Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Malvina Tsamouri | + 3156 word(s) | 3156 | 2021-10-18 05:01:27 | | | |
| 2 | Bruce Ren | Meta information modification | 3156 | 2021-10-27 08:25:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tsamouri, M.M. Muscle-Invasive Urothelial Carcinoma in Dogs. Encyclopedia. Available online: https://encyclopedia.pub/entry/15447 (accessed on 14 January 2026).
Tsamouri MM. Muscle-Invasive Urothelial Carcinoma in Dogs. Encyclopedia. Available at: https://encyclopedia.pub/entry/15447. Accessed January 14, 2026.
Tsamouri, Maria Malvina. "Muscle-Invasive Urothelial Carcinoma in Dogs" Encyclopedia, https://encyclopedia.pub/entry/15447 (accessed January 14, 2026).
Tsamouri, M.M. (2021, October 27). Muscle-Invasive Urothelial Carcinoma in Dogs. In Encyclopedia. https://encyclopedia.pub/entry/15447
Tsamouri, Maria Malvina. "Muscle-Invasive Urothelial Carcinoma in Dogs." Encyclopedia. Web. 27 October, 2021.
Copy Citation
Muscle-invasive urothelial carcinoma (MIUC) is the most common type of bladder malignancy in humans, but also in dogs that represent a naturally occurring model for this disease. Dogs are immunocompetent animals that share risk factors, pathophysiological features, clinical signs and response to chemotherapeutics with human cancer patients.
urothelial carcinoma
bladder cancer
comparative oncology
basic & translational cancer research
naturally occurring models of cancer
canine cancer
molecular cancer therapeutics
1. Muscle-Invasive Urothelial Carcinoma in the Dog
MIUC is the most common type of urinary bladder malignancy in the dog, affecting greater than 50,000 dogs annually in the US [1] with a ratio of female: male dogs being approximately 1.8:1 [1]. This is in direct contrast to human patients where the ratio of female: male patients is approximately 0.3:1 [2]. Early diagnosis of this disease in the dog is often challenging due to presentation with non-specific clinical signs that resemble those of other lower urinary tract disease (LUTD), including bladder inflammation or infection as well as stones, crystals, or debris in the bladder or urethra. Common clinical signs include difficulty urinating, frequent attempts to urinate, blood in urine and concurrent bacterial infection [3]. MIUC is typically diagnosed late in the dog with >10% canine patients presenting with metastatic disease at the time of diagnosis [4]. Canine MIUC has poor clinical prognosis, partly due to the delay in conclusive diagnosis, and due to ineffective definitive therapeutic options [5].
1.1. Standard Treatment for MIUC in Dogs
Even though cystectomy is the standard of care for localized MIUC in humans [6] as stated above, this is not the case in canine MIUC for several reasons. Canine MIUC is usually located at the trigone area of the bladder (whereas human MIUC is located throughout the bladder), often projecting towards the urethra or the prostate gland, making it anatomically difficult to excise the tumor while maintaining a “negative” surgical margin (tumor-free perimeter at the area of the incision) [7]. Several procedures have been proposed for complete cystectomy and urinary diversion in dogs [8][9][10][11], but severe side effects along with excessive cost of the procedure render cystectomy an unattractive option for the canine patient. For tumors that extend through the urethra and block urine outflow, palliative options are available, such as tumor “debulking” through transurethral resection with electrocautery/ laser or placement of a urethral stent to restore urine potency but can cause urinary incontinence [12].
As a result, combination therapy with non-steroidal anti-inflammatory drugs (NSAIDS), with or without the addition of chemotherapeutics has been the standard of care for canine MIUC (Table 1). The most commonly used NSAID has been the cyclooxygenase (COX) -inhibitor piroxicam, but other drugs in this category have also been used as well, such as meloxicam, carprofen and deracoxib [7]. Chemotherapeutics that has been used in the clinic include carboplatin, cisplatin, mitoxantrone, doxorubicin, vinblastine and gemcitabine. Despite the different therapeutic protocols tried in the clinic, dogs often become resistant to therapy and the median life expectancy for canine MIUC is approximately 105 (90–120) days with single agent therapy and 205 (180–240) days when NSAID inhibition is combined with chemotherapy [7][12].
