
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María Soledad Soledad | + 2646 word(s) | 2646 | 2021-07-22 05:40:08 | | | |
| 2 | Peter Tang | + 13 word(s) | 2659 | 2021-11-02 04:57:05 | | |
Video Upload Options
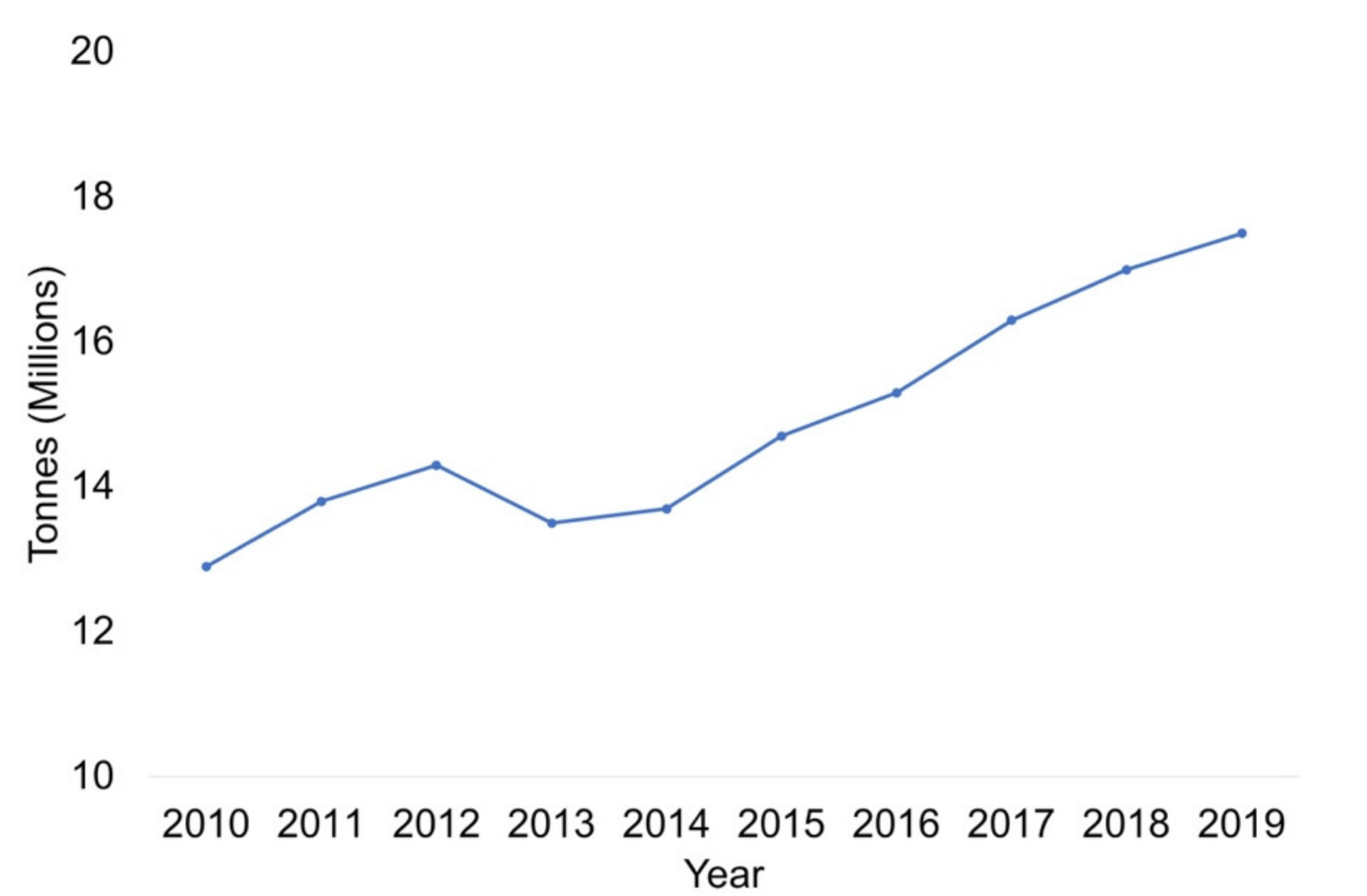
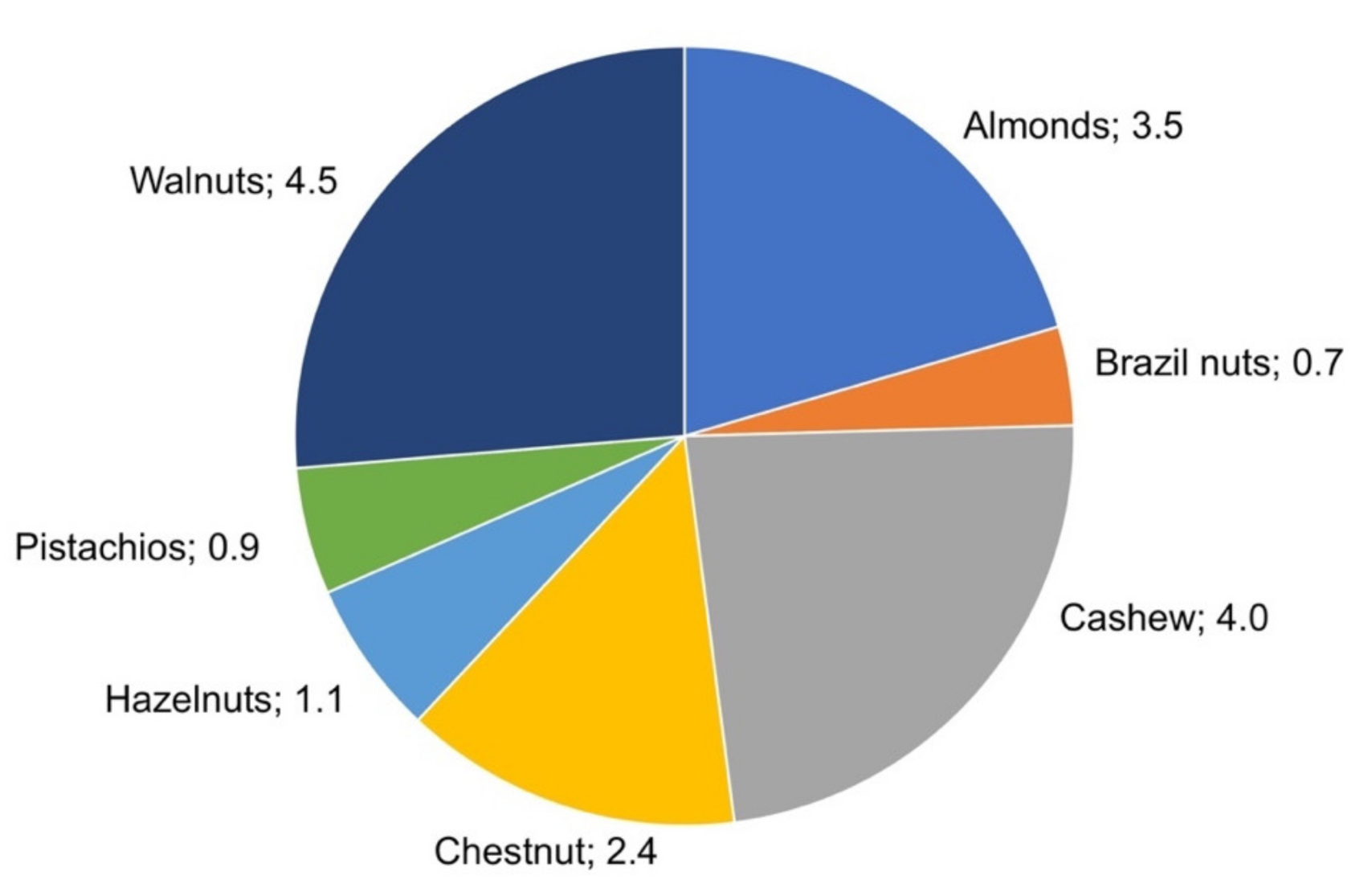
Nuts are indehiscent dry fruits with one seed and a thick, hard pericarp. The presence of nuts in diets has notably increased due to their composition, and the presence of antioxidants and their unsaturated fatty acid profile has led to a considerable increase in their consumption. The volatile profile of nuts is important from different points of view. It affects consumer’s selection, influences raw material selection for the production of composite foods, dictates variety selection in breeding programs, and, from a quality perspective, its changes can indicate food degradation or alteration.
1. Introduction
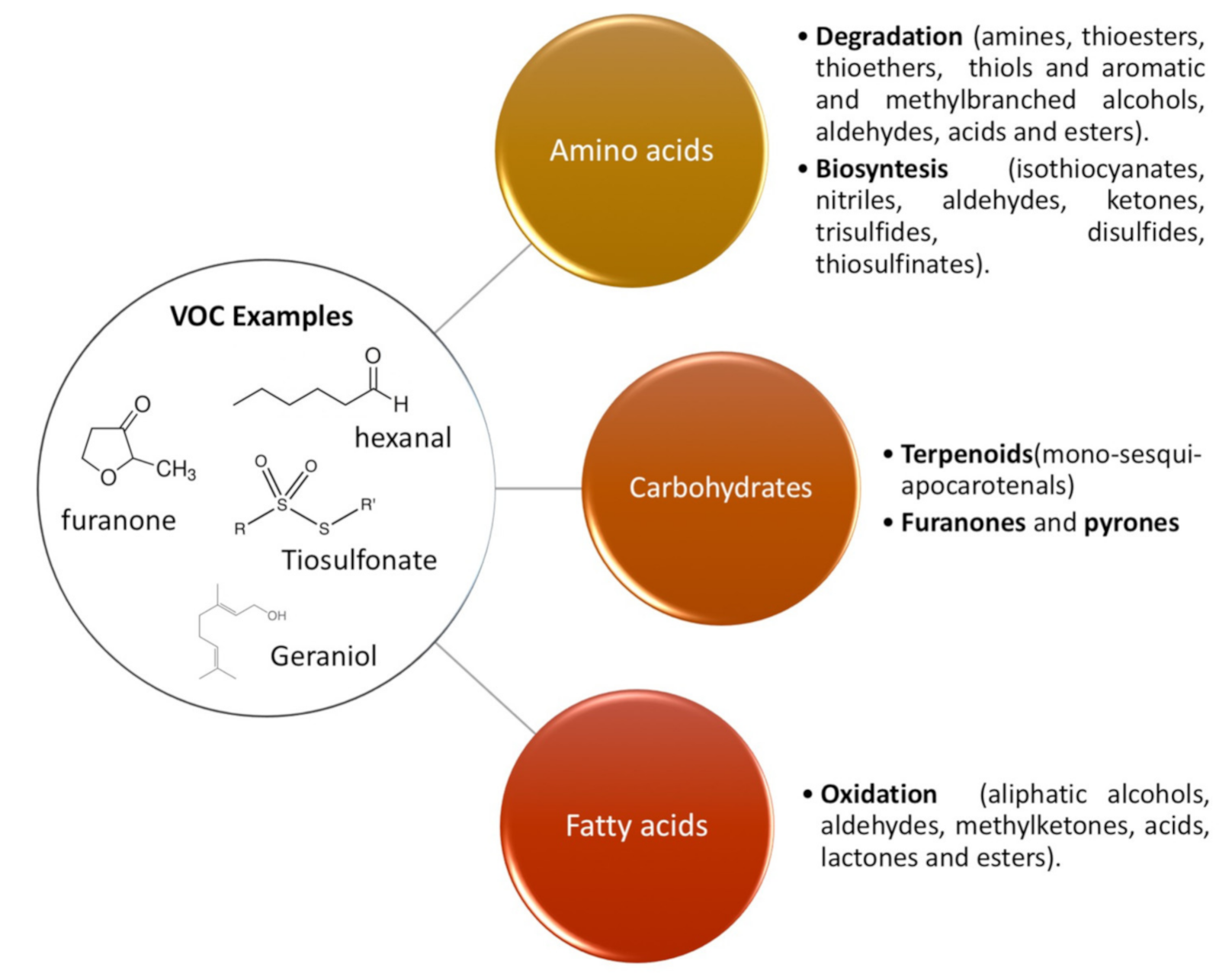
2. Main Volatile Organic Compounds (VOCs) Present in Nuts
3. Analysis of VOCs Present in Nuts
|
Equilibration Conditions |
Extraction Conditions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Type of Nut |
Sample Amount (g) |
Fiber |
Agitation |
Time (min) |
Temperature (°C) |
Time (min) |
Temperature (°C) |
Quantification |
Column |
Ref. |
|
Almond |
5.0 * |
1-cm 50/30 µm DVB/CAR/PDMS |
No |
40 |
24 |
30 |
24 |
(1) |
DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[15] |
|
Almond |
5.0 * |
1-cm 50/30 µm DVB/CAR/PDMS |
Yes |
45 |
40 |
45 |
40 |
(3) |
DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[16] |
|
Almond |
0.250 * |
1-cm 50/30 µm DVB/CAR/PDMS |
No |
15 |
25 |
30 |
25 |
(2) |
DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[17] |
|
Almond |
5.0 * |
1-cm 50/30 µm DVB/CAR/PDMS |
Yes |
45 |
40 |
45 |
40 |
(3) |
DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[10] |
