Rhus trilobata (RHTR) is a medicinal plant with cytotoxic activity in different cancer cell lines. However, the active compounds in this plant against ovarian cancer are unknown. In this study, we aimed to evaluate the antineoplastic activity of RHTR and identify its active metabolites against ovarian cancer. The aqueous extract (AE) and an active fraction (AF02) purified on C18-cartridges/ethyl acetate decreased the viability of SKOV-3 cells at 50 and 38 μg/mL, respectively, compared with CHO-K1 (> 50 μg/mL) in MTT assays and generated changes in the cell morphology with apoptosis induction in Hemacolor® and TUNEL assays (p ≤ 0.05, ANOVA). The metabolite profile of AF02 showed a higher abundance of flavonoid and lipid compounds compared with AE by UPLC-MS. Gallic acid and myricetin were the most active compounds in RHTR against SKOV-3 cells at 50 and 166 μg/mL, respectively (p ≤ 0.05, ANOVA). Antineoplastic studies in Nu/Nu female mice with subcutaneous SKOV-3 cells xenotransplant revealed that 200 mg/kg/i.p. of AE and AF02 inhibited ovarian tumor lesions from 37.6% to 49% after 28 days (p ≤ 0.05, ANOVA). In conclusion, RHTR has antineoplastic activity against ovarian cancer through a cytostatic effect related to gallic acid and myricetin. Therefore, RHTR could be a complementary treatment for this pathology.
1. Introduction
Ovarian cancer is the sixth most frequent tumor in women and the fourth cause of death in Mexico due to gynecological tumors
[1]. Ovarian tumors can be primary or metastatic and are classified based on their origin as epithelial, germinal, or stromal of the sexual cord tumors
[2]. Epithelial tumors are the most common type of ovarian neoplasm; among them, the serous subtype usually appears more frequently
[2]. Surgical resection is the principal treatment for this disease, followed by antitumoral chemotherapy with cytotoxic or cytostatic drugs
[1][3]. However, the surgent of resistance in neoplastic tissue limits ovarian cancer chemotherapy’s success
[3], making it necessary to search for alternative treatments or new therapeutic agents for this disease. Plants used for alternative medicine represent an option in the search for active compounds for cancer treatment. Recent work with
Rhus trilobata Nutt. (RHTR) demonstrated the presence of antineoplastic agents that could be new therapeutic candidates for the treatment of ovarian cancer that afflicts the female population in Mexico and around the world
[4].
In Mexico, RHTR (
Anacardiaceae family) is used for the treatment of gastrointestinal diseases and cancer. RHTR is known by the common name of
aciditos or
agritos due to the characteristic flavor of its fruit
[4]. Previous studies by Abbott et al. (1966) demonstrated the antineoplastic activity of RHTR in Syrian hamsters xenotransplanted with duodenum adenocarcinoma. Animals were treated with extracts of leaves (100 mg/kg/i.p.) or fruits (400 mg/kg/i.p.) for seven days; tumoral lesions decreased 33% with both extracts in comparison with control conditions
[5]. Subsequently, Pettit et al. (1974) isolated gallic acid (Ga) from RHTR leaves with column chromatography (Sephadex LH-20) and determined its biological activity; Ga decreased KB cell viability (IC
50: 3.1 μg/mL). The authors concluded that the medicinal properties of RHTR correspond to this compound
