
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daoliang Li | + 2583 word(s) | 2583 | 2021-09-24 05:15:01 | | | |
| 2 | Lindsay Dong | + 276 word(s) | 2859 | 2021-10-27 10:36:10 | | |
Video Upload Options
Automatic behavior monitoring, also called automated analytics or automated reporting, is the ability of an analytics platform to auto-detect relevant insights—anomalies, trends, patterns—and deliver them to users in real time, without users having to manually explore their data to find the answers they need. An analytics platform with automated behavior monitoring uses algorithms to auto-analyze datasets to search for notable changes in data.
1. Introduction
2. Important Behaviors in Crustacean Aquaculture
2.1. Feeding Behavior
2.2. Movement Rhythms
2.3. Reproductive Behavior
3. Behavior Monitoring Methods Based on Acoustic Technology
Autonomous acoustic monitoring is a technique using sound waves to remotely measure information. Acoustic technology has been widely used in species identification [30], biomass estimation [31], and behavior monitoring without causing stress to crustaceans [32]. For underwater monitoring, acoustic technology has key advantages over light waves and electromagnetic waves because of the long propagation distances [14]; another advantage of acoustic technology is that its measurement results are less affected by water turbidity and underwater light [33]. According to data acquisition methods, acoustic technology can be divided into passive acoustics and active acoustics. Active acoustics includes sonar, echo, and acoustic telemetry. Sonar and echo technology are more used to measure the density of crustaceans, and acoustic telemetry is more common to monitor crustacean behaviors.
3.1. Passive Acoustics
Many investigations have indicated that when some behaviors occur, crustaceans emit different sound frequencies, including feeding [34], mating [35], carapace vibrations [36], snap [37], and stick and slip friction [38][39][40]. With such variety of sound production mechanisms, the characteristics of the sounds produced by crustaceans are diverse [41][42]. According to the above theoretical basis, experts can identify crustacean behaviors via long-term acoustic monitoring of sounds.
The mechanisms and spectral characteristics of crustacean behaviors are heterogenous. In terms of feeding sounds, the physical production mechanism is that shrimp use mandibles and maxillae to tear feed pellets into pieces before entering the oral cavity [42]. Some scholars have used the sound spectral features of feeding as an indication of pellet consumption [43]. These experimental results show that the correlation between sound and feeding behavior can reach more than 95%. Although passive acoustic technology can provide guidance for measuring the relative intensity of feeding activity, it is unclear how accurate it is at estimating the quantity of consumed pellets from feeding sounds.
3.2. Acoustic Telemetry
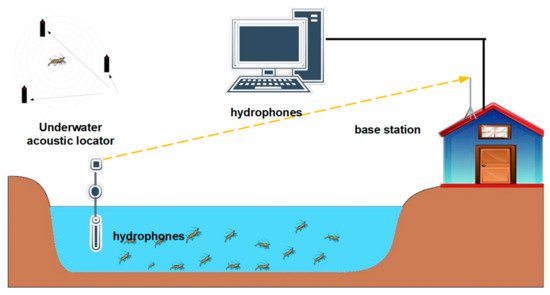
