
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nhut Minh Nguyen | + 4643 word(s) | 4643 | 2021-08-02 09:48:34 | | | |
| 2 | Nhut Minh Nguyen | + 1 word(s) | 4644 | 2021-10-30 13:21:55 | | | | |
| 3 | Vivi Li | -84 word(s) | 4560 | 2021-11-01 02:41:38 | | | | |
| 4 | Vivi Li | -1311 word(s) | 3249 | 2021-11-03 09:13:19 | | |
Video Upload Options
Chemical Looping Gasification is a process allowing for the conversion of solid feedstock (e.g. biomass) into N2-free, high-calorific syngas or producer gas. The process utilizes the ability of so-called oxygen carriers (e.g. ilmenite, iron ore) to take up and release oxygen in oxidizing and reducing atmospheres, respectively. Employing this characteristic, the oxygen carrier is cycled between two or more reactors to transport oxygen into the so-called fuel reactor, where the inlet feedstock is firstly gasified using steam or CO2, before intermediate gaseous products (e.g. H2, CH4) are further oxidized by the oxygen carrier, providing additional process heat to drive the endothermic gasification reactions. The loop is then closed as the reduced oxygen carrier is re-oxidized in a so-called air reactor, using the oxygen contained in ambient air, resulting in a stream of pure nitrogen at the air reactor outlet.
1. Process Fundamentals
The combustion of fossil fuels (coal, petroleum, and natural gas) contributes the largest share of greenhouse gas (GHG) emissions and currently, the mitigation of these emissions is one of the most challenging global issues. The Paris Agreement aims to limit the temperature increase to 1.5 °C above pre-industrial levels [1]. To reach this target, the increased use of renewable energy will play a key role in all energy systems, globally.
Biomass has been considered one of the most important primary and renewable energy resources for a renewable and sustainable energy future due to it being carbon-neutral renewable and its abundant quantity. Moreover, it offers the possibility to yield negative emissions when employed in combination with carbon capture and storage processes [2][3].
Biomass conversion pathways can typically be classified as biochemical and thermochemical processes. The thermochemical conversion processes mainly include combustion, gasification, and pyrolysis. Biomass thermochemical conversion processes are characterized by low efficiency mainly due to biomass properties such as high moisture content and relatively low energy density. Biomass gasification, a thermochemical conversion approach, is to convert efficiently the solid fuels into a combustible gas mixture, mainly CO and H2, which can be used as a feedstock in the production of chemicals or power generation. However, the drawback of conventional gasification technology is a demand for a large amount of heat supply for the production of high-quality syngas, making the process less attractive. Therefore, new technology is required to be more economically feasible to produce enriched hydrogen syngas from biomass.
Ishida et al. [4] firstly proposed the term “chemical looping” for the process, where a metal oxide is used as an oxygen transport medium to perform a redox reaction scheme for an increase of exergy efficiency in power generation. In the chemical looping concept, oxygen carriers (e.g., MexOy−1/MexOy) [2][3][5] are applied for oxygen transport, avoiding direct contact between fuels and air. Chemical looping processes can be used for power generation, production of syngas, chemicals, and liquid fuels through chemical looping combustion (CLC) [6][7][8], chemical looping reforming (CLR) [9][10], and chemical looping gasification (CLG) [6][11]. In the CLC process, metal/metal oxide as an oxygen carrier circulates between two reactors to completely combust fuels (gaseous and solid fuels), while the CLR is a process for the partial oxidation of hydrocarbon fuels to produce hydrogen. The CLG shares similar principles with the CLC and CLR, but the CLG can produce useful combustible gas from gaseous and solid carbonaceous materials through the partial oxidation process.
The typical mechanism operation of CLG of the biomass process is illustrated in Figure 1. The configuration mainly consists of an air reactor and a fuel reactor, where oxidation and reduction reactions take place, respectively.