[6]. Studies conducted by our research group revealed that the aqueous extract (AE) of RHTR stems contains quinic acid, myricetin (Myr), Ga, 1,2,3,4,6-pentakis-
O-gallioyl-
β-D-glucose (
β-PGG), quercetin, obtusaquinol, fisetin, margaric acid, and amentoflavone by UPLC-MS
E, which are compounds with biological activity already demonstrated by other studies
[4]. Additionally, AE-RHTR presented a selective activity against CACO-2 cells (IC
50: 5 μg/mL), and low toxicity (LD
50: 1141.5 mg/kg)
[4]. Thus, the biological activity observed in RHTR may be related to compounds such as phenolic acids or flavonoids and the synergic effects between both molecule types.
2. Biological Activity of AE-RHTR and Fractions in Cell Lines
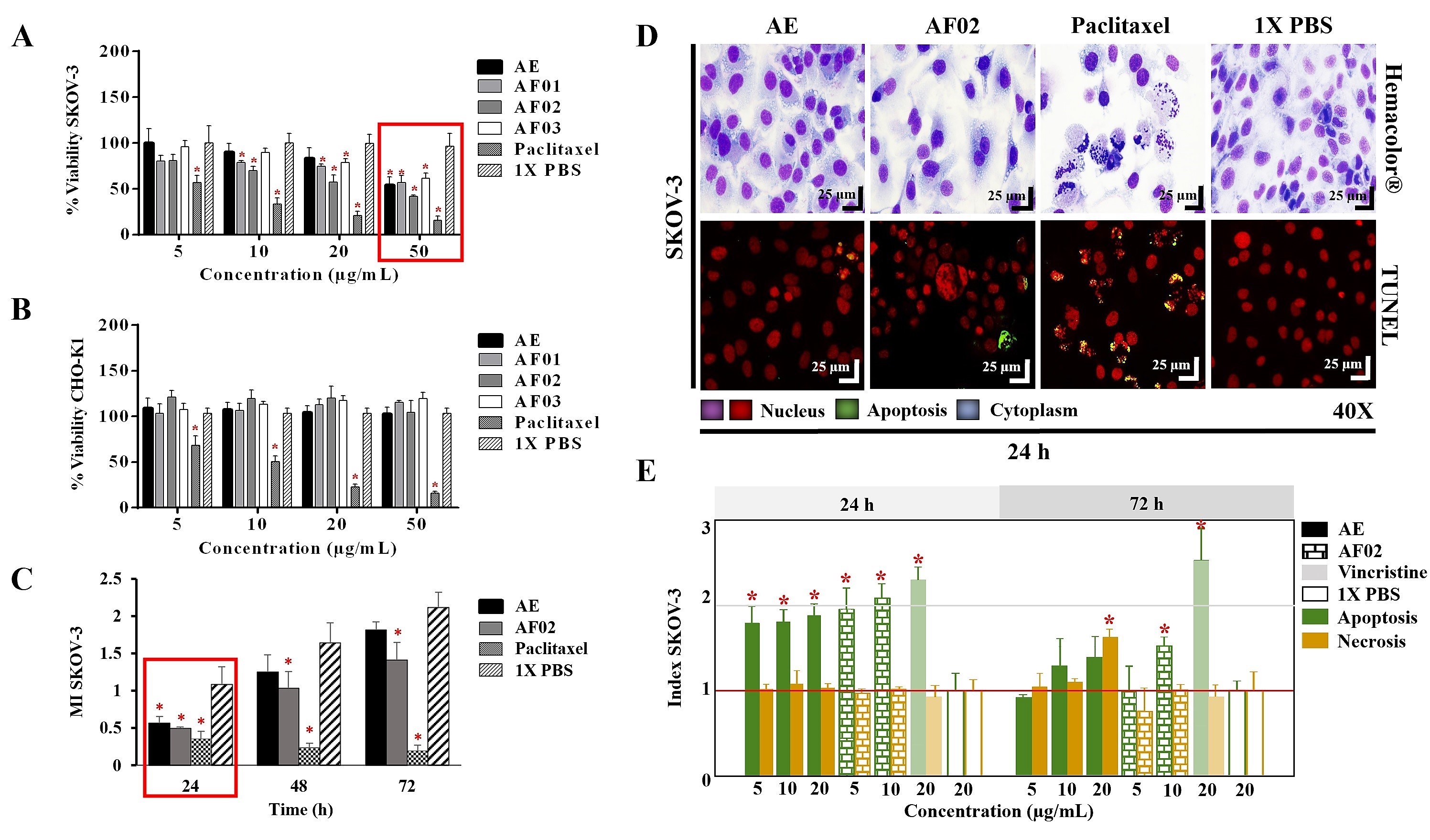
The AE and fractions from RHTR presented biological activity in SKOV-3 cells at 50 μg/mL (
Figure 1A). In comparison, the concentration required to affect the viability of CHO-K1 cells was greater than 50 μg/mL (
Figure 1B), demonstrating a selective effect. AE and AF02 were most active against SKOV-3 cells at 50 and 38 μg/mL, respectively, compared with 1× PBS (vehicle group) (
p < 0.05, Dunnett). However, AE and AF02 were found to only have a 24 h limited inhibitory effect on SKOV-3 cell proliferation (
Figure 1C). Additionally, SKOV-3 cells treated with AE and AF02 had a similar morphology to the vehicle group but with a considerable increase in cytoplasmic vesicles and an absence of cellular mitosis, which suggest a quiescent effect in both treatments (
Figure 1D). The apoptosis assays mainly demonstrated increased caspase-3/7 activity and nuclear DNA-fragmentation in cells treated with AE (8.47% ± 0.9%), AF02 (14.01% ± 3.7%), and paclitaxel (46.42% ± 5.0%) at 24 h in comparison with the vehicle group (2.47% ± 0.4%) (
p < 0.05, ANOVA;
Figure 1D,E). Necrotic events were present during treatments with both samples at 72 h (
Figure 1E), possibly related to stages of late apoptosis and not with true necrosis, which can promote inflammatory processes; however, additional studies are necessary to corroborate this finding. These results resemble those obtained with CACO-2 cells, where cell cycle arrest in G
1 and the appearance of a G
1 subpopulation related to apoptosis were observed. In contrast, in BEAS-2B cells, increasing the concentration up to 800 μg/mL was necessary to observe a similar effect
[4]. Therefore, AE and AF02 were selected to evaluate their antineoplastic activity.
Figure 1. Biological activity of RHTR in ovarian cancer. The IC50 of AE and fractions in SKOV-3 (A) and CHO-K1 (B) cell lines were obtained with dose–response viability curves at 24 h by MTT assay. The antiproliferative (C), cytotoxic, and apoptotic (E) activities of AE and AF02 were determined in SKOV-3 cells by an ApoTox-Glo™ Triplex Assay. The morphological changes with Hemacolor® rapid staining and TUNEL were observed at 24 h of treatment with AE (50 µg/mL) and AF02 (38 µg/mL), respectively (D). The results show the mean ± SD of three biological replicates (n = 3, in triplicates). * p ≤ 0.05 vs. the control group without treatment (1× PBS) (ANOVA). Paclitaxel or vincristine was used as the positive control. MI, mitotic index (MI = Abs sample/Abs control); RHTR, Rhus trilobata; AE, aqueous extract; AF, aqueous fraction (numbers indicate the fraction obtained after fractionation on the solid phase with solvents, as described in the Methodology section).