| Technology | Species | Application | Results or Accuracy | Culture Model | Acoustic Features/Principle | Reference |
|---|---|---|---|---|---|---|
| Passive acoustics | Tiger prawns | Feeding | R2 = 0.95 and R2 = 0.96 | Tank and pond | 3 kHz–7.6 kHz | [54] |
| Prawn | Confidence intervals: 98.4 ± 0.6 |
Pond | 51.2 kHz | [43] | ||
| Family Alpheidae | Snap | R = 0.71–0.92 | West Bay Marine Reserve | 1.5–20 kHz | [55] | |
| Red swamp crayfish | Intraspecific interactions and activities | 45% and p < 0.0001 | Tank and natural environment | Peak frequency = 28 kHz Bandwidth RMS = 20 kHz |
[56] | |
| European lobster | Seasonal activity | p < 0.05 | Site | Vemco 12 VR2W | [57] | |
| Japanese spiny lobster | Movement | Island | 0.04–21 kHz | [58] | ||
| Acoustic telemetry Active acoustics |
European spiny lobster | Home range | p < 0.001 | Protected area | Ultrasonic telemetry | [52] |
| American lobsters | (523.2 ± 78.1 m/day−1; r2 = 0.62, p = 0.0001) | Enclosure | VEMCO V8SC-2L | [49] | ||
| Lobster | SE = 0.09, p = 0.02 | Coast | Vemco V13P–L | [6] | ||
| European spiny lobster | Ranged from 1629.3 to 8641.3 m2 | Coast | Vemco V9P-1L 69 kHz | [50] | ||
| Spiny lobster | 923 versus 871 m/day, | Channel | Vemco V16 69 kHz | [24] | ||
| American lobsters | 51% moved <5 km, 19% moved 5–10 km, and 30% moved >10 km | Inshore | Vemco V13-1L 69 kHz | [59] | ||
| Lobster | The mean daily home range (n = 18) was 1002.0 ± 195.7 m2 (mean ± SEM) |
Castle | VRAP model, VEMCO | [60] | ||
| American lobsters | Home ranges (≈27.4−111.6 m2) | Castle | VRAP | |||
| Lobster Jasus lalandii | Nomadic behavior | p = 0.0002 | Aquarium | Vemco V8-2LR | [51] | |
| Lobster | Reproductive migrations | Three migrations per year by an individual female | Western Sambo Ecological Reserve | Vemco V16 69 kHz | [53] | |
| Lobster | Feeding | 90% confidence level (p = 0.09, K–S test) | Field enclosure | Vemco VR2W 69 kHz | [32] | |
| Spider crab | Migratory patterns | 70% recapture rate | Coast | VEMCO V16 | [61] | |
| Norway lobsters | Error < 1 m | European waters | Vemco | [62] | ||
| Lobster | Movement patterns | r2 = 0.82, DF = 70, p < 0.0001 |
2.5 km2 lobster | Vemco VRAP | [22] | |
| Spider crab | R = 0.353; R = 0.805 | Coast | VEMCO Ltd. | [63] | ||
| Blue crabs | R > 0.64; R = 0.71–0.97; R = 0.25–0.32 |
Coast | Tucson Arizona | [64] | ||
| Lobsters; crab | Tank | VEMCO Ltd. | [65] | |||
| Edible crab | p < 0.001–0.042 | Coast | Vemco VR 60 | [66] |
4. Behavior Monitoring Based on Machine Vision
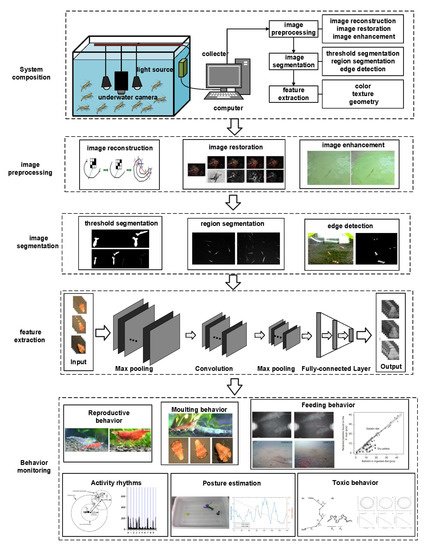
4.1. Machine Vision Based on Visible Light
4.1.1. Direct Behavior Monitoring
4.1.2. Indirect Behavior Monitoring
4.2. Machine Vision Based on Invisible Light
5. Electrosensors
5.1. Accelerometer
5.2. Electromyography
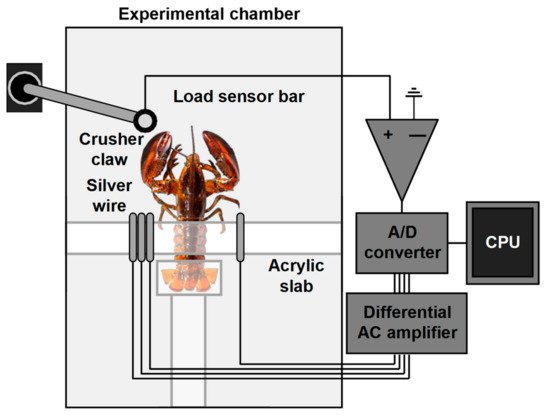
6. Other Methods
References
- FIGIS. FAO Statistics.Global Aquaculture Production 1950–2015. 2015. Available online: http://www.fao.org/figis/ (accessed on 7 November 2017).