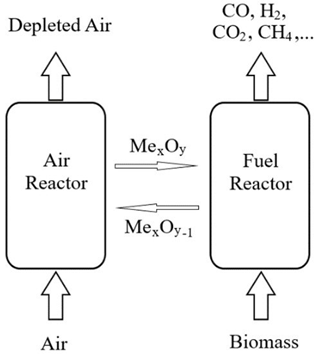
Figure 1. Schematic principles of Biomass chemical looping gasification process.
In the fuel reactor, a metal oxide as an oxygen carrier is reduced to provide oxygen for fuel conversion.
Then, the reduced metal oxide is circulated to the air reactor to be re-oxidized before a new cycle. The general chemical reactions in the fuel reactor and air reactor are shown as follows.
Biomass + MexOy MexOy−1 + H2 + CO + CO2 + CH4 (1)
MexOy−1 + ½ O2 → MexOy (2)
where MexOy is the oxidized and MexOy−1 represents the reduced form of oxygen carrier [12].
Biomass-derived chemical looping gasification is a novel technology to convert biomass into renewable hydrogen-enriched syngas. Since most reactions in the fuel reactor are endothermic, they require a large energy supply for stable operation. On the other hand, oxidation reactions in the air reactor are exothermic. Thus, the oxygen carrier, which can circulate between two reactors in the CLG process, can transfer not only oxygen but also heat from the air reactor to the fuel reactor through oxidation-reduction reactions [6][13][14]. Additionally, looping materials (metal oxides) can reduce the amount of tar that causes serious problems in a biomass gasification system, considerably. Metal oxides act as an effective catalyst for tar cracking and reduction in tar formation during chemical looping gasification. Mendiara et al. [15] found that the tar content was reduced around 2.4% per degree Celsius with the presence of iron ore. The NiFe2O4 showed a dual-function of oxidation-catalyst for toluene reduction and significantly promotes toluene converted into carbon and H2 [16]. Although biomass-based chemical looping gasification currently remains several challenges, the potential achievements may outweigh the challenges. Many studies have been carried out to investigate its nature and to solve operational challenges for the commercialization of this technology. The efforts focus on the development of biomass gasification processes for large-scale applications, improvement in reactivity and stability, as well as the multifunctional nature of looping materials, holistic evaluation for the economic feasibility of biomass-based chemical looping systems, and various types of biomass feedstock for chemical looping processes [6]. Therefore, biomass chemical looping technology has notable potentials to convert biomass-based materials into valuable products and effectively mitigate CO2 emissions in the atmosphere.
2. Process Configurations
The biomass chemical looping gasification concept aims to produce high-quality syngas for further applications. The contact between the fuel and the oxygen carrier plays a key role in the chemical looping system, especially BCLG. Hence, the selection of the reactor configuration is an important criterion for chemical looping processes. The essential requirements for selecting an appropriate BCLG with a continuous operation are as follows [8][13][17]:
- There should be sufficient particle circulation between the fuel reactor and the air reactor.
- There should be sufficient contact between the fuel/air and the solid oxygen carriers to achieve maximum conversion.
- High temperature and high-pressure operations must be carried out to achieve higher overall efficiency.
- There should be limited gas leakage between the fuel reactor and the air reactor.
As a part of a chemical looping system, a reactor is a crucial factor that affects process performance. Two common types of reactors have been proposed for chemical looping applications, namely fixed-bed and fluidized-bed reactors. Fixed-bed reactors are the simplest type of reactor in chemical looping processes ranging from laboratory-scale to pilot plant-scale and commercial-scale. In this type of reactor, the solid materials are stationary and are alternately exposed to reducing and oxidizing conditions through periodic switching of feed streams [18]. The major advantages of the fixed-bed reactor are that separation of gas and solid particles are not required, which allows for better utilization of oxygen carrier. To achieve a high process energy efficiency and continuous operation, two or more fixed bed reactors in parallel can be installed in the system. However, this reactor configuration has not been used widely for BCLG since it shows heat and mass transfer limitations and demands high temperature and a complex flow switching system. In fluidized bed systems, solids behave like a fluid by passing gas or liquid upwards through the bed of particles. The fluidized-bed reactor is extensively used in chemical looping processes. Its advantages over the fixed-bed reactors are uniform temperature distribution, more effective mixing, and higher heat and mass transfer. The behavior of a fluidized bed strongly depends on flow gas velocity and solid properties. Among fluidization regimes, bubbling, turbulent, and fast fluidization are mainly applied in chemical looping processes. However, one of the serious problems in the stable operation of the fluidized-bed reactor is particle segregation leading to poor fluidization. Based on the key requirements and types of reactors in the system mentioned above, it could be proposed to be accomplished in three configurations like a two-reactor system, three-reactor system, and alternating packed or fluidized bed reactor (Figure 2). Many researchers have carried out their studies in different types of reactor systems and different types of oxygen carriers for CLG fitting biomass fuels to evaluate the feasibility of the BCLG process.