|
Almond |
3.0 * |
1-cm 50/30 µm DVB/CAR/PDMS |
No |
10 |
40 |
30 |
40 |
(1) |
TRB-5MS (30 m × 0.25 mm × 0.25 μm) |
[18] |
|
Beechnut, hazelnut, pistachio and walnut |
10.0 ** |
1-cm 50/30 µm DVB/CAR/PDMS |
Yes |
60 |
25 |
60 |
25 |
No |
RTx-5 (60 m × 0.25 mm × 0.25 μm) |
[19] |
|
Hazelnut |
0.1 * |
1-cm 75 µm CAR/PDMS |
No |
10 |
60 |
10 |
60 |
(3) |
DB-Wax (30 m × 0.25 mm × 0.5 μm) |
[20] |
|
Hazelnut |
1.5 * |
2-cm 50/30 µm DVB/CAR/PDMS |
No |
20 |
50 |
20 |
50 |
(3) º |
MEGA-WAX™ (30 m × 0.20 mm × 0.20 μm) |
[21] |
|
Peanut |
5.0 * |
PDMS/DVB |
No |
30 |
60 |
15 |
60 |
(3) |
DB-5 |
[22] |
|
Peanut |
5.0 * |
50/30 µm DVB/CAR/PDMS |
Yes |
1440 |
25 |
20 |
21 |
(1) |
SUPELCOWAX™ 10 (30 m, 0.25 mm, 0.25 mm) |
[23] |
|
Peanut |
3.0 ** |
1-cm 65 µm PDMS/DVB |
Yes |
10 |
50 |
40 |
50 |
(1) |
DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[24] |
|
Peanut |
5.0 ** |
2-cm 50/30 µm DVB/CAR/PDMS |
No |
30 |
80 |
10 |
80 |
(1) |
RTX-5MS (30 m × 0.25 mm × 0.25 μm) |
[25] |
|
Peanut |
5.0 ** |
1-cm 50/30 µm DVB/CAR/PDMS |
Yes |
30 |
50 |
30 |
50 |
No |
DB-17MS (60 m × 0.25 mm × 0.25 μm) |
[26] |
|
Peanut |
0.2 * |
2-cm DVB/CAR/PDMS |
Yes |
8 h |
20 |
50 |
60 |
(1) |
dB5-MS semi-polar (60 m × 0.32 mm × 1 μm) |
[27] |
|
Peanut |
5.0 ** |
1-cm 65 µm DVB/CAR/PDMS |
No |
20 |
80 |
60 |
80 |
No |
HP-5 (30 m × 0.25 mm × 0.25 μm) |
[28] |
|
Pistachio |
15.0 * |
50/30 µm DVB/CAR/PDMS |
Yes |
15 |
50 |
120 |
50 |
No |
HP-5 (30 m × 0.32 mm × 0.25 μm) |
[13] |
|
Pistachio |
8.0 * |
50/30 µm DVB/CAR/PDMS |
No |
- |
- |
60 |
83 |
No |
HP-5MS (30 m × 0.25 mm × 0.25 μm) |
[29] |
|
Pistachio |
10.0 * |
50/30 µm DVB/CAR/PDMS |
15 |
50 |
120 |
50 |
(1) |
Equity-5 (30 m × 0.25 mm × 0.25 μm) |
[30] |
|
|
Pistachio |
24.5 * |
PDMS-DVB |
No |
30 |
30 |
20 |
30 |
No |
Agilent DB-1 (60 m × 0.320 mm × 0.25 μm) |