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020.
- Kubec, J.; Kouba, A.; Buric, M. Communication, behaviour, and decision making in crayfish: A review. Zool. Anz. 2019, 278, 28–37.
- Gherardi, F.; Aquiloni, L.; Tricarico, E. Behavioral plasticity, behavioral syndromes and animal personality in crustacean decapods: An imperfect map is better than no map. Curr. Zool. 2012, 58, 567–579.
- Briones-Fourzan, P.; Dominguez-Gallegos, R.; Lozano-Alvarez, E. Aggressive behaviour of spotted spiny lobsters (Panulirus guttatus) in different social contexts: The influence of sex, size, and missing limbs. Ices J. Mar. Sci. 2015, 72, 155–163.
- Moland, E.; Carlson, S.M.; Villegas-Rios, D.; Wiig, J.R.; Olsen, E.M. Harvest selection on multiple traits in the wild revealed by aquatic animal telemetry. Ecol. Evol. 2019, 9, 6480–6491.
- Antonucci, F.; Costa, C. Precision aquaculture: A short review on engineering innovations. Aquac. Int. 2020, 28, 41–57.
- Parra, L.; Lloret, G.; Lloret, J.; Rodilla, M. Physical Sensors for Precision Aquaculture: A Review. IEEE Sens. J. 2018, 18, 3915–3923.
- Howe, B.M.; Miksis-Olds, J.; Rehm, E.; Sagen, H.; Worcester, P.F.; Haralabus, G. Observing the Oceans Acoustically. Front. Mar. Sci. 2019, 6, 22.
- Sbragaglia, V.; Aguzzi, J.; Garcia, J.A.; Sarria, D.; Gomariz, S.; Costa, C.; Menesatti, P.; Vilaro, M.; Manuel, A.; Sarda, F. An automated multi-flume actograph for the study of behavioral rhythms of burrowing organisms. J. Exp. Mar. Biol. Ecol. 2013, 446, 177–185.
- Lyons, G.N.; Halsey, L.G.; Pope, E.C.; Eddington, J.D.; Houghton, J.D.R. Energy expenditure during activity in the American lobster Homarus americanus: Correlations with body acceleration. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 278–284.
- Gutzler, B.C.; Butler, M.J. Accelerometry as a tool for studying lobster behavior: Preliminary results from the Florida Keys, FL (USA). Lobster Newsl. 2014, 27, 8–9.
- Briffa, M. Contests in crustaceans: Assessments, decisions and their underlying mechanisms. In Animal Contests; Cambridge University Press Location: Cambridge, UK, 2013; pp. 86–112.
- Kuklina, I.; Kouba, A.; Kozak, P. Real-time monitoring of water quality using fish and crayfish as bio-indicators: A review. Environ. Monit. Assess. 2013, 185, 5043–5053.
- Cuellar-Anjel, J.; Corteel, M.; Galli, L.; Alday-Sanz, V.; Hasson, K.W. Principal Shrimp Infectious Diseases, Diagnosis and Management. In The Shrimp Book; CABI: Wallingford, UK, 2010; pp. 517–621.
- Yan, S.; Alfredsen, J.A. Real time lobster posture estimation for behavior research. In Proceedings of the Eighth International Conference on Graphic and Image Processing, Tokyo, Japan, 8 February 2017; Pham, T.D., Vozenilek, V., Zeng, Z., Eds.; SPIE: Bellingham, WA, USA, 2017; 10225.