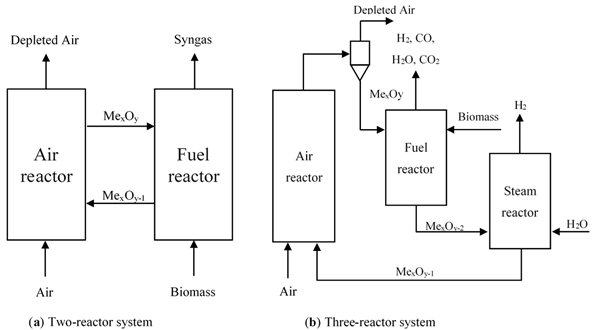
Figure 2. BCLG configurations for hydrogen enrich gas production: (a) Two-reactor system and (b) Three-reactor system.
2.1 Two-Reactor System
The two-reactor system is the most popular configuration for BCLG as shown in Figure 2a [19][20][21][22]. The configuration consists of two fluidized bed reactors as the air reactor and the fuel reactor, respectively. In the air reactor, the oxygen carrier materials are oxidized by oxygen from the air. The oxidized form of the oxygen carrier is transferred to the fuel reactor to react with biomass to produce a gaseous mixture and the reduced form of metal oxides, then they are returned to the air reactor for regeneration. Additionally, two loop-seal devices are installed between the air reactor and fuel reactor to prevent gas mixing between two reactors. This configuration is based on the reactivity of the oxygen carrier considering that the residence time of the oxygen carrier required for the reduction reaction is higher than for the oxidation. The air reactor, a fast fluidized bed reactor, has two objectives: to give the driving force for the solid material circulation and provide sufficient oxygen and heat for fuel conversion in the fuel reactor [7]. Interconnected fluid fluidized bed reactors, a type of two-reactor system, comprise mainly two fluidized bed reactors. This configuration normally consists of a high-velocity riser and a low velocity bubbling fluidized-bed as the air reactor and fuel reactor, respectively, being the most popular configuration among all the various types [20][23][24][25][26]. Biomass gasification takes place in the fuel reactor while the oxygen carrier is oxidized inside the air reactor. The loop-seals are installed to prevent gas leakage between the air reactor and the fuel reactor. Additionally, cyclones are used to remove solid particles from the gas stream.
2.2 Three-Reactor System
The three-reactor system can generate pure hydrogen and syngas separately and simultaneously. It shares similar principles with the chemical looping reforming of methane configuration [10] as shown in Figure 2b. A simplistic mechanism of the three-reactor system for BCLG combined with water splitting is illustrated in Figure 3. Biomass is partially oxidized by the lattice oxygen of the metal oxides in the fuel reactor to produce syngas as shown in Reaction (1), but the reduced oxygen carrier is oxidized by steam to regenerate lattice oxygen and produce H2 in the steam reactor Reaction (3) instead of in the air reactor. Afterward, the oxygen carrier is fully oxidized in the air reactor Reaction (2) before continuing the next cycle.
