
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gianmarco Marocchi | + 2140 word(s) | 2140 | 2021-10-20 04:30:05 | | | |
| 2 | Catherine Yang | -22 word(s) | 2118 | 2021-11-02 02:22:00 | | |
Video Upload Options
Over the last decades, contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) has emerged as an important diagnostic tool for the diagnosis and differentiation of several gastrointestinal diseases. The key advantage of CH-EUS is that the influx and washout of contrast in the target lesion can be observed in real time, accurately depicting microvasculature. CH-EUS is established as an evidence-based technique complementary to B-mode EUS to differentiate solid appearing structures, to characterize mass lesions, and to improve the staging of gastrointestinal and pancreatobiliary cancer. In the last few years, interest has increased in the use of CH-EUS in interventional procedures such as tissue acquisition, tumor ablation, biliary drainage, and the management of pancreatic fluid collections.
1. Introduction
Over the last decades, the development of contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) has emerged as an important diagnostic tool for the diagnosis and differentiation of several gastrointestinal diseases [1][2][3][4].
The key advantage of CH-EUS is that the influx and washout of contrast in the target lesion can be observed in real time, accurately depicting microvasculature. Thus, CH-EUS is established as an evidence-based technique complementary to B-mode EUS to differentiate solid appearing structures, to characterize mass lesions, to improve the staging of gastrointestinal and pancreatobiliary cancer, and to real-time guide diagnostic EUS [5]. Indeed, its main field of application, with high-quality level of evidence, is the differential diagnosis between benign and malignant lesions, especially for pancreatic ones [4].
However, despite its well-known diagnostic role, over the years, interest has increased in the use of CH-EUS in interventional procedures. Several studies have explored its potential benefit in EUS-guided tissue acquisition, in the management of pancreatic fluid collection (PFC), biliary and pancreatic duct drainage, drainage of gallbladder, celiac plexus neurolysis/blockage, drainage of mediastinal and intra-abdominal abscesses and collections, and in targeted cancer chemotherapy and radiotherapy. Moreover, guidelines on CH-EUS have been recently published from the Asian Federation of Societies for Ultrasound in Medicine and Biology [6], providing evidence-based information on technical aspects and indications. In parallel to the widespread of interventional EUS-guided procedures, indeed, CH-EUS could represent an important tool to delineate real-time vascular perfusion and to enhance imaging, in order to have a proper guidance and reduce potential complications.
2. CH-EUS and Tissue Acquisition
Some years far from its introduction, CH-EUS is now considered an extremely useful tool in the characterization of gastrointestinal lesions. As far as CH-EUS-guided tissue acquisition is concerned, the majority of the published studies have evaluated its role in solid pancreatic lesions. Some authors have initially investigated if lesion enhancement during CH-EUS could detect pancreatic adenocarcinoma better than EUS-guided fine needle aspiration (FNA) [7], while others have wondered if CH-EUS could improve the diagnostic yield of EUS-FNA. Kitano et al. [8] showed that the sensitivity in the diagnosis of pancreatic adenocarcinoma increased from 92.2% to 100% combining EUS-FNA histological diagnosis and CH-EUS characteristics.
The ability of CH-EUS to depict the macro- and micro-vascularization of solid lesions in a real-time manner allows the detection of avascular areas, which represent fibrotic or necrotic areas that harbor poor diagnostic potential and that should be avoided during tissue acquisition. Indeed, it has been demonstrated that EUS-FNA has lower sensitivity for pancreatic adenocarcinoma with avascular areas on CH-EUS [9]. In this perspective, it was postulated that CH-EUS could be used to guide EUS-FNA ( Figure 1 ).
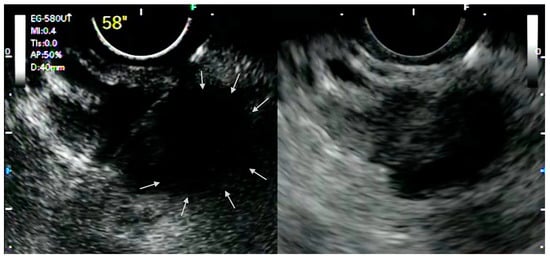
Although there is evidence for solid pancreatic masses, the role of CH-EUS for the guidance of FNA in other conditions still needs to be investigated. It is known that the presence of underlying chronic pancreatitis or the presence of a previously placed biliary stent could reduce the diagnostic yield of EUS-guided tissue acquisition. In these settings, CH-EUS was demonstrated to improve the detection of pancreatic lesions, helping to delineate the margins of a suspected lesion [10]. However, there is still little evidence, and larger dedicated studies are needed.