[31] |
|
Pistachio |
1.5 ** |
2-cm 50/30 µm DVB/CAR/PDMS |
Yes |
- |
- |
30 |
40 |
(1) |
DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[2] |
|
Walnut |
0.5 * |
50/30 µm DVB/CAR/PDMS |
Yes |
15 |
50 |
30 |
60 |
(1) |
RTX-5MS (30 m × 0.25 mm × 0.25 μm) |
[32] |
|
Walnut |
3.0 ** (mL) |
1-cm 50/30 µm DVB/CAR/PDMS |
No |
10 |
50 |
30 |
50 |
No |
HP-INNOWAX (30 m × 0.25 mm × 0.25 μm) |
[33] |
|
Walnut |
1.0 ** |
65 µm PDMS/DVB |
No |
- |
- |
30 |
50 |
No |
CP-Wax52CB (30 m × 0.25 mm × 0.25 μm) |
[14] |
|
Pecan |
2.0 * |
50/30 µm DVB/CAR/PDMS |
Yes |
30 |
25 |
30 |
65 |
(1) |
HP-5 (30 m × 0.25 mm × 0.25 μm) |
[34] |
Sample pre-treatment: * Only grinding ** Grinding and oil extraction. Quantification: 1: Semi-quantification with IS, 2: (1) corrected by sample amount, 3: quantification. º: IS was employed. DVB: Divinylbenzene, CAR: Carboxen, PDMS: Polydimethylsiloxane.
4. Effect of Harvesting Conditions and Healthy State of Nuts on Volatile Profile
|
Nut |
Main VOCs |
Ref. |
|---|---|---|
|
Almond |
1-Hexanol, 3-methyl-1-butanol, nonanal, 2-methyl-1-propanol, 1-propanol |
[17] |
|
Benzaldehyde, hexanal, 1,2-propanediol, 1-chloro-2-propanol, 3-methyl-1-butanol, pentanal, 2-heptanone, 1-hexanol. |
[15] |
|
|
Hexanal, 3-methyl-1-butanol, benzaldehyde, heptanal, nonanal, 1-octanol, 2-octanone |
[18] |
|
|
Chestnut |
ϒ-terpinene, phenylaldehyde, hexanal, furfural, α-terpinene |
[35] |
|
Hazelnut |
α-tujone, β-tujone, 2-pentanone, acetic acid, 3-methyl-2- butanol, n-decane |
[21] |
|
Peanut |
Hexanoic acid, 2-ethyl-1-hexanol, 1-hexanol, pentanal, hexanal, palmitic acid, 2-ethyl-5-methylpyrazine, heptanal |
[24] |
|
2-Propanone, α-pinene, benzene, α-terpinolene, hexanal, d-limonene |
[36] |
|
|
Toluene, α-limonene, γ-terpinene, p-cymene, nonanal, β, pinene, hexanoic acid |
[25] |
|
|