- Bardera, G.; Usman, N.; Owen, M.; Pountney, D.; Sloman, K.A.; Alexander, M.E. The importance of behaviour in improving the production of shrimp in aquaculture. Rev. Aquac. 2019, 11, 1104–1132.
- Silva, P.F.; Medeiros, M.d.S.; Alves Silva, H.P.; Arruda, M.d.F. A study of feeding in the shrimp Farfantepenaeus subtilis indicates the value of species level behavioral data for optimizing culture management. Mar. Freshw. Behav. Physiol. 2012, 45, 121–134.
- Karadal, O.; Turkmen, G. Effects of feeding frequency on growth performance and molting cycle of two different size classes of red swamp crayfish (Procambarus clarkii). LimnoFish J. Limnol. Freshw. Fish. Res. 2018, 4, 140–145.
- Santos, A.d.A.; Lopez-Olmeda, J.F.; Sanchez-Vazquez, F.J.; Fortes-Silva, R. Synchronization to light and mealtime of the circadian rhythms of self-feeding behavior and locomotor activity of white shrimps (Litopenaeus vannamei). Comp. Biochem. Physiology. A Mol. Integr. Physiol. 2016, 199, 54–61.
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059.
- Morse, B.L.; Comeau, M.; Rochette, R. Ontogenetic changes in movement patterns and activity levels of American lobsters (Homarus americanus) in Anse-Bleue, southern Gulf of St. Lawrence. J. Exp. Mar. Biol. Ecol. 2018, 505, 12–23.
- Childress, M.J.J.S.H. Behaviour; Cambridge Dictionary: Cambridge, UK, 2006; p. 112.
- Bertelsen, R.D.; Hornbeck, J. Using acoustic tagging to determine adult spiny lobster (Panulirus argus) movement patterns in the Western Sambo Ecological Reserve (Florida, United States). New Zealand J. Mar. Freshw. Res. 2009, 43, 35–46.
- Ghanawi, J.; Saoud, I.P. Molting, reproductive biology, and hatchery management of redclaw crayfish Cherax quadricarinatus (von Martens 1868). Aquaculture 2012, 358, 183–195.
- Farhadi, A.; Harlioglu, M.M. Photoperiod affects gamete production, and protein and lipid metabolism in male narrow-clawed Crayfish Pontastacus leptodactylus (Eschscholtz, 1823). Anim. Reprod. Sci. 2019, 211, 106204.
- Mellan, D.; Warren, A.; Buckholt, M.A.; Mathews, L.M. Sexual History Affects Mating Behavior and Mate Choice in the Crayfish Orconectes limosus. Ethology 2014, 120, 681–692.
- Katoh, E. Sex, pheromone and aggression in Norway lobsters (Nephrops norvegicus): For a better future of Scampi. Ph.D. Thesis, University of Hull, Hull, UK, 2011.
- Stebbing, P.D.; Bentley, M.G.; Watson, G.J. Mating behaviour and evidence for a female released courtship pheromone in the signal crayfish Pacifastacus leniusculus. J. Chem. Ecol. 2003, 29, 465–475.
- Horne, J.K. Acoustic approaches to remote species identification: A review. Fish. Oceanogr. 2000, 9, 356–371.
- Tan, C.S.; Lau, P.Y.; Correia, P.L.; Campos, A. Automatic analysis of deep-water remotely operated vehicle footage for estimation of Norway lobster abundance. Front. Inf. Technol. Electron. Eng. 2018, 19, 1042–1055.
- McMahan, M.D.; Brady, D.C.; Cowan, D.F.; Grabowski, J.H.; Sherwood, G.D. Using acoustic telemetry to observe the effects of a groundfish predator (Atlantic cod, Gadus morhua) on movement of the American lobster (Homarus americanus). Can. J. Fish. Aquat. Sci. 2013, 70, 1625–1634.
- Li, D.; Hao, Y.; Duan, Y. Nonintrusive methods for biomass estimation in aquaculture with emphasis on fish: A review. Rev. Aquac. 2020, 12, 1390–1411.
