
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jakub Żółkiewicz | + 1471 word(s) | 1471 | 2020-08-07 05:47:52 | | | |
| 2 | Nora Tang | -18 word(s) | 1453 | 2020-11-05 11:19:29 | | |
Video Upload Options
Postbiotics include any substance released by or produced through the metabolic activity of the microorganism, which exerts a beneficial effect on the host, directly or indirectly. As postbiotics do not contain live microorganisms, the risks associated with their intake are minimized. Postbiotics play a vital role in the maturation of the immune system, affect barrier tightness and the intestinal ecosystem, and indirectly shape the structure of the microbiota. Postbiotics display pleiotropic effects, including their immunomodulatory, anti-inflammatory, antioxidant, and anti-cancer properties. As such, postbiotics may be useful in treating or preventing many disease entities, including those for which effective causal therapy has not yet been found.
1. Introduction
The assemblage of microorganisms that inhabit the human body, their genomes and metabolites, as well as the environment in which they live, is called the microbiota. Microorganisms that are part of the microbiome can be isolated from all areas in constant contact with the external environment (e.g., the skin, upper respiratory tract, or urogenital tract). However, they are most abundant in the gastrointestinal tract. Our interdependent relationship with the intestinal microbiota is established during the first three years of life [1]. The human body provides a stable, nutrient-rich environment for the inhabiting microorganisms, and in return, receives a number of benefits. These benefits include stimulation of the immune system, improved digestion and absorption of food, reduced growth of pathogenic flora, and maintenance of intestinal barrier integrity. These beneficial effects of the interaction between the microbiota and the gastrointestinal tract can be observed not only locally, but also in distant organs, due to systemic distribution of substances and cells produced in the intestine. This phenomenon is called the gut-organ axis, according to which we can distinguish the gut-brain, gut-skin, gut-lung axis, and so on.
Several factors can affect the composition of the microbiota starting from the perinatal period, including the composition of the maternal gut microbiota, the mode of delivery and type of food the mother consumes, antibiotic therapy, and stress [2]. Moreover, many studies have shown that an imbalance in the intestinal microbiota—dysbiosis—can lead to the development of allergic or autoimmune diseases (e.g., inflammatory bowel disease, type 1 diabetes, among others), cancer, and psychiatric disorders [3]. As such, therapeutic strategies and preparations that affect the composition of the microbiota, and thus, the patient’s well-being, have become increasingly popular.
As summarized in Figure 1, there are currently three main ways in which the microbiota can be modulated, i.e., through the use of prebiotics, probiotics, synbiotics, or postbiotics. Prebiotics are used by microorganisms as food, and, at the same time, can exert a beneficial effect on the health of the host. Currently available prebiotics include human milk oligosaccharides (HMO), lactulose, and inulin derivatives. In contrast, probiotics directly impact the gut microbiome through the selective delivery of beneficial microorganisms to the gastrointestinal tract. According to the 2002 World Health Organization (WHO) definition, probiotics are live microorganisms administered in the appropriate amounts, which have a positive effect on host health. In clinical practice, the most commonly used probiotics are bacteria of the genera Lactobacillus, Bifidobacterium, and Streptococcus, as well as yeast Saccharomyces. Despite several meta-analyses confirming the clinical effectiveness of probiotics in various diseases (including acute gastrointestinal infection and inflammatory bowel diseases [4][5]), individual reports are increasingly undermining their effectiveness and safety, especially in high-risk patients [6]. Therefore, there is increasing interest in a surrogate group of preparations: Postbiotics.

The concept of postbiotics is based on the observation that the beneficial effects of the microbiota are mediated by the secretion of various metabolites. However, its precise definition remains under discussion. According to Tsilingiri et al., postbiotics include any substance released by or produced through the metabolic activity of the microorganism, which exerts a beneficial effect on the host, directly or indirectly [7]. For the purposes of this article, we assume that postbiotics include all substances of bacterial or fungal origin that confer beneficial effect to the host and do not meet the definition of a probiotic and are not exclusively of a prebiotic nature (Figure 2).