In conclusion, CH-EUS has been evaluated as an image-enhancing method that ameliorates the performance of EUS-guided tissue acquisition, above all for pancreatic diseases, leading to promising results downsized by the most recent studies. Rather than being constantly applied, the use of CH-EUS for the guidance of EUS-FNA may be reserved to selected cases, especially when the probability of conventional EUS-FNA failure seems to be higher.
3. CH-EUS and Tumor Ablation
EUS-guided tumor ablation is an emerging treatment modality initially introduced for malignant pancreatic lesions unsuitable for surgery [11]. The ability of CH-EUS to delineate tumor perfusion dynamics in a real-time manner and to detect enhancing lesions poorly visible on B-mode EUS is supposed to be useful in performing EUS-guided tumor ablation.
To date, only one study and a few case reports reported the use CH-EUS in the field of tumor ablation. Choi et al. described the use of CH-EUS for the guidance and monitoring of EUS-guided radiofrequency ablation (RFA) of solid abdominal tumors [12]. Nineteen patients (13 pancreatic neuroendocrine tumors, 2 solid pseudopapillary neoplasm, 1 pancreatic insulinoma, 2 adrenal adenomas, and 1 adrenal metastasis from hepatocellular carcinoma) underwent EUS-guided RFA preceded by CH-EUS evaluation. Early treatment response was assessed at 5 and 7 days. CH-EUS showed an absence of enhancement in 7 cases and the presence of residual enhancing foci in 12 cases, indicating the complete response or presence of viable tumor, respectively. In those cases of residual tumors, additional RFA sessions were performed. At 1-year follow-up, a complete response was achieved in 68.4% of cases with a median of two RFA sessions. CH-EUS showed the advantage of performing the assessment of early therapeutic response and the identification of residual viable lesions to target in additional RFA sessions. Two case reports [13][14] described the use of EUS-guided tumor ablation for the treatment of a perianastomotic colorectal cancer metastasis using RFA and the treatment of a hepatocellular carcinoma with ethanol injection. In both cases, CH-EUS was used after ablation to confirm the success of the procedure and to exclude the presence of remnant neoplastic tissue.
Concluding , first experiences with the addition of CH-EUS to EUS-guided tumor ablation have showed interesting results, as contrast enhancement of intratumoral vessels gives fundamental information both on the results of the ablation and on the potential residual neoplastic tissue to target in retreatment. Further studies are needed in this setting to confirm this promising results.
4. CH-EUS and Hepatobiliary Interventions
Indeed, it has been shown that CH-EUS following standard EUS can improve the diagnostic accuracy of gallbladder diseases, especially for the differential diagnosis of benign and malignant lesions, with an increased sensitivity from 82% to 100% when combining both modalities [15].
While the application of CH-EUS as a diagnostic tool in gallbladder thickening is well established, there are still limited data about its role in interventional gallbladder EUS procedures. Transmural EUS-guided gallbladder drainage (EUS-GBD) is a safe and effective interventional endoscopic option for the treatment of patients with acute cholecystitis and considered at high risk for cholecystectomy. Its clinical application is currently endorsed by the Tokyo Guidelines, which introduced EUS-GBD in the treatment pathway, especially for fragile patients affected by grade 2 and 3 of acute cholecystitis [16][17].
Firstly described in 2007 [18], in the last decade, EUS-GBD has gained popularity progressively, in particular after the introduction of specific lumen apposing metal stents (LAMS), reaching optimal technical and clinical success rates that rank respectively in the range of 93–97% and 94–98% [19] and representing nowadays an interesting alternative to endoscopic transpapillary gallbladder drainage (ET-GBD), which is preferred to percutaneous trans-hepatic gallbladder drainage (PT-GBD) when surgery is not considered an option [20].