- Jezequel, Y.; Bonnel, J.; Coston-Guarini, J.; Guarini, J.-M.; Chauvaud, L. Sound characterization of the European lobster Homarus gammarus in tanks. Aquatic Biology 2018, 27, 13–23.
- Popper, A.N.; Salmon, M.; Horch, K.W. Acoustic detection and communication by decapod crustaceans. J. Comp. Physiol. A-Neuroethol. Sens. Neural Behav. Physiol. 2001, 187, 83–89.
- Coquereau, L.; Grall, J.; Clavier, J.; Jolivet, A.; Chauvaud, L. Acoustic behaviours of large crustaceans in NE Atlantic coastal habitats. Aquat. Biol. 2016, 25, 151–163.
- Patek, S.N.; Oakley, T.H. Comparative tests of evolutionary trade-offs in a palinurid lobster acoustic system. Evolution 2003, 57, 2082–2100.
- Patek, S.N. Spiny lobsters stick and slip to make sound—These crustaceans can scare off predators even when their usual armour turns soft. Nature 2001, 411, 153–154.
- Patek, S.N. Squeaking with a sliding joint: Mechanics and motor control of sound production in palinurid lobsters. J. Exp. Biol. 2002, 205, 2375–2385.
- Patek, S.N.; Baio, J.E. The acoustic mechanics of stick-slip friction in the California spiny lobster (Panulirus interruptus). J. Exp. Biol. 2007, 210, 3538–3546.
- Patek, S.N.; Caldwell, R.L. The stomatopod rumble: Low frequency sound production in Hemisquilla californiensis. Mar. Freshw. Behav. Physiol. 2006, 39, 99–111.
- Patek, S.N.; Shipp, L.E.; Staaterman, E.R. The acoustics and acoustic behavior of the California spiny lobster (Panulirus interruptus). J. Acoust. Soc. Am. 2009, 125, 3434–3443.
- Smith, D.V.; Shahriar, M.S. A context aware sound classifier applied to prawn feed monitoring and energy disaggregation. Knowl. Based Syst. 2013, 52, 21–31.
- Hawkins, A.D.; Maclennan, D.N.; Urquhart, G.G.; Robb, C. Tracking cod gadusmorhua l in a scottish sea loch. J. Fish. Biol. 1974, 6, 225–236.
- Donaldson, M.R.; Hinch, S.G.; Suski, C.D.; Fisk, A.T.; Heupel, M.R.; Cooke, S.J. Making connections in aquatic ecosystems with acoustic telemetry monitoring. Front. Ecol. Environ. 2014, 12, 565–573.
- Atkinson, L.J.; Mayfield, S.; Cockcroft, A.C. The potential for using acoustic tracking to monitor the movement of the West Coast rock lobster Jasus lalandii. Afr. J. Mar. Sci. 2005, 27, 401–408.
- Hellstrom, G.; Klaminder, J.; Jonsson, M.; Fick, J.; Brodin, T. Upscaling behavioural studies to the field using acoustic telemetry. Aquat. Toxicol. 2016, 170, 384–389.
- Cooke, S.J.; Hinch, S.G.; Wikelski, M.; Andrews, R.D.; Kuchel, L.J.; Wolcott, T.G.; Butler, P.J. Biotelemetry: A mechanistic approach to ecology. Trends Ecol. Evol. 2004, 19, 334–343.
- Scopel, D.A.; Golet, W.J.; Watson, W.H., III. Home range dynamics of the American lobster, Homarus americanus. Mar. Freshw. Behav. Physiol. 2009, 42, 63–80.
- Giacalone, V.M.; Barausse, A.; Gristina, M.; Pipitone, C.; Visconti, V.; Badalamenti, F.; D’Anna, G. Diel activity and short-distance movement pattern of the European spiny lobster, Palinurus elephas, acoustically tracked. Mar. Ecol. Evol. Perspect. 2015, 36, 389–399.