3. Antineoplastic Activity of RHTR in Mice Xenotransplanted with SKOV-3 Cells
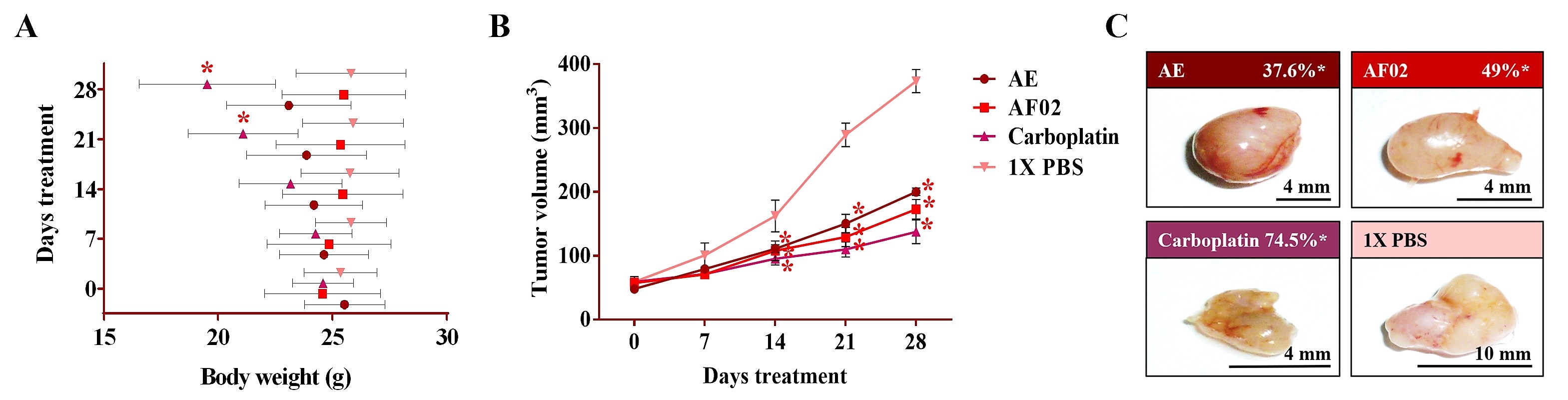
After 24 h of treatment, the rodents showed no behavioral changes. Additionally, the analyses of their bodyweight showed that the group treated with AE experienced a 9.5% decrease, but a 3.7% increase in the group with AF02. However, these changes were not significant compared to the control group treated with 1× PBS vehicle (p > 0.05, Dunnett; Figure 2A). At the end of treatments, mice were euthanized for tumor lesions recovery and to perform a macroscopic analysis of the developed lesions (Figure 2C). Similar characteristics in all tumors were present in the treated groups with an ovoid or smooth-surfaced morphology and the presence of vascularity (Figure 2C). However, minor differences in the color of tumors were found: pink for AE and whitish for AF02; groups treated with carboplatin and vehicle presented a yellow tumor with a nodular surface (Figure 2C). Analysis of tumoral weight showed that greater masses were found in the vehicle group, followed by AE, AF02, and carboplatin (p > 0.05, Tukey; Table 1). These results correlate with the tumor volume of lesions present in rodents along with the treatment time (Figure 2B). The inhibition percentage in tumoral growth with AE, AF02, and carboplatin was 37.6%, 49%, and 74.5%, respectively, compared with the control group for 14 and 28 days of treatment (p ≤ 0.05, Dunnett; Figure 2B,C). These findings demonstrate changes in the disease evolution directly related to the treatments used in the study. Similarly, these results agree with those obtained in tumoral length (Table 1), which demonstrated a cytostatic effect of the treatments.
Figure 2. Antineoplastic activity of AE and AF02 from RHTR in ovarian cancer. The bodyweight of rodents was monitored with an electronic bascule for 28 days (A). The tumor volume was determined with a Vernier caliper in mice treated with AE and AF02 at 200 mg/kg/i.p./day (Tumoral volume = [Larger diameter × (Shorter diameter)2]/2) (B). Morphological changes in tumor lesions were analyzed at the end of treatments, and the percent inhibition was calculated (C). Results show the mean ± SD of two biological replicates (n = 5). * p ≤ 0.05 vs. the control group without treatment (1× PBS) (ANOVA). The positive control was carboplatin (50 mg/kg/i.p./3 alternating days per week in mice). RHTR, Rhus trilobata; AE, aqueous extract; AF02, aqueous fraction-02 (active fraction obtained with ethyl acetate).
Table 1. Morphological characteristics of ovarian tumor lesions treated with RHTR.
| Treatments |
AE |
AF02 |
Carboplatin |
1× PBS |
| Weight (g) |
0.24 ± 0.09 * |
0.19 ± 0.10 * |
0.14 ± 0.06 * |
0.34 ± 0.09 |
| Larger diameter (mm) |
9.07 ± 0.25 * |
7.40 ± 0.95 * |
3.70 ± 0.26 * |
14.53 ± 2.4 |
| Tumor volume (mm3) |
201.4 ± 11.6 * |
172.7 ± 15.3 * |
137.3 ± 18.6 * |
373.3 ± 18.1 |
| Attenuation coefficient (HU) |
47 ± 2.16 |
48.8 ± 0.83 |
48.7 ± 0.95 |
47 ± 2.1 |
| Vascularity |
+ |
+ |
+ |
+++ |
| Fibrosis |
+ |
+ |
+ |
+ |
| Morphology |
Cystic |
Cystic |
Solid |
Cystic |