Table 1. Established and proposed therapeutic targets in canine MIUC.
| Target | Drug |
|---|---|
| COX-1/ COX-2 | Piroxicam, Meloxicam, Carprofen |
| COX-2 | Firocoxib, Mavacoxib |
| COX- 5-LOX | Tepoxalin |
| DNA damage repair mechanisms | Cisplatin, Mitoxantrone, Doxorubicin |
| Microtubular proteins | Vinblastin |
| DNA synthesis | Gemcitabine |
| CCR4 | Mogamulizumab |
| Survivin | EZN-3042 |
| Pan- ErbB | Sapatinib |
| PDGFR, VEGFR, KIT, Flt3 | SU11654 |
| BRAF | Vemurafenib, Dabrafenib |
| Pan- RAF | LY3009120 |
| MEK | Selumetinib, Trametinib |
| ERK | SCH772984 |
| P-38 | SB239063 |
| JNK | SP600125 |
| Nectin-4 | rMV-SLAMblind |
Even though radiation therapy is a standard therapy used in humans with MIUC, it has not been used as commonly in dogs with MIUC. Besides being less clinically available than in human medicine, external beam radiotherapy caused gastrointestinal adverse effects to the pelvic region, that were ameliorated by using more finely fractionated dosing schemes or by delivering a lower total dose [13][14] as well as using intensity-modulated radiation therapy [15]. The addition of radiotherapy to piroxicam and mitoxantrone was better tolerated by the animals, but it did not add a clinical advantage to the administration of these drugs without radiotherapy [16]. A pilot study in 4 dogs with MIUC involved the use of neoadjuvant chemotherapy (gemcitabine/piroxicam), external-beam radiation and adjuvant chemotherapy (carboplatin) with promising results [17].
Finally, in contrast to human MIUC, immune checkpoint inhibition is not part of standard-of-care treatment for canine MIUC. After establishing the efficacy of numerous anti-PD-1/PD-L1 antibodies for the treatment of human malignancies (including MIUC [18]), several monoclonal antibodies were developed against canine PD-L1/PD-1 [19][20][21]. PD-L1 protein expression was detected in 100% (20/20) of canine MIUC tissues [21], so PD-L1/PD-1 blockade holds promise in canine MIUC similarly to human MIUC. Clinical immunomodulation of canine MIUC was recently examined using mogamulizumab, an anti- CC chemokine receptor 4 (CCR4) monoclonal antibody [22]. Mogamulizumab was administered in combination with piroxicam (n = 14 dogs) and compared to 14 dogs treated with piroxicam alone. Administration of mogamulizumab and piroxicam increased overall survival [474 (≥259) and 241 (108–516) respectively] and progression-free survival [189 (91–397) and 76 (21–161) respectively] in comparison to treatment with piroxicam alone, holding promise for further exploring this area of immunotherapy in canine MIUC. Overall, there is the urgent need for more effective and tolerated therapeutic options in canine MIUC.
1.2. Similarities between Canine and Human MIUC
Several factors point to using MIUC in the dog as a naturally occurring model for human MIUC: predisposing risk factors, clinical presentation, pathophysiological characteristics, genetic and epigenetic regulation, metastatic behavior and response to chemo- and immunotherapies [23][24][25][26]. Dogs are immunocompetent animals that live in the same environment as humans, come in contact with millions of antigens daily and receive multiple vaccinations starting at a very young age [27]. Dogs are exposed to cigarette smoke, pesticides, and other chemicals that are known risk factors for human BlCa [28]. Therefore, they represent a more comparable naturally occurring model to humans than immunodeficient mice. not only for conventional chemotherapeutic approaches but also as models for immunotherapy [27]. Therefore, dog BlCa reflect human BlCa in studies of environmental risk factors and can partake in clinical trials involving the use of novel therapeutics prior to starting human clinical trials.