2,5-Dimethylpyrazine, nonanal, hexanal, 2-ethyl-5-methylpyrazine, octanal, 2,5-dimethyl-3-ethylpyrazine |
[28] |
|
|
Hexanal, benzaldehyde, benzenacetaldehyde, 2,5-dimethylpyrazine, 2-heptenal, 2-ethyl-5-methylpyrazine, trimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine |
[27] |
|
|
Pistachio |
9-Octadecenoic acid, α-pinene, 1-methyl-1H-pyrrole, α-terpinolene, limonene, dimethyl-2H-pyran-2-one, 2-octenal, 2-hexenal |
[29] |
|
α-Pinene, α-terpinolene, 1H-pyrrole, ethyl-alcohol, limonene, hexane |
[30] |
|
|
α-Pinene, β-pinene, 2-ethyl-1-hexanol, α-terpineol, camphene, hexanoic acid |
[2] |
|
|
Walnut |
Hexanal, hexanoic acid, 1-pentanol, 2-octenal, pentanal, 2-pentylfuran, propanoic acid |
[33] |
|
2-Octenal, hexanoic acid, hexanal, 2-decenal, 1-octen-3-ol, nonanal |
[14] |
5. Volatile Profile of Nuts after Thermal Treatments
|
Thermal Processing |
Nut |
Processing Conditions |
Main VOCs |
Ref. |
|---|---|---|---|---|
|
Hot-air roasting |
Hazelnut |
34, 18, 13 min at 130, 140 and 150 °C |
2,3-pentanedione, 2-acetyl-1-pyrroline, dimethyl sulphide, 2-furfurylthiol, 3-methylbutanal, 2-nonenal, 2-decenal, hydroxy-2,5-dimethyl-3(2H)-furanone |
[50] |
|
20, 25 and 30 min at 160 °C |
2-methylpropanal, 2-methylbutanal, 3-methylbutanal, 2,5-methylpyrazine |
[51] |
||
|
40 min at 140 °C |
2-methylbutanal, 3-methylbutanal, 2,5-methylpyrazine, furfuryl alcohol, 2-methylpropanal, ehtyl acetate, 2,3-pentanedione, 2-methylpyrazine, 2,5-methylpyrazine, furfural, 1-hydroxy-2-propanone |
[21] |
||
|
Almond |
5 min at 177 °C |
Benzyl alcohol, benzaldehyde, 1-octen-3-ol, toluene, dimethylpyrazine, 1-butanol, hexanal |
[52] |
|
|
5–10 min at 170–190 °C |
Hexanal, 2-methyl-butanal, 2-methyl-pyrazine, 2,5-dimethyl-pyrazine, furfural |
[53] |
||
|
10 min at 190 °C |
2,5-dimethyl-pyrazine, trimethylpyrazine |
[39] |
||
|
33 min at 138 °C |