3.1. Imaging and Histopathological Studies of Tumor Lesions
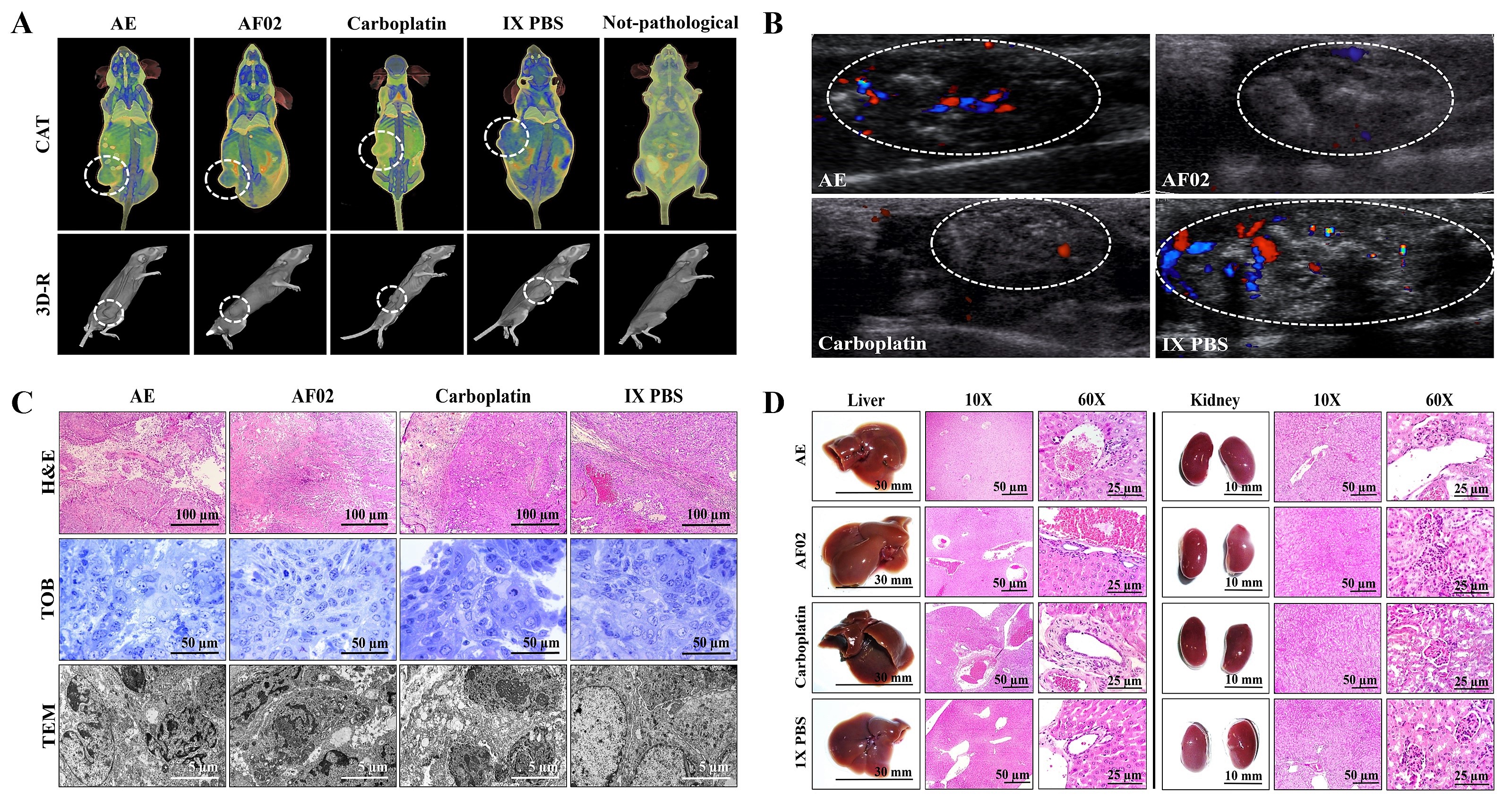
Images by NMR showed ovoid tumors located in the subcutaneous tissue, with attenuation units related to soft tissues (≈50 HU;
Table 1). Treatments with AE and AF02 induced regular edges in tumoral lesions, whereas groups treated with carboplatin and vehicle were mainly lobulated edges (
Figure 3A). Additionally, the necrosis percentage was higher in mice treated with vehicle (35.16%), whereas those treated with AE, AF02, and carboplatin were 30.68%, 20.54%, and 5.19%, respectively (
Figure 3A). Images by USG corroborated the presence of subcutaneous ovoid tumors, with partially defined edges and heterogeneous content (hyperechoic areas with diffuse distribution concerning fibrosis) in the different groups (
Figure 3B). Vascularity presence was observed in all tumors (seen as red and blue color); however, the highest vascularity was found in mice treated with vehicle compared with AE, whereas the lower vascularity was observed in those animals treated with AF02 or carboplatin (
Figure 3B). The histological analysis of cystic lesions located in the reticular dermis of rodents revealed a stroma/parenchyma of mixed composition and without residual organ (
Figure 3C). The abundant presence of loose fibrous tissue was observed in all tumors with a predominance of comedo-type necrosis for the vehicle (25%), decreasing by AE and AF02 treatment (10%), or basaloid-type histological pattern after carboplatin (
Figure 3C, H&E). Additionally, the adjacent stroma in tumors presented retraction artifacts (cracks) and signs of acute/moderate chronic inflammation by variable cellular infiltrate (
Figure 3C, H&E). Other characteristics observed in all tumors were the proliferation of basaloid cells (basement membrane cells-like) organized in a solid pattern that grew to push the stroma, as well as palisade cells on the tumor periphery with a radial orientation at superior parallel axes (
Figure 3C, H&E and TOB). Similarly, most cells presented mixed nuclei (small and hyperchromatic) with vesicular chromatin and a poorly defined wide eosinophilic cytoplasm (
Figure 3C, H&E). RHTR treatments induced atypical mitosis and apoptosis in comparison with the control group (
Figure 3C, TOB and TEM). All these characteristics observed in tumors correspond to poorly differentiated carcinomas (possibly of the serous papillary type)
[7]. The first-choice treatment for ovarian cancer (with anaplasia IV degree) is paclitaxel and carboplatin since both compounds exert their effects through different mechanisms of action
[1]. Carboplatin can generate DNA adducts to prevent cell proliferation
[8], and paclitaxel can bind to
β-tubulin to stabilize the microtubules and block the mitosis, which triggers cell death by apoptosis
[9]. Therefore, the similar effect that induced the RHTR compounds present in AE and AF02 to drugs used in chemotherapy for ovarian cancer (particularly with carboplatin) makes them suitable candidates for more comprehensive structural studies and sheds light on their potential application against this disease.