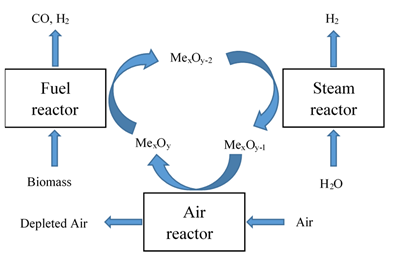
Figure 3. Process scheme of the three-reactor system for BCLG.
The reaction in the steam reactor can be illustrated as follows:
Steam reactor: MexOy−2 + H2O → H2 + MexOy−1 (3)
where MexOy is an oxygen carrier, MexOy−1 and MexOy−2 are the corresponding reduced form of oxygen carriers with different reduction degrees, i.e., the strongly reduced oxygen carriers (MexOy−2) leaving the fuel reactor are partially oxidized in the steam reactor (MexOy−1) before full regeneration in the air reactor.
Oxygen carrier material used in the process demands a sufficiently high reactivity with biomass, good performance for water splitting to generate hydrogen, and high stability during redox cycles. Additionally, the material should have good resistance to carbon deposition because it may cause contamination of the hydrogen produced. Some metal oxides have been considered as possible oxygen carriers for this configuration such as Fe3O4, WO3, SnO2, Ni-ferrites, (Zn, Mn)-ferrites, Cu-ferrites, and Ce based oxides [10]. A study was developed in a fixed bed reactor by He et al. [27] to combine BLCG and water/CO2 splitting using NiFe2O4 as an oxygen carrier. In this study, experimental investigations were carried out separately in three steps. Firstly, biomass was reduced by NiFe2O4 to generate syngas in the presence of steam/CO2. Afterward, the reduced form of oxygen carrier was oxidized partially by steam/CO2 to produce H2/CO, then it was fully oxidized by air in the oxidation step. During the investigations, syngas and H2/CO were obtained separately in different steps. The authors also proposed phase transitions corresponding to different reduction degrees of oxygen carrier at different steps as follows:
NiFe2O4 → metallic Fe(Ni)/FeOx → Ni1.43Fe1.7O4/Ni → NiFe2O4
The three-reactor configuration has been considered a promising approach since it can produce syngas and pure hydrogen simultaneously. However, there have been very few studies on this configuration. Some research related to the three-reactor system has been focused on gaseous fuels, coal, and FeO/Fe3O4/Fe2O3 materials as looing materials [28][29][30][31][32][33][34].
2.3 Packed and Fluidized-Bed
The packed bed configuration reactor can be applied for the chemical looping gasification of biomass. A simple configuration of this type is shown in Figure 4. The system comprises at least two reactors in parallel working alternately to continuously produce syngas. Each reactor works alternately in reduction and oxidation cycles and intermittently alternated with short periods of mild fluidization of the bed after each cycle to level off temperature and concentration profiles [8]. The main advantages of this technology include the separation of gas and particles and the ability to work under high pressure, whereas this technology requires high temperature and a high flow gas switching system [7].
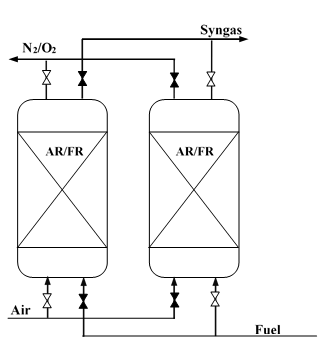
Figure 4. Simple configuration of alternating packed or fluidized bed reactors.
Yan et al. [35] used a fixed bed reactor to study the performance of Al2O3/BaFe2O4 as a synthesized oxygen carrier in BCLG for hydrogen-rich syngas production. Liu et al. [36] conducted a study of chemical looping co-gasification of pinewood and polyethylene in a fixed reactor. The effects of operating conditions during the BCLG process were reported in the investigation. A study of CLG of biomass char using NiO-modified iron ore as an oxygen carrier was carried out by Huang et al. [37]. They reported the reduction of oxygen carrier by biomass char in TGA and a fixed-bed reactor. Liu et al. [38] developed Ca2Fe2O5 with Mg/Al/Zn oxides as support materials for BCLG in a fixed bed reaction. This work mainly investigated the effects of Mg/Al/Zn oxides on the reactivity of Ca2Fe2O5 and the BCLG performance. Wang et al. [39] presented experimental results in a bubbling fluidized bed reactor for CLG of sawdust pellet with high volatile and low ash content as fuel. It was found that higher reaction temperatures increased gas production, while the amount of liquid and solid decreased.