1.3. Differences between Canine and Human MIUC
There are also differences between dog and human BlCa that need to be kept in mind. In contrast to human BlCa where most cases are represented by low-grade, superficial UC, more than 90% of canine BlCa cases are intermediate to high grade MIUC [24]. Further, MIUC in humans can be found in various locations in the bladder, predominantly on the lateral bladder walls [29], whereas tumors in dogs are mostly located in the trigone area of the bladder, usually extending through the urethra (or the prostate for male dogs) [23]. There are several theories that could explain this phenomenon. First of all, this could be attributed to the different orientation of the bladder in dogs compared to humans [30] and therefore the “pooling” of urine in the trigone area in the former. In addition, studies in rodents to identify potential stem cell niches in the bladder, showed that slow-cycling, progenitor cells (EdU retaining) were concentrated in the trigone area, close to the urethra (although found throughout the bladder) [31]. Another study showed that cells harvested from the caudal area of the bladder (including bladder neck and trigone areas), had higher proliferative and clonogenic capacity than those harvested from the cephalic area, properties that could indicate stemness. In contrast, human patients are thought to shed renal cells into the bladder—these include stem cells (called urine-derived stem cells) that can insert themselves and self-renew at different parts of the bladder [32]. There also appear to be some demographic genetic differences—for example, more female dogs suffer from BlCa compared to male dogs—whereas in human patients it is the other way around. This may be related to the fact that most male dogs are castrated at a fairly young age and therefore would not respond to hormones as human patients would, perhaps providing a protective effect [33]. Therefore, dog patients will not follow hormone dependent aspects of human MIUC such as those recently reported on [33].
Despite these differences, there are several similarities in the signaling pathways that lead to MIUC development in both canine and human patients that make the use of canines in preclinical trials for MIUC more promising. Note that canine patients will not usually model non-muscle invasive BlCa (NMIUC) prevalent in human patients as very few dogs are diagnosed with non-muscle invasive disease; however, they can serve as good models for MIUC. This review will outline the most important pathways that regulate tumor initiation and progression in dogs with UC, identifying similarities and differences with the homologous pathways in human MIUC.
2. Cell Cycle Regulation and Evasion of Apoptosis
Maintaining a balance between cell proliferation and cell death is crucial for tissue homeostasis. Signaling for cell proliferation starts with the binding of extracellular growth signals to cell membrane receptors that are then transmitted to the nucleus through signal transduction pathways. In the nucleus, phosphorylation of a cascade of cyclins/CDK complexes (Figure 1) leads to cell cycle progression and cellular proliferation under the control of the tumor suppressor genes p53 and Rb [34]. However, if the conditions for cell division are not optimal, the tumor suppressors can induce cell cycle arrest and even condemn the cells to apoptosis if normal growth conditions are not restored or if cellular defects are irreversible [35]. The inactivation of these tumor suppressor genes represent the key molecular features of MIUC [36] and have been studied in detail in human disease [37]. Comparison of the process of cell cycle progression and arrest as well as apoptosis and survival demonstrate a closely related process in canine and human MIUC.
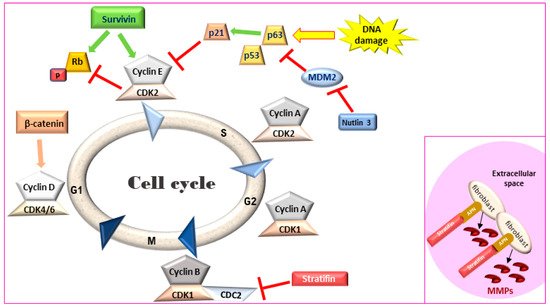
Figure 1. Cell cycle regulation. Survivin leads to an accelerated S phase and phosphorylates Rb thereby blocking its action. Stratifin blocks CDK1-Cyclin B complex causing G2/M arrest. Inset: In the extracellular space, stratifin can bind to aminopeptidase N (APN) on the plasma membrane of stromal fibroblasts and lead to the production of matrix metalloproteinases (MMPs). Rb: retinoblastoma, MDM2: mouse double minute 2 homology, CDK: cyclin-dependent kinase.