Figure 3. Imaging and histopathologic studies of ovarian tumor lesions and organs of Nu/Nu mice treated with RHTR. Analyses with CAT (A) and USG (B) were performed to observe densitometric and morphological changes in the tumoral lesions, as well as to rule out metastatic processes during the treatments. The tumor lesions are delimited with a white circle. In the Doppler color modality, the arterial and venous flow are indicated in red and blue, respectively. Differential histological patterns were observed in the tumor lesions (C) and the absence of tissue damage in the liver and kidneys (D) with treatment with AE and AF02 from RHTR at 200 mg/kg/i.p. for 28 days. Results show the mean ± SD of two biological replicates (n = 5). The positive control was carboplatin (50 mg/kg/i.p./3 alternating days per week in mice). RHTR, Rhus trilobata; AE, aqueous extract; AF02, aqueous fraction-02 (active fraction obtained with ethyl acetate); CAT, computer axial tomographic; 3D-R, 3D reconstruction; USG, ultrasonography; H&E, hematoxylin and eosin; TOB, toluidine blue; MET, transmission electron microscopy.
3.2. Morphometric and Paraclinical Studies of Mice Treated with RHTR
Anatomical lesions (
Figure 3D) or morphometric changes (
Table S1) in the liver and kidneys were absent in mice treated with RHTR. Additionally, histopathological lesions in the organs were not found by comparison with the control group (1× PBS;
Figure 3D). Finally, paraclinical studies revealed slight leukopenia in mice treated with AE and AF02 at 28 days of administration concerning reference values for mice (
p ≤ 0.05, ANOVA;
Table 2). The biochemical analysis demonstrated a decrease in albumin for mice treated with AE and AF02, whereas urea decreased with AE treatment at the end of the study (
p ≤ 0.05, ANOVA;
Table 2). The results suggest slight hematopoiesis suppression in the bone marrow with AE and AF02 treatments, possibly by the quickly replicative phenotype of blood cells
[10]. Additionally, paraclinical studies did not reveal hepatic dysfunction or renal failure, so the use of RHTR as an alternative treatment for ovarian cancer could be considered.
Table 2. Paraclinical studies in Nu/Nu mice treated with RHTR.
| Treatments |
AE |
AF02 |
Carboplatin |
1× PBS |
Reference
Range (mean) |
| Glucose (mg/dL) |
72 ± 23.9 |
114 ± 21.2 * |
107 ± 14.8 * |
78.3 ± 16.6 |
63–176 (89) |
| Triglycerides (mg/dL) |
71.5 ± 26.2 |
80 ± 11.35 |
103.7 ± 12.7 * |
87.6 ± 12.01 |
55–115 (85) |
| Cholesterol (mg/dL) |
66 ± 25.4 * |
62 ± 2.52 * |
108.6 ± 19.4 * |
85.5 ± 13.4 |
26–82 (64) |
| Protein (g/dL) |
4.48 ± 0.6 |
4.42 ± 0.62 |
5.1 ± 1.46 |
4.7 ± 0.56 |
4–8.6 (6.2) |
| Albumin (g/dL) |
2.1 ± 0.8 * |
2.03 ± 0.35 * |
1.33 ± 0.29 |
1.36 ± 0.21 |
2.5–4 (3.2) |
| AST (GOT) (U/L) |
132.6 ± 45.7 |
145.7 ± 7.69 |
162.9 ± 8.2 |
149.8 ± 27.29 |
55–251 (139) |
| ALT (GPT) (U/L) |
41.2 ± 9.8 |
32.43 ± 19.2 |
64.4 ± 6.87 * |
40.75 ± 14.5 |
17–77 (47) |
| Total bilirubin (mg/dL) |
0.47 ± 0.07 * |
0.39 ± 0.08 * |
0.58 ± 0.2 |
0.67 ± 0.16 |
0.20–1.0 (0.6) |
| Alkaline phosphatase (U/L) |
46.5 ± 23.2 * |
83.6 ± 24.04 |
66.6 ± 15.6 |
71 ± 10.36 |
9–88 (48.5) |
| Creatinine (mg/dL) |
0.74 ± 0.4 |
0.7 ± 0.25 |
0.37 ± 0.26 * |
0.65 ± 0.2 |
0.2–0.9 (0.5) |
| Urea (mg/dL) |
40.8 ± 8.2 |
57.6 ± 10.7 |
59.4 ± 9.99 |
47.9 ± 7.16 |
46.9–73 (60.1) |
| BUN (g/dL) |
19 ± 3.8 |
26.8 ± 4.7 |
27.8 ± 4.63 |
22.36 ± 3.32 |
11–27 (19) |
| Hemoglobin (g/dL) |
15.2 ± 0.98 |
12.35 ± 2.2 |
13.1 ± 1.61 |
14.4 ± 2.13 |
10–17 (13.1) |
| Hematocrit (%) |
48.3 ± 7.92 |
43.6 ± 17.8 |
40.57 ± 4.16 |
44.52 ± 2.17 |
39–49 (40.4) |
| Erythrocytes (×106/mm3) |
7.24 ± 1.06 |
7.67 ± 2.26 |
7.81 ± 0.88 |
7.92 ± 1.93 |
8.3 |
| Leukocytes (×100/mm3) |
3600 ± 903 * |
4242.6 ± 980 |
2750 ± 777 * |
5720 ± 684 |
5–12 (6.33) |
| Platelets (×106/μL) |
736 ± 1.414 |
689 ± 5.425 |
759 ± 19.143 |
710 ± 6.716 |
116 |
| PCT |
0.53 ± 0.07 |
0.49 ± 0.10 |
0.55 ± 0.29 |
0.55 ± 0.30 |
-- |
4. Purified Metabolites in RHTR
Methyl gallate (methyl 3,4,5-trihydroxybenzoate, C
8H
8O
5) was purified from RHTR and identified by nuclear magnetic resonance (NMR). The signal obtained was
1H-NMR (300 MHz, CD
3OD) δ: 7.07 (H-2, H-6), δ: 4.9 (H-3, H-4, H-5), δ: 3.8 (H-8) (
Figure S1A).