3. Looping materials in chemical looping gasification of biomass
In the chemical looping gasification process, metal oxides as oxygen carriers transfer lattice oxygen and heat from the air reactor to the fuel reactor through oxidation-reduction reactions to generate the syngas. Metal oxide materials play a key role in the chemical looping redox processes. These metal oxide materials can be classified as chemical looping gasification (BCLG) and oxygen uncoupling chemical looping gasification (OU-BCLG) based on the properties of looping materials. Firstly, biomass fuel is decomposed and cracked down at high temperatures mainly into different fractions of gas, tar, and char, which can be simplified into volatiles and char. The volatile matter in the biomass, which is reduced to the release of hydrocarbon gases, is constituted by complex organic substances and can be condensed at a sufficiently low temperature to liquid tars. The gaseous fraction is an incondensable mixture of gases at ambient temperature and accounts typically for 70–90 wt.% of the feedstock [40][41]. This mixture of gases consists mainly of hydrogen, carbon monoxide, carbon dioxide, and light hydrocarbon. Afterward, these products react with oxygen carrier particles. Two main types of reactions occur simultaneously in the fuel reactor: homogeneous and heterogeneous. There are two reaction pathways proposed between the oxygen carrier and biomass in the fuel reactor: direct reduction of oxygen carrier by biomass and reduction of oxygen carrier by gaseous biomass gasification products [6]. The first pathway consists of two main reaction types, reactions of volatile matter released from biomass with oxygen carrier (Reaction (5)), and direct solid-solid reactions. Since solid-solid reactions are very limited due to low contact efficiency, the impact on the final gas composition is negligible in comparison to heterogeneous reactions. Due to a relatively high fraction of volatile matter in biomass, it is a benefit for biomass that a higher proportion of the fuel can directly react with oxygen carriers in a CLG system [6]. The second pathway is an indirect reaction between biomass and oxygen carrier. Firstly, char is gasified with H2O/CO2 to produce mainly H2/CO (Reactions (6) and (7)), and then gaseous products can readily react with oxygen carrier (Reactions (8) to (10)). The general reactions occur in the fuel reactor.
|
Biomass → Volatiles + Char (mainly C) + H2O |
(4) |
|
Volatiles + MexOy → CO2 + H2O + MexOy-1 |
(5) |
|
Char + CO2 → 2CO |
(6) |
|
Char + H2O → H2 + CO |
(7) |
|
CO + MexOy → CO2 + MexOy-1 |
(8) |
|
H2 + MexOy → H2O + MexOy-1 |
(9) |
|
CH4 + 3MexOy → CO+2H2O + 3MexOy-1 |
(10) |
The other concept proposed is oxygen uncoupling biomass chemical looping gasification (OU-BCLG). The system is based on the use of an oxygen carrier that can release gaseous oxygen in the fuel reactor to oxidize the fuel.
|
2MexOy ⇌ 2MexOy-1 + O2 |
(11) |
|
Char + O2 → CO + CO2 |
(12) |
|
Volatiles + O2 → CO + CO2 + H2O |
(13) |
In the OU-BCLG process, the oxygen carrier releases oxygen according to reaction (11), and the biomass fuel can be decomposed simultaneously into volatiles and char as shown in reaction (4). Afterward, the devolatilization products are oxidized to CO, CO2, and H2O according to reactions (12) and (13). The remaining char is gasified by CO2 and H2O to produce CO and H2. Moreover, the fuel reactor in both concepts should be fluidized by H2O, CO2, or their mixture, which also acts as gasifying agents to accelerate biomass gasification.