Hexanal, benzeneacetaldehyde, 2,5-dimethyl-pyrazine, nonanal |
[15] |
||
|
28 and 38 min at 138 °C |
2-methylbutanal, 3-methylbutanal, hexanal, benzaldehyde, furfural, 2-phenyl acetaldehyde |
[15] |
||
|
28, 33 and 38 min at 138 °C |
2-methylbutanal, 3-methylbutanal, hexanal, benzaldehyde, furfural, 2-phenyl acetaldehyde |
[54] |
||
|
Chestnut |
25 min at 200 °C |
Hexanal, butylacetate, ethylbenzene, 2-hydroxy-2-cyclopenten-1-one |
[54] |
|
|
Pistachio with and without salt |
90 min at 120 °C |
α-pinene, limonene, 3-carene |
[55] |
|
|
Cashew |
3 and 9 min at 143 °C |
Methylbutanal, hexanal, acetaldehyde, heptane, ethanol, pentane, acetone |
[56] |
|
|
Microwave roasting |
Almond |
120 V for 2 min |
Benzyl alcohol, methional, benzaldehyde, dimethylpyrazine, nonanal, undecane, 1-octen-3-ol, 1,4-butyrolactone |
[52] |
|
Pistachio |
480 or 640 W for 2, 3 and 4 min |
α-pinene, limonene, nonanal |
[57] |
|
|
Hot air-assisted radio frequency |
Almond |
15 min at 120–130 °C |
2,5-dimethyl-pyrazine, toluene, hexanal and heptane |
[58] |
|
Deep-frying |
Almond |
5 min at 135 °C |
Benzyl alcohol, methional, benzaldehyde, 1-butanol, 1-octen-3-ol |
[52] |
|
10–15 min at 160–200 °C |
Hexanal, 2-methyl-butanal, 3-methyl-butanal, 2,5-dimethyl-pyrazine, 1-pentanol |
[53] |
||
|
Chestnut |
15 min at 240 °C |
Hexanal, octanal, nonanal, furfural, 3-heptanone, 4-hydroxy-2-butanone |
[59] |
References
- Rowan, D.D. Volatile Metabolites. Metabolites 2011, 1, 41–63.
- Ojeda-Amador, R.M.; Fregapane, G.; Salvador, M.D. Influence of Cultivar and Technological Conditions on the Volatile Profile of Virgin Pistachio Oils. Food Chem. 2020, 311.
- Sud Ali, N.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Sendra, E. Flavors and Aromas. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Carrillo-López, A., Eds.; Elsevier: London, UK, 2019; pp. 385–404.