13C-NMR (300 MHz, CD
3OD) δ: 122.3 (C-1), δ: 110.9 (C-2, C-6), δ: 147.3 (C-3, C-5), δ: 140.6 (C-) 4), δ: 169.8 (C-7), δ: 53.1 (C-8) (
Figure S1B). The identification pattern obtained is similar to those reported by other authors
[13][14]. Methyl gallate is present in several species of the
Rhus genus
[15] and exhibited antioxidant, antimicrobial, and antineoplastic activity in various investigations
[16][17][18]; therefore, the biological activity of methyl gallate was evaluated in SKOV-3 cells.
5. Phytochemical Composition and in Silico Analysis of AF02-RHTR
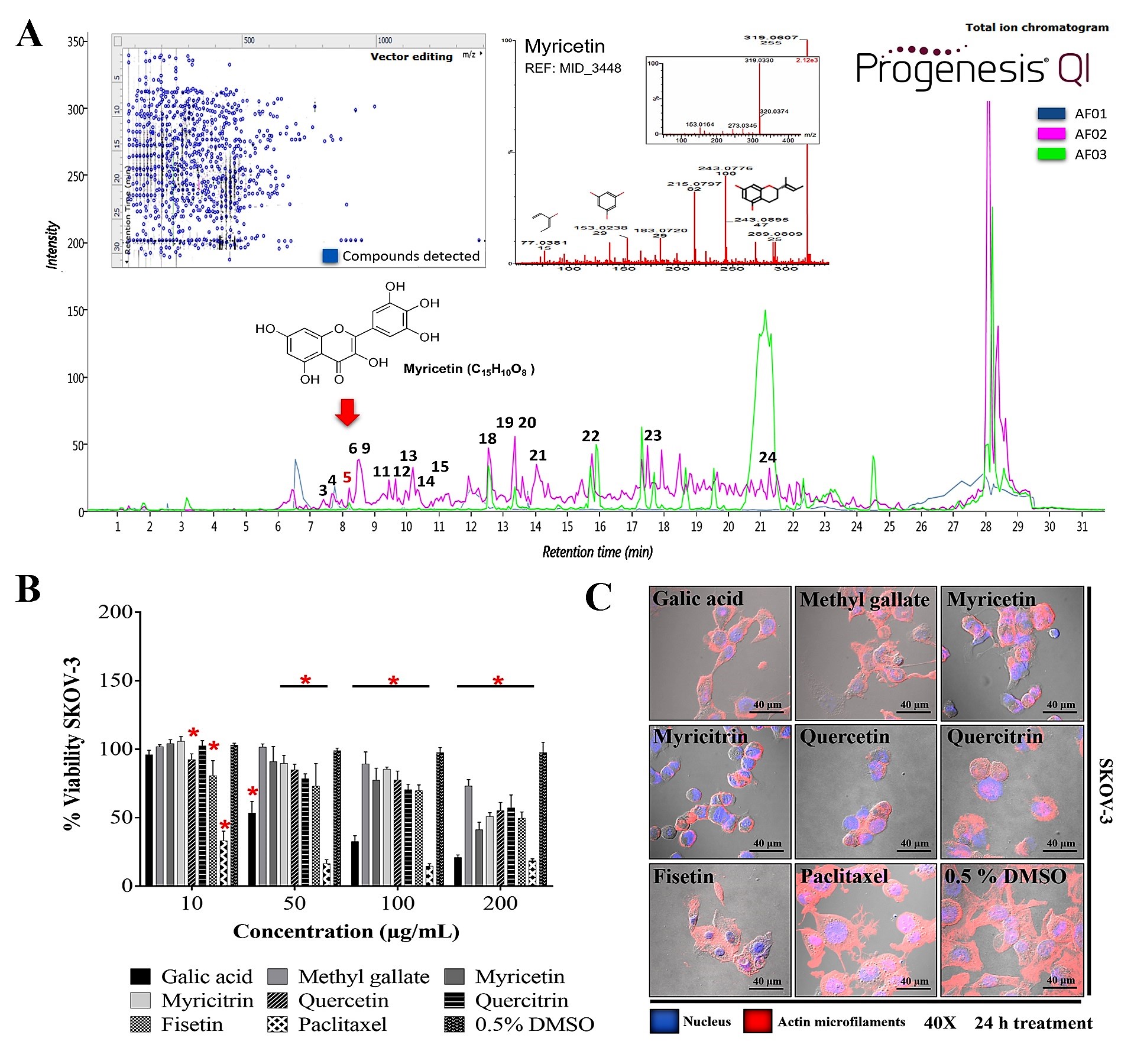
The metabolite profile of AF02 showed a higher abundance of flavonoid and lipid compounds (
Table 3). Additionally, a comparative study between the metabolite profile of AE and AF02 showed that epigallocatechin 3-cinnamate (3), 3,5-digalloylepicatechin (4), Myr (5), myricitrin (6), quercetin (9), hibiscoquinone A (11), quercitrin (12), myricetin 3-(4’’-galloylrhamnoside) (13), obtusaquinol (14), epifisetinidol-(4
β→8)-catechin (15), 12
S-hydroxy-16-heptadecynoic acid (18), (-)-pinellic acid (19), 11,14-eicosadienoic acid (20), amentoflavone (21), 3-(1,1-dimethylallyl)-8-(3,3-dimethylallyl)xanthyletin (22), lignocerate (23), and 2
R-hydroxy-9
Z,12
Z-octadecadienoic acid (24) had a high relative abundance (double) in AF02 (
Table 3,
Figure 4A). These compounds were identified by their fragmentation patterns and the matching of their retention times with analytical standards (
Figure S2). A comprehensive analysis of the fractions revealed that compound 5 (RT: 8.27 min) was present in all fractions and had higher relative abundance in AF02 (
Figure 4A). Myr is a compound that was reported to have cytotoxic activity against different cancer cell lines
[19]. Therefore, Myr could be related to the antineoplastic activity of RHTR on ovarian cancer studied in this project. To delimit the scope of this study, the experimental design focused on an in silico analysis and bibliographic search of the medicinal properties of the major metabolites in AF02-RHTR to determine its possible activity against ovarian cancer (
Table 3). However, the choice of this design most likely generated a bias in which possibly, unintentionally, some active compounds in low concentration or the synergistic effect that can be generated by the combined presence of several compounds were omitted. This opens the possibility for future studies aimed at elucidating these two possibilities, either by our own group or by members of the community interested in studying natural compounds with potential anticancer bioactivity. Compounds that presented a higher score in the drug-likeness model were: 4 (DLMS: 1.52), 6 (DLMS: 0.78), 9 (DLMS: 0.93), 12 (DLMS: 1.04), 13 (DLMS: 1.12), 15 (DLMS: 0.61), and 21 (DLMS: 0.51) (
Table 3). However, Myr presented a –0.04 value, possibly due to the low specificity of its pharmacological effect. These compounds are classified as flavonoids and were identified in other species of the
Rhus genus, which mainly show antioxidant, anti-inflammatory, and cytotoxic activities (
Table 3)
[15]. Consequently, compounds 5 (RT: 8.27 min), 6 (RT: 8.47 min), 9 (RT: 8.60 min), 12 (RT: 9.45 min), and 16 (RT: 11.92 min) were selected to further evaluate their cytotoxic activity against SKOV-3 cells.
