.
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587.
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199.
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346.
- Fry, S.M.; Milholland, R.D. Multiplication and translocation of Xylella fastidiosa in petioles and stems of grapevine resistant, tolerant, and susceptible to Pierce’s disease. Phytopathology 1990, 80, 61–65.
- Colnaghi-Simionato, A.V.; da Silva, D.S.; Lambais, M.R.; Carrilho, E. Characterization of a putative Xylella fastidiosa diffusible signal factor by HRGC‐EI‐MS. J. Mass Spectrom. 2007, 42, 1375–1381.
- Killiny, N.; Martinez, R.H.; Dumenyo, C.K.; Cooksey, D.A.; Almeida, R.P.P. The exopolysaccharide of Xylella fastidiosa is essential for biofilm formation, plant virulence, and vector transmission. Mol. Plant. Microbe Interact. 2013, 26, 1044–1053.
- Mendes, J.S.; Santiago, A.S.; Toledo, M.A.; Horta, M.A.; de Souza, A.A.; Tasic, L.; de Souza, A.P. In vitro determination of ex-tracellular proteins from Xylella fastidiosa. Front. Microbiol. 2016, 7, 2090.
- Scala, V.; Pucci, N.; Salustri, M.; Modesti, V.; L’Aurora, A.; Scortichini, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; Loreti, S. Xylella fastidiosa subsp. pauca and olive produced lipids moderate the switch adhesive versus non-adhesive state and viceversa. PLoS ONE 2020, 15, e0233013.
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicolì, F.; Vergine, M.; Negro, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by Xylella fastidiosa. Sci. Rep. 2019, 9, 1–11.
- Cariddi, C.; Saponari, M.; Boscia, D.; De Stradis, A.; Loconsole, G.; Nigro, F.; Porcelli, F.; Potere, O.; Martelli, G.P. Isolation of a Xylella fastidiosa strain infecting olive and oleander in Apulia, Italy. J. Plant. Pathol. 2014, 96, 425–429.
- EFSA, P.L.H. Scientific opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, 3989.
- Jeger, M.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen‐Schmutz, K.; Gilioli, G.; Grégoire, J.C.; Miret, J.A.J.; McLeod, A.; Navarro, M.N.; et al. Updated pest categorisation of Xylella fastidiosa. EFSA J. 2018, 16, e05357.
- Poblete, T.; Camino, C.; Beck, P.S.A.; Hornero, A.; Kattenborn, T.; Saponari, M.; Boscia, D.; Navas-Cortes, J.A.; Zarco-Tejada, P.J. Detection of Xylella fastidiosa infection symptoms with airborne multispectral and thermal imagery: Assessing bandset reduction performance from hyperspectral analysis. ISPRS J. Photogramm. Remote Sens. 2020, 162, 27–40.
- Clark, R.E.; Basu, S.; Lee, B.W.; Crowder, D.W. Tri‐trophic interactions mediate the spread of a vector‐borne plant pathogen. Ecology 2019, 100, e02879.
- Crowder, D.W.; Li, J.; Borer, E.T.; Finke, D.L.; Sharon, R.; Pattemore, D.E.; Medlock, J. Species interactions affect the spread of vector‐borne plant pathogens independent of transmission mode. Ecology 2019, 100, e02782.
- Cunty, A.; Legendre, B.; de Jerphanion, P.; Juteau, V.; Forveille, A.; Germain, J.F.; Ramel, J.M.; Reynaud, P.; Olivier, V.; Poliakoff, F. Xylella fastidiosa subspecies and sequence types detected in Philaenus spumarius and in infected plants in France share the same locations. Plant. Pathol. 2020, 69, 1798–1811.
- Acquasanta, F.; Bacci, L.; Baser, N.; Carmignano, P.M.; Cavalieri, V.; Cioffi, M.; Convertini, S.; D’Accolti, A.; Dal Maso, E.; Diana, F. et al. Tradizione e Innovazione nel Controllo del Philaenus spumarius Linnaeus, 1758 (Hemiptera Aphrophoridae). In Atti Giornate Fitopatologiche I, Proceedings of the Giornate Fitopatologiche, Chianciano Terme, Italy, 6–9 March 2018, pp. 181–190.
- Bucci, E.M. Effectiveness of the monitoring of X. fastidiosa subsp. pauca in the olive orchards of Southern Italy (Apulia). Rend. Lincei Sci. Fis. Nat. 2019, 30, 681–688.
- Kogan, M. Integrated pest management: Historical perspectives and contemporary developments. Ann. Rev. Entomol. 1998, 43, 243–270.
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; D’Attoma, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D.; et al. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci. Rep. 2017, 7, 1–13.
- Pedigo, L.P.; Rice, M.E. Entomology and Pest Management, 6th ed.; Waveland Press, Inc.: Long Grove, IL, USA, 2009.
- McCravy, K.W. A review of sampling and monitoring methods for beneficial arthropods in agroecosystems. Insects 2018, 9, 170.
- Ruesink, W.G.; Kogan, M. The quantitative basis of pest management: Sampling and measuring. In Introduction to Insect Pest Management; Metcalf, R.L., Luckmann, W.H., Eds.; J. Wiley & Sons: New York, NY, USA, 1975; pp. 309–351.
- Schotzko, D.J.; O’Keeffe, L.E. Comparison of Sweep Net., D.-Vac., and Absolute Sampling., and Diel Variation of Sweep Net Sampling Estimates in Lentils for Pea Aphid (Homoptera: Aphididae)., Nabids (Hemiptera: Nabidae)., Lady Beetles (Cole-optera: Coccinellidae)., and Lacewings (Neuroptera: Chrysopidae). J. Econ. Entomol. 1989, 82, 491–506.
- Ausden, M.; Drake, M. Invertebrates. In Ecological Census Techniques: A Handbook, 2nd ed.; Sutherland, W.J. Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 214–289.
- Kretzschmar, G.P. Soybean insects in Minnesota with special reference to sampling techniques. J. Econ. Entomol. 1948, 41, 586–591.
- Pedigo, L.P.; Lentz, G.L.; Stone, J.D.; Cox, D.F. Green Clover worm Populations in Iowa Soybean with Special Reference to Sampling Procedure. J. Econ. Entomol. 1972, 65, 414–421.
- Bubici, G.; Prigigallo, M.I.; Garganese, F.; Nugnes, F.; Jansen, M.; Porcelli, F. First report of Aleurocanthus spiniferus on Ailanthus altissima: Profiling of the insect microbiome and MicroRNAs. Insects 2020, 11, 161.
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). Insects 2020, 11, 509.
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.; Blua, M.J.; Mizell III, R.F.; Andersen, P.C. The biology of xylem fluid–feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Ann. Rev. Entomol. 2004, 49, 243–270.
- Schneider, K.; Mourits, M.; van der Werf, W.; Oude-Lansinka, A. Analysis on consumer impact from Xylella fastidiosa subspecies pauca. Ecol. Econom. 2021, 185, 1–11.
- Scholten, R.; Martinez-Sanchez, L. Monitoring the impact of Xylella on Apulia’s olive orchards using MODIS satellite data supported by weather data. In Proceedings of the 2nd European Conference on Xylella fastidiosa, Ajaccio, France, 29–30 October 2019. Available online: http://www.efsa.europa.eu/sites/default/files/event/191029-xylella/S6.P1_BECK.pdf (accessed on 16 June 2021).