- Haley, C.N.; Blamey, L.K.; Atkinson, L.J.; Branch, G.M. Dietary change of the rock lobster Jasus lalandii after an ‘invasive’ geographic shift: Effects of size, density and food availability. Estuar. Coast. Shelf Sci. 2011, 93, 160–170.
- Giacalone, V.M.; Zenone, A.; Badalamenti, F.; Ciancio, J.; Buffa, G.; Gristina, M.; Pipitone, C.; D’Anna, G. Homing and Home Range Of The European Spiny Lobster, Palinurus Elephas (Decapoda, Palinuridae) Acoustically Tracked. Crustaceana 2019, 92, 463–476.
- Bertelsen, R.D. Characterizing daily movements, nomadic movements, and reproductive migrations of Panulirus argus around the Western Sambo Ecological Reserve (Florida, USA) using acoustic telemetry. Fish. Res. 2013, 144, 91–102.
- Smith, D.V.; Tabrett, S. The use of passive acoustics to measure feed consumption by Penaeus monodon (giant tiger prawn) in cultured systems. Aquac. Eng. 2013, 57, 38–47.
- Bohnenstiehl, D.R.; Lillis, A.; Eggleston, D.B. The Curious Acoustic Behavior of Estuarine Snapping Shrimp: Temporal Patterns of Snapping Shrimp Sound in Sub-Tidal Oyster Reef Habitat. PLoS ONE 2016, 11, e0143691.
- Buscaino, G.; Filiciotto, F.; Buffa, G.; Di Stefano, V.; Maccarrone, V.; Buscaino, C.; Mazzola, S.; Alonge, G.; D’Angelo, S.; Maccarrone, V. The underwater acoustic activities of the red swamp crayfish Procambarus clarkii. J. Acoust. Soc. Am. 2012, 132, 1792–1798.
- Skerritt, D.J.; Robertson, P.A.; Mill, A.C.; Polunin, N.V.C.; Fitzsimmons, C. Fine-scale movement, activity patterns and home-ranges of European lobster Homarus gammarus. Mar. Ecol. Prog. Ser. 2015, 536, 203–219.
- Kikuchi, M.; Akamatsu, T.; Takase, T. Passive acoustic monitoring of Japanese spiny lobster stridulating sounds. Fish. Sci. 2015, 81, 229–234.
- Goldstein, J.S.; Watson, W.H., III. Seasonal movements of American lobsters in southern Gulf of Maine coastal waters: Patterns, environmental triggers, and implications for larval release. Mar. Ecol. Prog. Ser. 2015, 524, 197–211.
- Watson, W.H., III.; Golet, W.; Scopel, D.; Jury, S. Use of ultrasonic telemetry to determine the area of bait influence and trapping area of American lobster, Homarus americanus, traps. New Zealand J. Mar. Freshw. Res. 2009, 43, 411–418.
- Gonzalez-Gurriaran, E.; Freire, J.; Bernardez, C. Migratory patterns of female spider crabs maja squinado detected using electronic tags and telemetry. J. Crustacean Biol. 2002, 22, 91–97.
- Masmitja, I.; Navarro, J.; Gomariz, S.; Aguzzi, J.; Kieft, B.; O’Reilly, T.; Katija, K.; Bouvet, P.J.; Fannjiang, C.; Vigo, M.; et al. Mobile robotic platforms for the acoustic tracking of deep-sea demersal fishery resources. Sci. Robot. 2020, 5, eabc3701.
- Gonzalezgurriaran, E.; FREIRE, J. Movement patterns and habitat utilization in the spider crab Maja squinado (Herbst) (Decapoda, Maji-dae) measured by ultrasonic telemetry. J. Exp. Mar. Biol. Ecol. 1994, 184, 269–291.
- Hines, A.H.; Wolcott, T.G.; González-Gurriarán, E.; González-Escalante, J.L.; Freire, J. Movement patterns and migrations in crabs teleme-try of juvenile and adult behavior in Callinectes sapidus and Maja squinado. J. Mar. Biol. Assoc. U. K. 1995, 75, 27–42.