Figure 4. Metabolite profile of RHTR fractions and biological activity in cell lines. Total ion chromatograms (ESI+) for AF01, AF02, and AF03 revealed the presence of myricetin (RT: 8.27 min) in all of them (A). Compounds with higher relative abundance in AF02 are indicated (A). The vector map indicates 215 putative identifications of 555 compounds found in AF02 (A). Comparisons with the fragmentation pattern of myricetin in AF02 and an analytical standard (A). The IC50 of metabolites from RHTR was determined by dose-response viability curves with an MTT assay for 24 h in SKOV-3 cells (B), and morphological changes were determined by immunofluorescence with phalloidin-rhodamine (actin microfilaments) and DAPI (nucleus) in the same conditions (C). Results show the mean ± SD of three biological replicates (n = 3, in triplicates); * p ≤ 0.05 vs. the vehicle group without treatment (0.5% DMSO) (ANOVA). RHTR, Rhus trilobata; AF, aqueous fraction (numbers indicate the fraction obtained after fractionation on solid-phase with solvents, as described in the Methodology).
Table 3. Major phytochemical compounds in AF02-RHTR by UPLC-MSE.
| No. |
RT (min) |
PI |
Compound Name |
Biological Activity |
Presence in Plants |
DLMS |
| 1 |
6.18 |
HMDB41635 |
myricetin 3-arabinoside |
Antioxidant, anticarcinogenic |
Rhus spp. |
0.90 |
| 2 |
6.44 |
CSID70398 |
methyl gallate * |
Antioxidant |
Rhus spp. |
−0.49 |
| 3 |
7.05 |
HMDB38831 |
epigallocatechin 3-cinnamate |
Antioxidant, antibacterial |
Ocotea porosa |
0.24 |
| 4 |
7.36 |
CSID3525015 |
3,5-digalloylepicatechin |
Antioxidant |
-- |
1.52 |
| 5 |
8.27 |
CHEBI:18152 |
myricetin * |
Antioxidant, antineoplastic |
Rhus spp. |
−0.04 |
| 6 |
8.47 |
LMPK12112436 |
myricitrin * |
Antioxidant, antineoplastic |
Rhus spp. |
0.78 |
| 7 |
8.52 |
LMPK12110568 |
quercetin 3-(2’’’-galloylglucosyl)-(1→2)-alpha-L-arabinofuranoside |
Antioxidant |
Euphorbia pachyrhiza |
1.24 |
| 8 |
8.52 |
CHEBI18082 |
1,2,3,4,6-pentakis-O-galloyl-β-D-glucose |
Antineoplastic |
Rhus spp. |
0.35 |
| 9 |
8.60 |
CHEBI:16243 |
quercetin * |
Antioxidant, anti-inflammatory, antineoplastic |
Rhus spp. |
0.93 |
| 10 |
9.13 |
CSID24784962 |
4-O-digalloyl-1,2,3,6-tetra-O-β-D-galloylglucose |
Antibacterial, antineoplastic, antithrombotic |
Rhus typhina |
0.35 |
| 11 |
9.45 |
CHEBI:5715 |
hibiscoquinone a |
Antioxidant |
Hibiscus spp. |
−0.52 |
| 12 |
9.45 |
LMPK12112171 |
quercitrin * |
Antioxidant, antineoplastic |
Rhus spp. |
1.04 |
| 13 |
9.96 |
LMPK12112447 |
myricetin 3-(4’’-galloylrhamnoside) |
Antioxidant |
-- |
1.12 |
| 14 |
10.20 |
LMPK12100067 |
obtusaquinol |
Antiparasitic, nitric oxide inhibitor |
Dalbergia spp. |
−0.03 |
| 15 |
10.94 |
CSID35013429 |
epifisetinidol-(4β→8)-catechin |
Antibacterial, α-amylase and lipase inhibitor |
Cotinus coggyria |
0.61 |
| 16 |
11.92 |
LMPK12111566 |
fisetin * |
Antineoplastic, antioxidant, antiangiogenic |
Rhus spp. |
0.76 |
| 17 |
12.54 |
LMFA01010048 |
margaric acid |
Antioxidant, antifungal |
Rhus typhina |
−0.33 |
| 18 |
12.54 |
LMFA01050146 |
12S-hydroxy-16-heptadecynoic acid |
Anti-inflammatory |
-- |
−0.38 |
| 19 |
13.37 |
CSID8034429 |
(-)-pinellic acid |
Metabolism, adjuvant activity |
Pinelliae tuber |
−1.08 |
| 20 |
13.37 |
LMFA01030130 |
11,14-eicosadienoic acid |
Antioxidant, anti-inflammatory |
Rhus typhina |
−0.08 |
| 21 |
13.9 |
LMPK12040009 |
amentoflavone |
Antioxidant, anti-inflammatory |
Rhus spp. |
0.51 |
| 22 |
15.94 |
CSID10297786 |
3-(1,1-dimethylallyl)-8-(3,3-dimethylallyl) xanthyletin |
-- |
Ruta graveolens |
−1.31 |
| 23 |
17.7 |
CHEBI:31014 |
lignocerate |
Anti-inflammatory |
Oleandra neriiformis |
−0.33 |
| 24 |
21.28 |
LMFA02000057 |
2R-hydroxy-9Z,12Z-octadecadienoic acid |
Anti-allergic |
Brassica campestris |
−0.96 |
6. Biological Activity of Active Metabolites from RHTR in Cell Lines
The biological activity of purified compounds in RHTR such as Ga
[6] and methyl gallate (2) was evaluated. Additionally, compounds selected from the in silico analysis, such as 5, 6, 9, 12, and 16, were evaluated in cancer and normal epithelial cells. The results showed that the compounds had biological activity in SKOV-3 (