- Mansurova, M.; Ebert, B.E.; Blank, L.M.; Ibáñez, A.J. A Breath of Information: The Volatilome. Curr. Genet. 2018, 64, 959–964.
- Phenol-Explorer Database Phenol-Explorer Database. Available online: http://phenol-explorer.eu/ (accessed on 15 January 2021).
- USDA Database. Available online: Https://Fdc.Nal.Usda.Gov/ (accessed on 20 February 2021).
- FAOSTAT Food and Agriculture Organization of the United States. FAOSTAT Database. Available online: http://www.fao.org/faostat/en/#data (accessed on 20 February 2021).
- Beltrán, A.; Ramos, M.; Grané, N.; Martín, M.L.; Garrigós, M.C. Monitoring the Oxidation of Almond Oils by HS-SPME–GC–MS and ATR-FTIR: Application of Volatile Compounds Determination to Cultivar Authenticity. Food Chem. 2011, 126, 603–609.
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant. J. 2008, 54, 712–732.
- Franklin, L.M.; King, E.S.; Chapman, D.; Byrnes, N.; Huang, G.; Mitchell, A.E. Flavor and Acceptance of Roasted California Almonds during Accelerated Storage. J. Agric. Food Chem. 2018, 66, 1222–1232.
- Beltrán, A.; Maestre, S.E.; Grané, N.; Valdés, A.; Prats, M.S. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods 2021, 10, 153.
- Lubes, G.; Goodarzi, M. Analysis of Volatile Compounds by Advanced Analytical Techniques and Multivariate Chemometrics. Chem. Rev. 2017, 117, 6399–6422.
- Aceña, L.; Vera, L.; Guasch, J.; Busto, O.; Mestres, M. Comparative Study of Two Extraction Techniques to Obtain Representative Aroma Extracts for Being Analysed by Gas Chromatography-Olfactometry: Application to Roasted Pistachio Aroma. J. Chromatogr. A 2010, 1217, 7781–7787.
- Mu, H.; Gao, H.; Chen, H.; Fang, X.; Zhou, Y.; Wu, W.; Han, Q. Study on the Volatile Oxidation Compounds and Quantitative Prediction of Oxidation Parameters in Walnut (Carya cathayensis Sarg.) Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1–9.
- Lee, J.; Xiao, L.; Zhang, G.; Ebeler, S.E.; Mitchell, A.E. Influence of Storage on Volatile Profiles in Roasted Almonds (Prunus dulcis). J. Agric. Food Chem. 2014, 62, 11236–11245.
- Franklin, L.M.; Chapman, D.M.; King, E.S.; Mau, M.; Huang, G.; Mitchell, A.E. Chemical and Sensory Characterization of Oxidative Changes In Roasted Almonds Undergoing Accelerated Shelf Life. J. Agric. Food Chem. 2017, 65, 2549–2563.
- Rogel-Castillo, C.; Luo, K.; Huang, G.; Mitchell, A.E. Effect of Drying Moisture Exposed Almonds on the Development of the Quality Defect Concealed Damage. J. Agric. Food Chem. 2017, 65, 8948–8956.
- Oliveira, I.; Malheiro, R.; Meyer, A.S.; Pereira, J.A.; Gonçalves, B. Application of Chemometric Tools for the Comparison of Volatile Profile from Raw and Roasted Regional and Foreign Almond Cultivars (Prunus dulcis). J. Food Sci. Technol. 2019, 56, 3764–3776.
- Stuebiger, G.; Buchbauer, G.; Krist, S.; Bail, S.; Unterweger, H. Characterization of Volatile Compounds and Triacylglycerol Profiles of Nut Oils Using SPME-GC-MS and MALDI-TOF-MS. Eur. J. Lipid Sci. Technol. 2009, 111, 170–182.
- Pastorelli, S.; Valzacchi, S.; Rodriguez, A.; Simoneau, C. Solid-Phase Microextraction Method for the Determination of Hexanal in Hazelnuts as an Indicator of the Interaction of Active Packaging Materials with Food Aroma Compounds. Food Addit. Contam. 2006, 23, 1236–1241.
- Nicolotti, L.; Cordero, C.; Bicchi, C.; Rubiolo, P.; Sgorbini, B.; Liberto, E. Volatile Profiling of High Quality Hazelnuts (Corylus avellana, L.): Chemical Indices of Roasting. Food Chem. 2013, 138, 1723–1733.
- Baker, G.L.; Cornell, J.A.; Gorbet, D.W.; O’Keefe, S.F.; Sims, C.A.; Talcott, S.T. Determination of Pyrazine and Flavor Variations in Peanut Genotypes during Roasting. J. Food Sci. 2003, 68, 394–400.
- Abegaz, E.G.; Kerr, W.L.; Koehler, P.E. The Role of Moisture in Flavor Changes of Model Peanut Confections during Storage. LWT Food Sci. Technol. 2004, 37, 215–225.