As for EUS-GBD, few data are available on the usefulness of CH-EUS on this setting, although the use of contrast enhancement agents may be helpful in optimizing EUS examination in order to safely perform EUS-guided drainage, reducing the risk of intraprocedural adverse events.
5. CH-EUS and Pancreatic Fluid Collections
PFCs are one of the main local complications of acute pancreatitis, and the literature is counting on a continuous understanding of their morphology and technical improvements to handle them. Thanks to the widespread techniques and the introduction of dedicated devices on the market, EUS has become an essential part of both the diagnostic and therapeutic work-out of PFC [21][22][23].
In the last decades, we have perceived a change of paradigm in management of PFC, in parallel to the wide spread of both diagnostic and interventional EUS, allowing an effective and less invasive approach than other conventional treatment.
Over the years, EUS has gained a pivotal role both for the assessment and the treatment of PFC [24]. Thanks to its high detailed spatial resolution, indeed, EUS provides substantial information on the content of PFC, distinguishing among liquid collections, namely pseudocyst (PCs) and walled-off pancreatic necrosis (WOPN), and on the presence of a mature capsule; the correct assessment of the morphology and content of the PFC is to date essential for the choice of the best treatment option [21][22].
EUS has led a better characterization of PFC, especially a better definition of the content of the collections, providing additional, and perhaps more accurate (compared with other radiological techniques such as CT-scan) assessment of solid component within the collections [25]. A study by Rana et al., comparing magnetic resonance (MRI), EUS, and US showed that MRI underestimated the amount of necrosis in patients with <40% solid debris, comparing to EUS and US. US showed an accuracy comparable to EUS and MRI for the evaluation of necrotic collections, although limitations were related to the suboptimal detection of collections and the inability to characterize collections with high solid content or air. Moreover, in the same study, EUS was more accurate for the diagnosis of venous collaterals around the collection, which was important in order to reduce the risk of bleeding due to inadvertent puncture during drainage [26].
Moreover, it has been reported that thanks to its accuracy in characterizing PFC, EUS performed prior to intervention can modify the management in up to one-third of patients because of a change of diagnosis or identification of anatomical and vascular factors precluding endoscopic management [27][28].
Therefore, in the setting of PFC, CH-EUS could provide a deeper definition of the collection, the content, and vascular complication (e.g., pseudoaneurysms, splenic vein thrombosis, etc.) that could potentially increase the risks of adverse events of the endoscopic treatment.
In 2017, Minaga et al. reported a case of EUS-guided drainage of an infected WOPN under CH-EUS guidance. There is an inability to depict the target lesion and its margins in B-mode due to heterogeneous echogenicity; thus, a CH-EUS was performed to enhance the contrast between the targeted WOPN and the surrounding tissues, enabling the assessment of the microvasculature and hemodynamics of the collection in real time and the ability to safely puncture it and perform the EUS-guided drainage [29].
Moreover, in 2011, Badea et al. reported a case of a 33-year-old male with recurrent episodes of acute pancreatitis on alcohol-induced chronic pancreatitis complicated by PC and bleeding from the major papilla. After both abdominal ultrasound and EUS, the use of a contrast agent allowed revealing the presence of a pseudoaneurysm of the splenic artery within the PC. Due to the continuous hemorrhage, the patient underwent surgery, and there was a diagnosis of wirsungorrhagia due to the rupture of the pseudoaneurysm of the splenic artery in the cyst [30].
In conclusion, as outlined previously, CH-EUS can be successfully used for the evaluation and diagnostic and therapeutic work-up of PFC, and contrast enhancement can also be used to help this evaluation, adding more key points toward a better treatment. Finally, the addition of contrast agents to EUS may aid the interpretation of vascularity and its complications, even though the literature on this topic is limited to a few cases.
6. Conclusions and Future Perspectives
Contrast-enhanced EUS could be a valid tool for endosonographers that approach interventional EUS-guided procedures. In fact, in parallel to the increasing invasiveness of EUS, it would be useful to have a detailed roadmap that may both increase the accuracy and efficacy of EUS-guided procedures and may help to predict all those conditions that may be associated to an increased risk of complications. Furthermore, inter-observer agreement in CH-EUS has already been reported as satisfactory among endosonographers, even between experienced and non-experienced ones [31], so it is reasonable to expect that this is true also for CH-EUS when applied to interventional procedures, but further studies should address this issue.