- Rotllant, G.; Aguzzi, J.; Sarria, D.; Gisbert, E.; Sbragaglia, V.; Del Rio, J.; Simeo, C.G.; Manuel, A.; Molino, E.; Costa, C.; et al. Pilot acoustic tracking study on adult spiny lobsters (Palinurus mauritanicus) and spider crabs (Maja squinado) within an artificial reef. Hydrobiologia 2015, 742, 27–38.
- Ungfors, A.; Hallback, H.; Nilsson, P.G. Movement of adult edible crab (Cancer pagurus l.) at the swedish west coast by mark-recapture and acoustic tracking. Fish. Res. 2007, 84, 345–357.
- Myrberg, A.A. Underwater Television—Tool for Marine Biologist. Bull. Mar. Sci. 1973, 23, 824–836.
- Saberioon, M.; Gholizadeh, A.; Cisar, P.; Pautsina, A.; Urban, J. Application of machine vision systems in aquaculture with emphasis on fish: State-of-the-art and key issues. Rev. Aquac. 2017, 9, 369–387.
- Trenkel, V.M.; Cotter, J. Choosing survey time series for populations as part of an ecosystem approach to fishery management. Aquat. Living Resour. 2009, 22, 121–126.
- Jouffre, D.; Borges, M.d.F.; Bundy, A.; Coll, M.; Diallo, I.; Fulton, E.A.; Guitton, J.; Labrosse, P.; Abdellahi, K.O.M.; Masumbuko, B.; et al. Estimating EAF indicators from scientific trawl surveys: Theoretical and practical concerns. Ices J. Mar. Sci. 2010, 67, 796–806.
- Aguzzi, J.; Costa, C.; Fujiwara, Y.; Iwase, R.; Ramirez-Llorda, E.; Menesatti, P. A Novel Morphometry-Based Protocol of Automated Video-Image Analysis for Species Recognition and Activity Rhythms Monitoring in Deep-Sea Fauna. Sensors 2009, 9, 8438–8455.
- Brosnan, T.; Sun, D.W. Improving quality inspection of food products by computer vision—A review. J. Food Eng. 2004, 61, 3–16.
- Oppedal, F.; Dempster, T.; Stien, L.H. Environmental drivers of Atlantic salmon behaviour in sea-cages: A review. Aquaculture 2011, 311, 1–18.
- Salierno, J.D.; Gipson, G.T.; Kane, A.S. Quantitative movement analysis of social behavior in mummichog, Fundulus heteroclitus. J. Ethol. 2008, 26, 35–42.
- Valletta, J.J.; Torney, C.; Kings, M.; Thornton, A.; Madden, J. Applications of machine learning in animal behaviour studies. Anim. Behav. 2017, 124, 203–220.
- Gage, J.D.; Bett, B.J. Deep-Sea Benthic Sampling. In Methods for the Study of Marine Benthos, 3rd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2005; pp. 273–325.
- Karplus, I.; Barki, A. Male morphotypes and alternative mating tactics in freshwater prawns of the genus Macrobrachium: A review. Rev. Aquac. 2019, 11, 925–940.
- Qiao, X.; Rauschenbach, T.; Li, D. Review of Underwater Machine Vision Technology and Its Applications. Mar. Technol. Soc. J. 2017, 51, 75–97.
- Egmont-Petersen, M.; de Ridder, D.; Handels, H. Image processing with neural networks—A review. Pattern Recognit. 2002, 35, 2279–2301.
- Yan, F.; Iliyasu, A.M.; Khan, A.R.; Yang, H. Measurements-based Moving Target Detection in Quantum Video. Int. J. Theor. Phys. 2016, 55, 2162–2173.
- Aguzzi, J.; Costa, C.; Robert, K.; Matabos, M.; Antonucci, F.; Juniper, S.K.; Menesatti, P. Automated Image Analysis for the Detection of Benthic Crustaceans and Bacterial Mat Coverage Using the VENUS Undersea Cabled Network. Sensors 2011, 11, 10534–10556.