There are a limited number of oxygen carrier materials, which have the property of releasing oxygen that can meet the requirement for multiple cycles of oxygen uncoupling processes. They must be reversible in the reactions of releasing and oxidizing oxygen. In comparison with oxygen carrier for the normal BCLG process, the metal oxides used in OU-BCLG have a suitable equilibrium partial pressure of gas-phase oxygen at a temperature range of 800–1200 °C. Thus, there are three metal oxide system could be used in the OU-BCLG system such as CuO/Cu2O, Mn2O3/Mn3O4, and Co3O4/CoO. Their reversible reactions are as follows [7]:
|
4CuO ⇌ 2Cu2O + O2 |
(14) |
|
6Mn2O3 ⇌ 4Mn3O4 + O2 |
(15) |
|
2Co3O4 ⇌ 6CoO + O2 |
(16) |
In chemical looping gasification, an oxygen carrier is used to not only provide the oxygen needed for gasification to extremely improve the quality of syngas but also as a thermal carrier that increases heat balance between the two reactors [21]. Furthermore, some metal oxide oxygen carriers may have a catalytic effect on biomass tar cracking [42][43][44]. Thus, the selection of an appropriate oxygen carrier is one of the most important criteria for the good performance of the chemical looping process. The preferable properties of an oxygen carrier for system performance should be as follows: [7][8][45][46][47]
- Sufficient oxygen transport capacity.
- Favorable thermodynamics and reactivity regarding the solid fuel for the reduction reactions.
- High reactivity in the oxidation reactions.
- Selectivity towards CO and H2.
- Resistance to attrition to minimize losses of elutriated solid.
- Minimal carbon deposition.
- Good fluidization characteristics (no presence of agglomeration) and high melting points.
- Reasonable cyclability/circulation for using over several redox reactions.
- Low cost and long lifetime.
- Environmentally friendly properties.
- High mechanical strength and resistance to frictional stresses.
- The capability of converting biomass to gaseous products.
- Propensity to convert methane
References
- The Paris Agreement 2015. United Nations2015.
- Mendiara T, García-Labiano F, Abad A, Gayán P, de Diego LF, Izquierdo MT, et al. Negative CO2 emissions through the use of biofuels in chemical looping technology: A review. Applied Energy. 2018;232:657-84.
- Mattison T, Hildor F, Li Y, Linderholm C. Negative emissions of carbon dioxide through chemical-looping combustion (CLC) and gasification (CLG) using oxygen carriers based on manganese and iron. Mitigation and Adaptation Strategies for Global Change. 2020;25:497-517.
- Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy. 1987;12:147 - 54.
- Ajay K, Jones D, Hanna M. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies. 2009;2.
- Zhao X, Zhou H, Sikarwar VS, Zhao M, Park A-HA, Fennell PS, et al. Biomass-based chemical looping technologies: the good, the bad and the future. Energy & Environmental Science. 2017;10:1885-910.
- Adánez J, Abad A, García-Labiano F, Gayán P, de Diego L. Progress in Chemical-Looping Combustion and Reforming Technologies. Fuel and Energy Abstracts. 2012;38.
- Present status and overview of Chemical Looping Combustion technology. Renewable and Sustainable Energy Reviews. 2016;59:597 - 619.
- Pujara M, Sheth M, Rachchh N, Bhoraniya R, Harichandan A. Chemical Looping Reforming (CLR) System for H2 Production—A Review. 2020. p. 267-76.
- Luo M, Yi Y, Wang S, Wang Z, Du M, Pan J, et al. Review of hydrogen production using chemical-looping technology. Renewable and Sustainable Energy Reviews. 2018;81:3186-214.
- Lin Y, Wang H, Wang Y, Huo R, Huang Z, Liu M, et al. Review of Biomass Chemical Looping Gasification in China. Energy & Fuels. 2020;34:7847-62.
- Cuadrat A, Abad A, García-Labiano F, Gayán P, de Diego L, Adánez J. Relevance of the coal rank on the performance of the in situ gasification chemical-looping combustion. Chemical Engineering Journal. 2012;s 195–196:91–102.