- Liu, X.J.; Jin, Q.Z.; Liu, Y.F.; Huang, J.H.; Wang, X.G.; Mao, W.Y.; Wang, S.S. Changes in Volatile Compounds of Peanut Oil during the Roasting Process for Production of Aromatic Roasted Peanut Oil. J. Food Sci. 2011, 76, 404–412.
- Costa De Camargo, A.; Aparecida Bismara Regitano-d’Arce, M.; Matias De Alencar, S.; Guidolin Canniatti-Brazaca, S.; Ferreira de Souza Vieira, T.M.; Shahidi, F. Chemical Changes and Oxidative Stability of Peanuts as Affected by the Dry-Blanching. J. Am. Oil Chem. Soc. 2016, 93, 1101–1109.
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring Oxidative Stability and Changes in Key Volatile Compounds in Edible Oils during Ambient Storage through HS-SPME/GC–MS. Int. J. Food Prop. 2018, 20, S2926–S2938.
- Lykomitros, D.; Fogliano, V.; Capuano, E. Drivers of Preference and Perception of Freshness in Roasted Peanuts (Arachis spp.) for European Consumers. J. Food Sci. 2018, 83, 1103–1115.
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of Hot and Cold-Pressed Processes on Volatile Compounds of Peanut Oil and Corresponding Analysis of Characteristic Flavor Components. LWT Food Sci. Technol. 2019, 112, 107648.
- Kendirci, P.; Onoǧur, T.A. Investigation of Volatile Compounds and Characterization of Flavor Profiles of Fresh Pistachio Nuts (Pistacia vera, L.). Int. J. Food Prop. 2011, 14, 319–330.
- Georgiadou, M.; Gardeli, C.; Komaitis, M.; Tsitsigiannis, D.I.; Paplomatas, E.J.; Sotirakoglou, K.; Yanniotis, S. Volatile Profiles of Healthy and Aflatoxin Contaminated Pistachios. Food Res. Int. 2015, 74, 89–96.
- Beck, J.J.; Willett, D.S.; Mahoney, N.E.; Gee, W.S. Silo-Stored Pistachios at Varying Humidity Levels Produce Distinct Volatile Biomarkers. J. Agric. Food Chem. 2017, 65, 551–556.
- Lee, J.; Vázquez-Araújo, L.; Adhikari, K.; Warmund, M.; Elmore, J. Volatile Compounds in Light, Medium, and Dark Black Walnut and Their Influence on the Sensory Aromatic Profile. J. Food Sci. 2011, 76, C199–C204.
- Zhou, Y.; Fan, W.; Chu, F.; Wang, C.; Pei, D. Identification of Volatile Oxidation Compounds as Potential Markers of Walnut Oil Quality. J. Food Sci. 2018, 83, 2745–2752.
- Gong, Y.; Kerrihard, A.L.; Pegg, R.B. Characterization of the Volatile Compounds in Raw and Roasted Georgia Pecans by HS-SPME-GC-MS. J. Food Sci. 2018, 83, 2753–2760.
- Krist, S.; Unterweger, H.; Bandion, F.; Buchbauer, G. Volatile Compound Analysis of SPME Headspace and Extract Samples from Roasted Italian Chestnuts (Castanea sativa Mill.) Using GC-MS. Eur. Food Res. Technol. 2004, 219, 470–473.
- Mexis, S.F.; Kontominas, M.G. Effect of Gamma Irradiation on the Physico-Chemical and Sensory Properties of Raw Shelled Peanuts (Arachis hypogaea, L.) and Pistachio Nuts (Pistacia vera, L.). J. Sci. Food Agric. 2009, 89, 867–875.
- Lipan, L.; Martín-Palomo, M.J.; Sánchez-Rodríguez, L.; Cano-Lamadrid, M.; Sendra, E.; Hernández, F.; Burló, F.; Vázquez-Araújo, L.; Andreu, L.; Carbonell-Barrachina, Á.A. Almond Fruit Quality Can Be Improved by Means of Deficit Irrigation Strategies. Agric. Water Manag. 2019, 217.
- Lipan, L.; García-Tejero, I.F.; Gutiérrez-Gordillo, S.; Demirbaş, N.; Sendra, E.; Hernández, F.; Durán-Zuazo, V.H.; Carbonell-Barrachina, A.A. Enhancing Nut Quality Parameters and Sensory Profiles in Three Almond Cultivars by Different Irrigation Regimes. J. Agric. Food Chem. 2020, 68.
- Lipan, L.; Cano-Lamadrid, M.; Vázquez-Araújo, L.; Łyczko, J.; Moriana, A.; Hernández, F.; García-García, E.; Carbonell-Barrachina, Á.A. Optimization of Roasting Conditions in HydroSOStainable Almonds Using Volatile and Descriptive Sensory Profiles and Consumer Acceptance. J. Food Sci. 2020, 85, 3969–3980.
- Şahan, A.; Bozkurt, H. Effects of Harvesting Time and Irrigation on Aroma Active Compounds and Quality Parameters of Pistachio. Sci. Hortic. 2020, 261, 108905.