2.1. p53 Family of Proteins—The Master Regulator of Cell Cycle
p53, an extensively studied tumor-suppressor and transcription factor in cancer, is activated in response to cellular insult, stimulating transcription of genes related to cell-cycle regulation, cell-cycle arrest, repair and eventually apoptosis to prevent accumulation of damaged or malignant cells. However, in cancer, p53 is often lost or mutated and p53 mutation is correlated with advanced tumor stage and grade in human BlCa [38]. It is commonly understood that mutant p53 can function in a dominant negative manner to pervert the function of the wild-type p53 protein, which is transiently expressed in response to irradiation or other forms of DNA damage, and then rapidly degraded. Two homologues of p53, p63 and p73, belong to a family of related transcription factors [39]. In canine cancer, the role of p53, p63, and p73 was first studied in 2009 and revealed that the sequences of these transcription factors were 87%, 99.6% and 81% homologous to their human counterparts respectively [40]. Both the wild-type and the mutant form of p53 were discovered in different canine cell lines. In addition, the direct p53 target, p21 share a 80% similarity between human and canine amino acid sequence [40].
Nuclear p53 IHC expression was identified in 26% (5/19) of canine bladder tumors but not in normal bladder tissue [41]. The expression of p63 was found to be significantly lower in canine MIUC tumors than in tissues from dogs with polypoid cystitis or healthy dogs (p < 0.01). Lower expression of p63 in IHC was significantly associated with vascular infiltration (p < 0.05), presence of metastasis (p < 0.01) and shorter dog survival (p < 0.05) when compared to dogs with higher p63 expression. It was concluded that p63 could serve as biomarker for the prognosis of canine UC (Table 2) [42]. In 2018, pathway analysis by RNA-seq identified the p53 pathway to be significantly downregulated (bias-corrected z score = −2.977) in canine MIUC tumors versus normal bladder tissue [43]. Considering the importance of this pathway in tumor initiation and progression for both humans and dogs, the p53 pathway and associated mutations need to be further elucidated in canine MIUC.
Table 2. Established and potential diagnostic and prognostic biomarkers for canine MIUC.
| Biomarker | Method of Detection | Tissue/Biofluid | Function |
|---|---|---|---|
| p63 | IHC | Tumor | Prognosis |
| Survivin (nuclear) | IHC | Tumor (↑) * | Diagnosis |
| Stratifin | IHC | Diagnosis | Diagnosis |
| uroplakin | IHC | Tumor (↓) | Diagnosis |
| ELISA | Urine (↑) | ||
| FGF | ELISA | Urine (↑) | Diagnosis |
| EGFR, HER-2 | RT-qPCR | Tumor (↑) | Diagnosis |
| IHC | |||
| PDGFR-β, KIT | IHC | Tumor (↑) | Diagnosis |
| BRAFV595E | Droplet PCR | Urine (+) # | Diagnosis |
| PCR | Plasma (+) | ||
| Choline | NMR | Urine (↑) | Diagnosis |
| Urea | NMR | Urine (↑) | Diagnosis |
| Methylguanidine | NMR | Urine (↑) | Diagnosis |
| Citrate | NMR | Urine (↑) | Diagnosis |
| Acetone | NMR | Urine (↑) | Diagnosis |
| β-hydroxybutyrate | NMR | Urine (↑) | Diagnosis |
| Oleic acid | DESI-MS/ TS-MS | Tumor (↑) | Diagnosis |
| Stearic acid | DESI-MS/ TS-MS | Tumor (↓) | Diagnosis |
2.2. Evasion of Apoptosis—The Role of Survivin
Survivin, a member of the inhibitors of apoptosis protein (IAP) family, is a regulator of cell division and proliferation and a suppressor of apoptosis. When survivin translocates to the nucleus, it leads to an accelerated S phase, CDK2/cyclin E activation and Rb phosphorylation (Figure 1) whereas survivin knockdown inhibits cell proliferation in a dose-dependent manner via cell-cycle arrest at the G2/M checkpoint and leads to apoptosis [44][45]. Although adult tissues express this protein in much lower levels [46], high survivin transcriptional and protein expression has been described in rapidly proliferating normal cells both during development and in tumors. Survivin expression in IHC was detected in human MIUC tumors (78%) but not in normal bladder tissue [47][48]. Survivin expression was correlated with higher histopathological grade, disease progression and poor overall survival [47][48][49][50], with strong nuclear staining correlated with a worse clinical outcome in human BlCa patients [51]. Survivin was detected in 100% of urine samples of human patients with MIUC but not in the urine of healthy individuals. Moreover, urinary survivin levels along with liquid-based cytology provided specificity and sensitivity of over 90% for human MIUC diagnosis [52][53].