- Zhou, C.; Yang, X.; Zhang, B.; Lin, K.; Xu, D.; Guo, Q.; Sun, C. An adaptive image enhancement method for a recirculating aquaculture system. Sci. Rep. 2017, 7, 1–11.
- Zhang, H.; Wu, C.; Jiang, D.; Zhao, L.; Gui, F. Monitoring waste cumulating in aquaculture ponds using image processing technology. Oceanol. Et Limnol. Sin. Hai Yang Yu Hu Chao 2016, 47, 374–379.
- Aguzzi, J.; Costa, C.; Menesatti, P.; Antonio Garcia, J.; Jose Chiesa, J.; Sarda, F. Monochromatic blue light entrains diel activity cycles in the Norway lobster, Nephrops norvegicus (L.) as measured by automated video-image analysis. Sci. Mar. 2009, 73, 773–783.
- Hung, C.-C.; Tsao, S.-C.; Huang, K.-H.; Jang, J.-P.; Chang, H.-K.; Dobbs, F.C. A highly sensitive underwater video system for use in turbid aquaculture ponds. Sci. Rep. 2016, 6.
- Huang, I.-J.; Hung, C.-C.; Kuang, S.-R.; Chang, Y.-N.; Huang, K.-Y.; Tsai, C.-R.; Feng, K.-L. The Prototype of a Smart Underwater Surveillance System for Shrimp Farming; IEEE: New York, NY, USA, 2018; pp. 177–180.
- Sarria, D.; del Rio, J.; Manuel, A.; Aguzzi, J.; Sarda, F.; Garcia, J.A. Studying the Behaviour of Norway Lobster Using RFID and Infrared Tracking Technologies; IEEE: New York, NY, USA, 2009; p. 4.
- Weiss, H.M.; Lozano-Alvarez, E.; Briones-Fourzan, P.; Negrete-Soto, F. Using red light with fixed-site video cameras to study the behavior of the spiny lobster, Panulirus argus, and associated animals at night and inside their shelters. Mar. Technol. Soc. J. 2006, 40, 86–95.
- Gleiss, A.C.; Morgan, D.L.; Whitty, J.M.; Keleher, J.J.; Fossette, S.; Hays, G.C. Are vertical migrations driven by circadian behaviour? Decoupling of activity and depth use in a large riverine elasmobranch, the freshwater sawfish (Pristis pristis). Hydrobiologia 2017, 787, 181–191.
- Zenone, A.; Ceraulo, M.; Ciancio, J.E.; Buscaino, G.; D’Anna, G.; Grammauta, R.; Mazzola, S.; Giacalone, V.M. The use of 3-axial accelerometers to evaluate sound production in European spiny lobster, Palinurus elephas. Ecol. Indic. 2019, 102, 519–527.
- Goldstein, J.S.; Dubofsky, E.A.; Spanier, E. Into a rhythm: Diel activity patterns and behaviour in Mediterranean slipper lobsters, Scyllarides latus. Ices J. Mar. Sci. 2015, 72, 147–154.
- Jury, S.H.; Langley, T.; Gutzler, B.C.; Goldstein, J.S.; Watson, W.H. Monitoring the behavior of freely moving lobsters with accelerometers. Bull. Mar. Sci. 2018, 94, 533–553.
- Pollak, D.J.; Feller, K.D.; Serbe, E.; Mircic, S.; Gage, G.J. An Electrophysiological Investigation of Power-Amplification in the Ballistic Mantis Shrimp Punch. J. Undergrad. Neurosci. Educ. JUNE A Publ. FUN Fac. Undergrad. Neurosci. 2019, 17, T12–T18.
- Chikamoto, K.; Kagaya, K.; Takahata, M. Electromyographic Characterization of Walking Behavior Initiated Spontaneously in Crayfish. Zool. Sci. 2008, 25, 783–792.
- Frisch, A.J.; Hobbs, J.-P.A. Long-term retention of internal elastorner tags in a wild population of painted crayfish (Panulirus versicolor Latreille) on the Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 2006, 339, 104–110.