In conclusion, the latest evidence suggests that CH-EUS could be a helpful tool to guide selected cases of EUS-guided tissue acquisition, to evaluate the efficacy of EUS-guided tumor ablation and the need for retreatment, and it provides adjunctive information for predicting technical success and potential adverse events in EUS-guided biliary drainage and endoscopic management of PFC.
As little evidence is still available on the role of CH-EUS in interventional EUS, there are many questions that do still have an ambiguous answer and which may be the objects of future studies.
References
- Dietrich, C.F.; Ignee, A.; Frey, H. Contrast-Enhanced Endoscopic Ultrasound with Low Mechanical Index: A New Technique. Z. Gastroenterol. 2005, 43, 1219–1223.
- Săftoiu, A.; Dietrich, C.F.; Vilmann, P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy 2012, 44, 612–617.
- Alvarez-Sanchez, M.V.; Napoleon, B. Contrast-enhanced harmonic endoscopic ultrasound imaging: Basic principles, present situation and future perspectives. World J. Gastroenterol. 2014, 20, 15549–15563.
- Fusaroli, P.; Napoleon, B.; Gincul, R.; Lefort, C.; Palazzo, L.; Palazzo, M.; Kitano, M.; Minaga, K.; Caletti, G.; Lisotti, A. The clinical impact of ultrasound contrast agents in EUS: A systematic review according to the levels of evidence. Gastrointest. Endosc. 2016, 84, 587–596.e10.
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.J.C.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: Update 2017 (long version). Ultraschall. Med. 2018, 39, e2–e44.
- Kitano, M.; Yamashita, Y.; Kamata, K.; Ang, T.L.; Imazu, H.; Ohno, E.; Hirooka, Y.; Fusaroli, P.; Seo, D.-W.; Napoléon, B.; et al. The Asian Federation of Societies for Ultrasound in Medicine and Biology (AFSUMB) Guidelines for Contrast-Enhanced Endoscopic Ultrasound. Ultrasound Med. Biol. 2021, 47, 1433–1447.
- Gincul, R.; Palazzo, M.; Pujol, B.; Tubach, F.; Palazzo, L.; Lefort, C.; Fumex, F.; Lombard, A.; Ribeiro, D.; Fabre, M.; et al. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: A prospective multicenter trial. Endoscopy 2014, 46, 373–379.
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of Small Solid Tumors in the Pancreas: The Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310.
- Kamata, K.; Takenaka, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Nishida, N.; Chikugo, T.; et al. Impact of avascular areas, as measured by contrast-enhanced harmonic EUS, on the accuracy of FNA for pancreatic adenocarcinoma. Gastrointest. Endosc. 2018, 87, 158–163.
- Fusaroli, P.; Spada, A.; Mancino, M.G.; Caletti, G. Contrast Harmonic Echo–Endoscopic Ultrasound Improves Accuracy in Diagnosis of Solid Pancreatic Masses. Clin. Gastroenterol. Hepatol. 2010, 8, 629–634.e2.
- Park, D.H.; Choi, J.-H.; Oh, D.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. Endoscopic Ultrasonography-Guided Ethanol Ablation for Small Pancreatic Neuroendocrine Tumors: Results of a Pilot Study. Clin. Endosc. 2015, 48, 158–164.
- Choi, J.-H.; Seo, D.-W.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasound for the Guidance and Monitoring of Endoscopic Radiofrequency Ablation. Gut Liver 2020, 14, 826–832.
- Mangiavillano, B.; Auriemma, F.; Scaltrini, F.; Bianchetti, M.; Di Leo, M.; Carrara, S.; Repici, A. Endoscopic Ultrasonography–Guided Radiofrequency Ablation for a Perianastomotic Neoplastic Colorectal Recurrence. Am. J. Gastroenterol. 2019, 114, 1709.
- Lisotti, A.; Piscaglia, F.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided ethanol injection for a small hepatocellular carcinoma. Endoscopy 2019, 51, E317–E318.
- Kamata, K.; Takenaka, M.; Kitano, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Nishida, N.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig. Endosc. 2018, 30, 98–106.