Canine survivin mRNA and protein are more than 90% homologous to the human counterparts [54]. Survivin expression and subcellular localization in canine MIUC was first assessed in 2008 between MIUC tumors and normal bladder tissue [55] as well as between MIUC tumors, cystitis and normal bladder tissue samples [56]. Rankin et al. reported that the difference in mRNA and cytoplasmic protein levels of survivin between MIUC samples and healthy controls did not reach statistical significance (p = 0.06 and p = 0.07 respectively) [55], However, 68% of MIUC samples had nuclear survivin localization that was not detected in any of the normal bladder tissue samples (p < 0.001), supporting the immunohistochemical studies in human BlCa [57]. In an additional study [56], nuclear survivin was also detected in 50% of cystitis tissues whereas cytoplasmic survivin was only detected in 8% of these tissues. In 2017, a study comparing canine tumors of different origins (epithelial, mesenchymal and round-cell tumors) showed that survivin was significantly increased in malignant versus benign tumors (p < 0.05) at the transcriptional level [58]. Finally, in 2020, the survivin inhibitor EZN-3042 was shown to be well-tolerated in a phase I clinical trial of dogs with lymphoma, opening new avenues for the clinical targeting of this protein in veterinary oncology [59].
2.3. Stratifin
Stratifin (also named 14-3-3-σ and human mammary epithelial marker) has a dual role in cancer progression. When stratifin is localized inside the cell, it acts as a negative regulator of cell-cycle progression by causing a G2/M arrest and preventing the cdc2-cyclin B1 complex from entering the nucleus, which is required for cell-cycle progression through mitosis. When stratifin is released in the extracellular space, it can bind to aminopeptidase N (APN) on the plasma membrane of stromal fibroblasts and lead to the production of matrix metalloproteinases (MMPs), a group of proteolytic enzymes that alter the extracellular matrix, promoting cancer cell invasion and metastasis (Figure 1—inset) [60]. Stratifin protein expression is downregulated in human MIUC tumors as compared to normal bladder tissue [61]. Similarly, stratifin is overexpressed in the cytoplasm and nuclei of normal urothelial cells but lost in 53% of canine UC tumors. However, some cells in the invasive front of canine MIUC tumors showed increased cytoplasmic staining for stratifin and increased p53 levels [41].
2.4. Conclusions from the Comparative Analysis of Cell Cycle and Apoptosis Pathways
The above literature demonstrates that similar pathways that regulate cell cycle progression and cell survival are affected in both dog and human bladder cancer. Given that most chemotherapeutic agents work in cells that are rapidly proliferating or are cell cycle specific, these therapies show potential for both species.