- Dieringer P, Marx F, Alobaid F, Ströhle J, Epple B. Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions. Applied Sciences. 2020;10.
- Protasova L, Snijkers F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel. 2016;181:75-93.
- Mendiara T, Abad A, de Diego LF, García-Labiano F, Gayán P, Adánez J. Biomass combustion in a CLC system using an iron ore as an oxygen carrier. International Journal of Greenhouse Gas Control. 2013;19:322-30.
- Huang Z, Zheng A, Deng Z, Wei G, Zhao K, Chen D, et al. In-situ Removal of Toluene as a Biomass Tar Model Compound Using NiFe2O4 for Application in Chemical Looping Gasification Oxygen Carrier. Energy. 2019;190:116360.
- Wolf J. CO2 Mitigation in Advanced Power Cycles. Universitetsservice US AB, Stockholm, 2004 KTH - Royal Institute of Technology; 2004.
- Noorman S, van Sint Annaland M, Kuipers. Packed Bed Reactor Technology for Chemical-Looping Combustion. Industrial & Engineering Chemistry Research. 2007;46:4212-20.
- Huijun G, Laihong S, Fei F, Shouxi J. Experiments on biomass gasification using chemical looping with nickel-based oxygen carrier in a 25 kWth reactor. Applied Thermal Engineering. 2015;85:52-60.
- Huseyin S, Wei G-q, Li H-b, He F, Huang Z. Chemical-looping gasification of biomass in a 10 kWth interconnected fluidized bed reactor using Fe2O3/Al2O3 oxygen carrier. Journal of Fuel Chemistry and Technology. 2014;42:922-31.
- Zeng J, Xiao R, Zhang H, Wang Y, Zeng D, Ma Z. Chemical looping pyrolysis-gasification of biomass for high H2/CO syngas production. Fuel Processing Technology. 2017;168:116-22.
- Hedayati A, Soleimanisalim AH, Linderholm CJ, Mattisson T, Lyngfelt A. Experimental evaluation of manganese ores for chemical looping conversion of synthetic biomass volatiles in a 300 W reactor system. Journal of Environmental Chemical Engineering. 2021;9:105112.
- Ge H, Guo W, Shen L, Song T, Xiao J. Biomass Gasification using Chemical Looping in a 25kWth Reactor with Natural Hematite as Oxygen Carrier. Chemical Engineering Journal. 2015;286.
- Wei G, He F, Zhao Z, Huang Z, Zheng A, Zhao K, et al. Performance of Fe–Ni bimetallic oxygen carriers for chemical looping gasification of biomass in a 10 kWth interconnected circulating fluidized bed reactor. International Journal of Hydrogen Energy. 2015;40.
- Condori O, García-Labiano F, de Diego LF, Izquierdo MT, Abad A, Adánez J. Biomass chemical looping gasification for syngas production using ilmenite as oxygen carrier in a 1.5 kWth unit. Chemical Engineering Journal. 2021;405:126679.
- Samprón I, de Diego LF, García-Labiano F, Izquierdo MT, Abad A, Adánez J. Biomass Chemical Looping Gasification of pine wood using a synthetic Fe2O3/Al2O3 oxygen carrier in a continuous unit. Bioresource Technology. 2020;316:123908.
- He F, Huang Z, Wei G, Zhao K, Wang G, Kong X, et al. Biomass chemical-looping gasification coupled with water/CO2-splitting using NiFe2O4 as an oxygen carrier. Energy Conversion and Management. 2019;201:112157.
- Wolf J, Yan J. Parametric study of chemical looping combustion for tri-generation of hydrogen, heat, and electrical power with CO2 capture. International Journal of Energy Research. 2005;29:739-53.
- Cormos C-C. Hydrogen production from fossil fuels with carbon capture and storage based on chemical looping systems. International Journal of Hydrogen Energy. 2011;36:5960-71.