- Carbonell-Barrachina, Á.A.; Memmi, H.; Noguera-Artiaga, L.; del Carmen Gijón-López, M.; Ciapa, R.; Pérez-López, D. Quality Attributes of Pistachio Nuts as Affected by Rootstock and Deficit Irrigation. J. Sci. Food Agric. 2015, 95.
- Polari, J.J.; Zhang, L.; Ferguson, L.; Maness, N.O.; Wang, S.C. Impact of Microclimate on Fatty Acids and Volatile Terpenes in “Kerman” and “Golden Hills” Pistachio (Pistacia vera) Kernels. J. Food Sci. 2019, 84, 1937–1942.
- Vas, G.; Vékey, K. Solid-Phase Microextraction: A Powerful Sample Preparation Tool Prior to Mass Spectrometric Analysis. J. Mass Spectrom. 2004, 39, 233–254.
- Beck, J.J.; Higbee, B.S.; Merrill, G.B.; Roitman, J.N. Comparison of Volatile Emissions from Undamaged and Mechanically Damaged Almonds. J. Sci. Food Agric. 2008, 88.
- Beck, J.J.; Merrill, G.B.; Higbee, B.S.; Light, D.M.; Gee, W.S. In Situ Seasonal Study of the Volatile Production of Almonds (Prunus dulcis) Var. ‘Nonpareil’ and Relationship to Navel Orangeworm. J. Agric. Food Chem. 2009, 57.
- Scott-Thomas, A.; Chambers, S.T. Volatile Organic Compounds: Upcoming Role in Diagnosis of Invasive Mould Infections. Curr. Fungal Infect. Rep. 2017, 11.
- Beck, J.J.; Willett, D.S.; Gee, W.S.; Mahoney, N.E.; Higbee, B.S. Differentiation of Volatile Profiles from Stockpiled Almonds at Varying Relative Humidity Levels Using Benchtop and Portable GC-MS. J. Agric. Food Chem. 2016, 64.
- Beck, J.J.; Mahoney, N.E.; Cook, D.; Gee, W.S. Volatile Analysis of Ground Almonds Contaminated with Naturally Occurring Fungi. J. Agric. Food Chem. 2011, 59.
- Valdés, A.; Beltrán, A.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Effect of Frying and Roasting Processes on the Oxidative Stability of Sunflower Seeds (Helianthus annuus) under Normal and Accelerated Storage Conditions. Foods 2021, 10, 944.
- Yang, J.; Pan, Z.; Takeoka, G.; MacKey, B.; Bingol, G.; Brandl, M.T.; Garcin, K.; McHugh, T.H.; Wang, H. Shelf-Life of Infrared Dry-Roasted Almonds. Food Chem. 2013, 138, 671–678.
- Marzocchi, S.; Pasini, F.; Verardo, V.; Ciemniewska-Żytkiewicz, H.; Caboni, M.F.; Romani, S. Effects of Different Roasting Conditions on Physical-Chemical Properties of Polish Hazelnuts (Corylus avellana, L. Var. Kataloński). LWT Food Sci. Technol. 2017, 77, 440–448.
- Agila, A.; Barringer, S. Effect of Roasting Conditions on Color and Volatile Profile Including HMF Level in Sweet Almonds (Prunus dulcis). J. Food Sci. 2012, 77, 1–8.
- Valdés, A.; Beltrán, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Monitoring the Oxidative Stability and Volatiles in Blanched, Roasted and Fried Almonds under Normal and Accelerated Storage Conditions by DSC, Thermogravimetric Analysis and ATR-FTIR. Eur. J. Lipid Sci. Technol. 2015, 117, 1199–1213.
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS Characterization of Volatiles in Raw and Dry-Roasted Almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39.
- Penci, M.C.; Martinez, M.L.; Fabani, M.P.; Feresin, G.E.; Tapia, A.; Ighani, M.; Ribotta, P.D.; Wunderlin, D.A. Matching Changes in Sensory Evaluation with Physical and Chemical Parameters: A Case Study: Argentinean Pistachio Nuts (Pistachia vera, L. Cv Kerman). Food Bioprocess. Technol. 2013, 6, 3305–3316.
- Agila, A.; Barringer, S.A. Volatile Profile of Cashews (Anacardium occidentale, L.) from Different Geographical Origins during Roasting. J. Food Sci. 2011, 76.
- Hojjati, M.; Noguera-Artiaga, L.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Effects of Microwave Roasting on Physicochemical Properties of Pistachios (Pistaciavera, L.). Food Sci. Biotechnol. 2015, 24, 1995–2001.
- Xu, Y.; Liao, M.; Wang, D.; Jiao, S. Physicochemical Quality and Volatile Flavor Compounds of Hot Air-Assisted Radio Frequency Roasted Almonds. J. Food Process. Preserv. 2020, 44.
- Li, Q.; Shi, X.; Zhao, Q.; Cui, Y.; Ouyang, J.; Xu, F. Effect of Cooking Methods on Nutritional Quality and Volatile Compounds of Chinese Chestnut (Castanea mollissima Blume). Food Chem. 2016, 201, 80–86.