3. Identifying the Urothelial Origin of Metastatic UC Cells-Uroplakin Family of Proteins
The urinary bladder wall comprises of five layers—the serosa, muscularis, submucosa, muscularis mucosa, and lamina propria (from outside to inside) [62]. The urothelium is a stratified layer of epithelial cells that covers the lamina propria (separated from it by a basement membrane) and consists of basal and intermediate cells with a superficial layer of “umbrella” cells that line the surface (Figure 2A) [63]. The umbrella cells are characterized by a highly specialized apical plasma membrane, the asymmetric unit membrane (AUM), which is a component of the permeability barrier that protects underlying tissues from noxious components of urine. The AUM is comprised mainly of four integral membrane proteins, the uroplakins (UP) Ia, Ib, II and III that form “plaques” on the surface of urothelial cells [64]. UPIa plays an important role in uropathogenic Escherichia coli (UPEC) pathogenesis while UPII and UPIII are type-1 transmembrane proteins that heterodimerize with UPIa and UPIb, respectively. UPIa and UPII appeared to be urothelium-specific, but UPIb was detected in several non-urothelial tissues [65]. UPII is a very specific marker for the identification of cells with urothelial origin of local or metastatic malignancies, and anti-uroplakin antibodies can potentially be used both for diagnostic and therapeutic purposes [66].
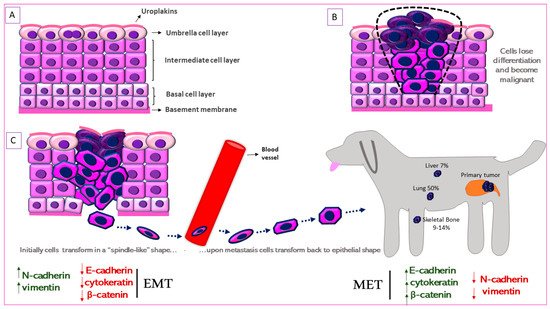
Figure 2. Schematic representation of Epithelial-to-Mesenchymal Transition (EMT) in canine MIUC. (A). Structure of normal urothelium. (B). Urothelial tumor outlined with the black dashed line. (C). EMT process in which the immotile urothelial cells upregulate mesenchymal (N-cadherin, vimentin) and downregulate epithelial (e-cadherin, cytokeratin) markers, acquire a “spindle-like” shape, become motile and infiltrate surrounding tissues and blood vessels. When they reach the metastatic site, the process is reversed (Mesenchymal-to-Epithelial Transition, MET) accompanied by the upregulation of mesenchymal and downregulation of epithelial markers. Some of the most common metastatic sites are depicted.
UPIII has a cytoplasmic domain that may function as a signal transducer. This integral membrane protein has been the gold-standard for identification of primary, anaplastic, cutaneous, subcutaneous and abdominal metastatic canine urothelial tumors [67][68][69]. Loss of tumor UPIII expression has been associated with higher tumor stage and grade and a metastatic phenotype in human BlCa (Figure 2B) [70]. On the other hand, urinary UPIII levels were significantly increased in human patients with BlCa compared to those with benign urological disease or healthy controls [71]. This indicates shedding of UPIII from the tumor into the urine. UPIII loss in BlCa is not confined to human patients but is seen in dogs with BlCa as well. Tumor classification was significantly associated with UPIII pattern (P = 1.49 × 10−18) as well as loss of UPIII (P = 1.27 × 10−4) in a study on a series of 99 canine proliferative urothelial lesions of the urinary bladder [72]. Furthermore, there were significant associations between depth of neoplastic cell infiltration into the bladder wall and overall UPIII pattern (P = 1.54 × 10−14), as well as loss of UPIII (P = 2.07 × 10−4) [72]. The expression of UPII and UPIII or loss thereof, may therefore be useful in both canine and human BlCa to identify cells of urothelial origin.
References
- Fulkerson, C.M.; Knapp, D.W. Management of Transitional Cell Carcinoma of the Urinary Bladder in Dogs: A Review. Vet. J. 2015, 205, 217–225.
- Key Statistics for Bladder Cancer. Available online: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html (accessed on 29 March 2021).