- Chiesa P, Lozza G, Malandrino A, Romano M, Piccolo V. Three-reactors chemical looping process for hydrogen production. International Journal of Hydrogen Energy. 2008;33:2233-45.
- Xiang W, Chen S, Xue Z, Sun X. Investigation of coal gasification hydrogen and electricity co-production plant with three-reactors chemical looping process. International Journal of Hydrogen Energy. 2010;35:8580-91.
- Fan L-S, Li F. Chemical Looping Technology and Its Fossil Energy Conversion Applications. Industrial & Engineering Chemistry Research. 2010;49:10200-11.
- Li F, Kim HR, Sridhar D, Wang F, Zeng L, Chen J, et al. Syngas Chemical Looping Gasification Process: Oxygen Carrier Particle Selection and Performance. Energy & Fuels. 2009;23:4182-9.
- Gupta P, Velazquez-Vargas LG, Fan L-S. Syngas Redox (SGR) Process to Produce Hydrogen from Coal Derived Syngas. Energy & Fuels. 2007;21:2900-8.
- Yan J, Sun R, Shen L, Bai H, Jiang S, Xiao Y, et al. Hydrogen-rich syngas production with tar elimination via biomass chemical looping gasification (BCLG) using BaFe2O4/Al2O3 as oxygen carrier. Chemical Engineering Journal. 2020;387:124107.
- Liu Q, Hu C, Peng B, Liu C, Li Z, Wu K, et al. High H2/CO ratio syngas production from chemical looping co-gasification of biomass and polyethylene with CaO/Fe2O3 oxygen carrier. Energy Conversion and Management. 2019;199:111951.
- Huang Z, Feng Y, Zhao K, Zheng A, Chang S, Wei G, et al. Biomass Char Direct Chemical Looping Gasification Using NiO-Modified Iron Ore as an Oxygen Carrier. Energy & Fuels. 2013;28:183–91.
- Liu G, Liao Y, Wu Y, Ma X. Enhancement of Ca2Fe2O5 oxygen carrier through Mg/Al/Zn oxide support for biomass chemical looping gasification. Energy Conversion and Management. 2019;195:262-73.
- Wang S, Song T, Yin S, Hartge E-U, Dymala T, Shen L, et al. Syngas, tar and char behavior in chemical looping gasification of sawdust pellet in fluidized bed. Fuel. 2020;270:117464.
- Roos C. Clean Heat and Power Using Biomass Gasification for Industrial and Agricultural Projects. 2010.
- Schmid JC, Wolfesberger U, Koppatz S, Pfeifer C, Hofbauer H. Variation of feedstock in a dual fluidized bed steam gasifier—influence on product gas, tar content, and composition. Environmental Progress & Sustainable Energy. 2012;31:205-15.
- Mendiara T, Johansen JM, Utrilla R, Jensen AD, Glarborg P. Evaluation of different oxygen carriers for biomass tar reforming (II): Carbon deposition in experiments with methane and other gases. Fuel. 2011;90:1370-82.
- Park HJ, Park SH, Sohn JM, Park J, Jeon J-K, Kim S-S, et al. Steam reforming of biomass gasification tar using benzene as a model compound over various Ni supported metal oxide catalysts. Bioresource Technology. 2010;101:S101-S3.
- Sonoyama N, Nobuta K, Kimura T, Hosokai S, Hayashi J-i, Tago T, et al. Production of chemicals by cracking pyrolytic tar from Loy Yang coal over iron oxide catalysts in a steam atmosphere. Fuel Processing Technology. 2011;92:771-5.
- Wang P, Means N, Shekhawat D, Berry D, Massoudi M. Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies. 2015;8:10605.
- Guo Q, Cheng Y, Liu Y, Jia W, Ryu H-J. Coal Chemical Looping Gasification for Syngas Generation Using an Iron-Based Oxygen Carrier. Industrial & Engineering Chemistry Research. 2013;53:78–86.
- Voitic G, Hacker V. Recent advancements in chemical looping water splitting for the production of hydrogen. RSC Advances. 2016;6:98267-96.