- Okamoto, K.; Suzuki, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Endo, I.; Iwashita, Y.; Hibi, T.; Pitt, H.A.; Umezawa, A.; et al. Tokyo Guidelines 2018: Flowchart for the management of acute cholecystitis. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 55–72.
- Mori, Y.; Itoi, T.; Baron, T.H.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Ukai, T.; Shikata, S.; Noguchi, Y.; Teoh, A.Y.B.; et al. Tokyo Guidelines 2018: Management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 87–95.
- Kwan, V.; Eisendrath, P.; Antaki, F.; Le Moine, O.; Devière, J. EUS-guided cholecystenterostomy: A new technique (with videos). Gastrointest. Endosc. 2007, 66, 582–586.
- Mohan, B.P.; Khan, S.R.; Trakroo, S.; Ponnada, S.; Jayaraj, M.; Asokkumar, R.; Adler, D.G. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: A systematic review and comparative meta-analysis. Endoscopy 2020, 52, 96–106.
- Teoh, A.Y.B.; Kitano, M.; Itoi, T.; Pérez-Miranda, M.; Ogura, T.; Chan, S.M.; Serna-Higuera, C.; Omoto, S.; Torres-Yuste, R.; Tsuichiya, T.; et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomized multicentre controlled superiority trial (DRAC 1). Gut 2020, 69, 1085–1091.
- Arvanitakis, M.; Dumonceau, J.-M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Ferreira, A.O.; Gyökeres, T.; et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018, 50, 524–546.
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75.e1.
- Fabbri, C.; Luigiano, C.; Lisotti, A.; Cennamo, V.; Virgilio, C.; Caletti, G.; Fusaroli, P. Endoscopic ultrasound-guided treatments: Are we getting evidence based-a systematic review. World J. Gastroenterol. 2014, 20, 8424–8448.
- Binda, C.; Coluccio, C.; Sbrancia, M.; Fabbri, C. Role of endoscopic ultrasonography in the management of peripancreatic collections. Diagnostic and therapeutic approach. Minerva Gastroenterol. 2021.
- Giovannini, M. Endoscopic Ultrasound–Guided Drainage of Pancreatic Fluid Collections. Gastrointest. Endosc. Clin. N. Am. 2018, 28, 157–169.
- Rana, S.S.; Chaudhary, V.; Sharma, R.; Sharma, V.; Chhabra, P.; Bhasin, D.K. Comparison of abdominal ultrasound, endoscopic ultrasound and magnetic resonance imaging in detection of necrotic debris in walled-off pancreatic necrosis. Gastroenterol. Rep. 2015, 4, 50–53.
- Zaheer, A.; Singh, V.K.; Qureshi, R.O.; Fishman, E.K. The revised Atlanta classification for acute pancreatitis: Updates in imaging terminology and guidelines. Abdom. Imaging 2013, 38, 125–136.
- Fockens, P.; Johnson, T.G.; Van Dullemen, H.M.; Huibregtse, K.; Tygat, G.N. Endosonographic imaging of pancreatic pseudocysts before endoscopic transmural drainage. Gastrointest. Endosc. 1997, 46, 412–416.
- Minaga, K.; Takenaka, M.; Omoto, S.; Miyata, T.; Kamata, K.; Yamao, K.; Imai, H.; Watanabe, T.; Kitano, M.; Kudo, M. A case of successful transluminal drainage of walled-off necrosis under contrast-enhanced harmonic endoscopic ultrasonography guidance. J. Med Ultrason. 2018, 45, 161–165.
- Badea, R.; Seicean, A.; Procopet, B.; Dina, L.; Osian, G. Pseudoaneurysm of Splenic Artery Ruptured in Pancreatic Pseudocyst and Complicated by Wirsungorrhagia: The Role of the Ultrasound Techniques and Contrast Substances. Ultraschall Med. Eur. J. Ultrasound 2010, 32, 205–207.
- Fusaroli, P.; Kypraios, D.; Mancino, M.G.; Spada, A.; Benini, M.C.; Bocus, P.; De Angelis, C.; Fabbri, C.; Grillo, A.; Marzioni, M.; et al. Interobserver agreement in contrast harmonic endoscopic ultrasound. J. Gastroenterol. Hepatol. 2012, 27, 1063–1069.