Figure 4B) and OVCAR-3 (
Figure S3A) cell lines at concentrations lower than 200 μg/mL (
Table 4). However, Ga (50/43 μg/mL) and Myr (166/94 μg/mL) had the highest activity compared with the vehicle group (0.5% DMSO) (
p ≤ 0.05, Dunnett). The biological activity of both compounds was corroborated in CACO-2 cells (
Figure S3B) at 25 and 62 μg/mL, respectively (
p ≤ 0.05, Tukey;
Table 4). Similarly, both compounds presented cytotoxic activity in CHO-K1 and BEAS-2B cells (
Figure S3C,D) at ~150 μg/mL, possibly through a genotoxic effect related to reactive oxygen species (ROS) production
[20][21]. The highest activity was observed with Myr at 33 μg/mL in CHO-K1 and Ga at 25 μg/mL in BEAS-2B; the lowest activity was found with quercitrin and methyl gallate at 200 μg/mL in both cell lines (
p ≤ 0.05, Dunnett) (
Table 4). These results demonstrated that the biological activity of the compounds considerably depends on the cellular phenotype. Cell morphology analysis in SKOV-3 revealed that the compounds induced cellular changes such as individualization, rounding, microfilaments loss, cytoplasm condensation, nuclear fragmentation, and cell monolayer breakdown after 24 h of treatment, and these effects were similar to those for the paclitaxel group (
Figure 4C). Cells treated with vehicle (0.5% DMSO) presented lamellipodia, filopodia, stress fibers, adhesion dots, mitosis, confluence, and cell monolayer formation (
Figure 4C).
Table 4. Biological and cytotoxic activity of metabolites from RHTR in cell lines.
| Sample/Cell Line |
SKOV-3 |
OVCAR-3 |
CACO-2 |
BEAS-2B |
CHO-K1 |
| Gallic acid |
50 (294) |
43 (253) |
25 (147) |
25 (147) |
100 (588) |
| Methyl gallate |
>200 (1,086) |
>200 (1,086) |
100 (543) |
200 (1,086) |
130 (706) |
| Myricetin |
166 (522) |
94 (295) |
62 (195) |
64 (201) |
33 (104) |
| Myricitrin |
197 (424) |
200 (431) |
>200 (431) |
160 (344) |
94 (202) |
| Quercetin |
>200 (662) |
200 (662) |
150 (496) |
189 (625) |
127 (420) |
| Quercitrin |
>200 (446) |
>200 (446) |
179 (399) |
>200 (446) |
>200 (446) |
| Fisetin |
200 (699) |
200 (699) |
100 (349) |
50 (175) |
46 (161) |
| Paclitaxel |
7 (8.20) |
8 (9.37) |
20 (23.42) |
8 (9.37) |
10 (11.71) |
The results showed that the principal metabolites in RHTR against ovarian cancer cells could be Ga and Myr. These compounds are active in multiple cancer cell lines through ROS generation (amongst other processes) that triggers cell death by apoptosis
[19][22]. Complementary studies by our research group in mouse models for ovarian cancer demonstrated that Ga and Myr at 50 mg/kg administered by the peritumoral route inhibited tumoral lesions (50% of progression), decreased vascularity, and induced apoptosis, with few toxicological effects, possibly by a mechanism related to carbonic anhydrase-IX or PI3K
[23]. However, there is the possibility that other metabolites in RHTR (majority or present) could have specific activity against different cancer types because cancer cells can develop a sensitivity or selective resistance to certain drugs classes related to the phenotypic and functional heterogeneity, as well as molecular features present in each neoplasm disease
[24]. Therefore, these findings could be a perspective to address in future studies. Similarly, additional studies are required to demonstrate if the antineoplastic activity observed in RHTR results from a synergistic mechanism among other abundant compounds in the plant such as
β-PGG (
Table 3)
[25][26]. Recent studies have demonstrated that
β-PGG can induce cell cycle arrest in breast cancer (MCF-7, 50 μM) in the G
1 phase by inhibiting kinase activity in the D/CDK4 and CDK2 complex, decreasing the phosphorylation in retinoblastoma protein (pRB), and increasing the levels of p27Kip, p21Cip, and p53
[27][28]. Thus,
β-PGG could be involved in the biological activity found in RHTR; however, more in-depth studies are required to confirm this claim. Finally, the results show that RHTR, Ga, and Myr could play a role in the alternative treatment of ovarian cancer.