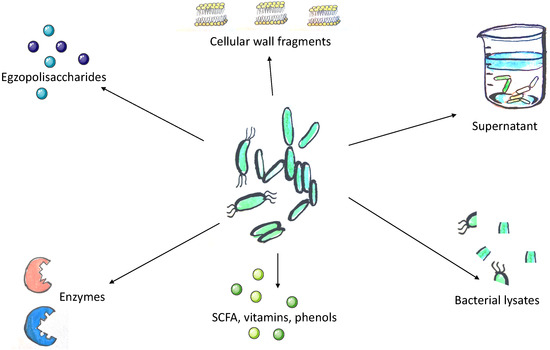
According to the current literature, postbiotics are not considered as synbiotics. Synbiotics are a combination of prebiotics and probiotics that are claimed to have a beneficial impact on gut microbiome. However, it is believed that postbiotics may also strengthen the intestinal microbiome [8], so we believe that term “synbiotics” should be reviewed and postbiotics should be incorporated in its definition.
Although postbiotics do not contain live microorganisms, they show a beneficial health effect through similar mechanisms that are characteristic of probiotics while minimizing the risks associated with their intake. Therefore, like prebiotics, postbiotics appear to lack serious side effects while maintaining similar effectiveness to probiotics (although currently there are no studies directly comparing substances belonging to both groups).
Here, we provided a critical review of the postbiotic drugs described in the literature, including their mechanisms of action, clinical characteristics, and potential therapeutic applications.
2. Future Clinical Applications
Postbiotics play a vital role in the maturation of the immune system, affect barrier tightness and the intestinal ecosystem, and indirectly shape the structure of the microbiota. As such, postbiotics may be useful in treating or preventing many disease entities, including those for which effective causal therapy has not yet been found (e.g., Alzheimer’s disease, inflammatory bowel disease, or multiple sclerosis). Indeed, clinical trials aimed at modifying the microbiota of patients suffering from the abovementioned diseases are currently underway, and the first results are promising [9][10].
Postbiotics may be particularly useful in infants, as the first months of life are critical for developing the proper structure of the microbiota. As the microbiota “matures” up to about three years of age [1], any abnormalities can be associated with short- and long-term consequences (e.g., necrotizing enterocolitis and asthma, respectively) [11]. Creating the appropriate environment for the formation of the correct microbiota appears crucial for the proper development and preservation of the child’s future well-being, and postbiotics can provide such conditions.
Postbiotics may also be useful in the prevention and treatment of SARS-CoV-2 infection, as the structure and metabolic activity of the intestinal microbiome may be related to the occurrence of biomarkers predicting the severe coronavirus disease 2019 (COVID-19) course [12].
The potential value of postbiotics is not limited to therapeutic applications. Indeed, the emergence of biological doping (and its detection) is an area of interest. A recent study in mice showed that the presence of bacteria of the genus Veillonella in the gut, which can metabolize lactic acid to propionate, significantly increased the animals’ physical performance [13]. A similar result was obtained by the enteral administration of propionic acid, indicating the possibility of using postbiotics to modify physical fitness and the independence of the observed effect from the presence of bacteria [13].
3. Summary
The use of metabolites or fragments derived from microorganisms (i.e., “postbiotics”) is an attractive therapeutic and preventive strategy in modern medicine. According to current data, such postbiotics have pleiotropic effects, including immunomodulatory, anti-inflammatory, antioxidant, and anti-cancer properties. Some of these properties are even in clinical use. The boundary between probiotics and postbiotics is blurred in some trials, as their impact on the results is often not evaluated separately. We expect further research into the biological activities of these metabolites will unveil novel uses for postbiotics in medicine and beyond.
References
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050.
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome during Pregnancy. MCN Am. J. Matern. Child Nurs. 2017, 42, 310–317.
- Bastiaanssen, T.F.S.; Cowan, C.S.M.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making Sense of the Microbiome in Psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52.
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400.
- Szajewska, H.; Kolodziej, M.; Gieruszczak-Bialek, D.; Skorka, A.; Ruszczynski, M.; Shamir, R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children-a 2019 update. Aliment. Pharmacol. Ther. 2019, 49, 1376–1384.
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547.
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107.
- Klemashevich, C.; Wu, C.; Howsmon, D.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr. Opin. Biotechnol. 2014, 26, 85–90.
- Melbye, P.; Olsson, A.; Hansen, T.H.; Søndergaard, H.B.; Bang Oturai, A. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 2019, 139, 208–219.
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126.
- DeWeerdt, S. How baby’s first microbes could be crucial to future health. Nature 2018, 555, S18–S19.
- Gou, W.; Fu, Y.; Yue, L.; Chen, G.-d.; Cai, X.; Shuai, M.; Xu, F.; Yi, X.; Chen, H.; Zhu, Y.J.; et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv 2020.
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109.