- Kent, M.S.; Zwingenberger, A.; Westropp, J.L.; Barrett, L.E.; Durbin-Johnson, B.P.; Ghosh, P.; Vinall, R.L. MicroRNA Profiling of Dogs with Transitional Cell Carcinoma of the Bladder Using Blood and Urine Samples. BMC Vet. Res. 2017, 13, 339.
- Knapp, D.W.; Glickman, N.W.; DeNicola, D.B.; Bonney, P.L.; Lin, T.L.; Glickman, L.T. Naturally-Occurring Canine Transitional Cell Carcinoma of the Urinary Bladder A Relevant Model of Human Invasive Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2000, 5, 47–59.
- Tsuboi, M.; Sakai, K.; Maeda, S.; Chambers, J.K.; Yonezawa, T.; Matsuki, N.; Uchida, K.; Nakayama, H. Assessment of HER2 Expression in Canine Urothelial Carcinoma of the Urinary Bladder. Vet. Pathol. 2019, 56, 369–376.
- Treatment of Bladder Cancer, by Stage. Available online: https://www.cancer.org/cancer/bladder-cancer/treating/by-stage.html (accessed on 28 June 2021).
- Knapp, D.W. Canine Bladder Cancer. Available online: https://www.vet.purdue.edu/pcop/files/docs/CanineUrinaryBladderCancer.pdf (accessed on 7 May 2021).
- Stone, E.A.; Withrow, S.J.; Page, R.L.; Schwarz, P.D.; Wheeler, S.L.; Seim, H.B. Ureterocolonic Anastomosis in Ten Dogs with Transitional Cell Carcinoma. Vet. Surg. VS 1988, 17, 147–153.
- Fries, C.L.; Binnington, A.G.; Valli, V.E.; Connolly, J.G.; Holmberg, D.L.; Pennock, P. Enterocystoplasty with Cystectomy and Subtotal Intracapsular Prostatectomy in the Male Dog. Vet. Surg. VS 1991, 20, 104–112.
- Saulnier-Troff, F.-G.; Busoni, V.; Hamaide, A. A Technique for Resection of Invasive Tumors Involving the Trigone Area of the Bladder in Dogs: Preliminary Results in Two Dogs. Vet. Surg. VS 2008, 37, 427–437.
- Boston, S.; Singh, A. Total Cystectomy for Treatment of Transitional Cell Carcinoma of the Urethra and Bladder Trigone in a Dog. Vet. Surg. VS 2014, 43, 294–300.
- Griffin, M.A.; Culp, W.T.N.; Rebhun, R.B. Lower Urinary Tract Neoplasia. Vet. Sci. 2018, 5, 96.
- Anderson, C.R.; McNiel, E.A.; Gillette, E.L.; Powers, B.E.; LaRue, S.M. Late Complications of Pelvic Irradiation in 16 Dogs. Vet. Radiol. Ultrasound 2002, 43, 187–192.
- Choy, K.; Fidel, J. Tolerability and Tumor Response of a Novel Low-Dose Palliative Radiation Therapy Protocol in Dogs with Transitional Cell Carcinoma of the Bladder and Urethra. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2016, 57, 341–351.
- Nolan, M.W.; Kogan, L.; Griffin, L.R.; Custis, J.T.; Harmon, J.F.; Biller, B.J.; LaRue, S.M. Intensity-Modulated and Image-Guided Radiation Therapy for Treatment of Genitourinary Carcinomas in Dogs. J. Vet. Intern. Med. 2012, 26, 987–995.
- Poirier, V.J.; Forrest, L.J.; Adams, W.M.; Vail, D.M. Piroxicam, Mitoxantrone, and Coarse Fraction Radiotherapy for the Treatment of Transitional Cell Carcinoma of the Bladder in 10 Dogs: A Pilot Study. J. Am. Anim. Hosp. Assoc. 2004, 40, 131–136.
- Marconato, L.; Nitzl, D.B.; Melzer-Ruess, K.J.; Keller, M.A.; Buchholz, J. Chemotherapy and Radiation Therapy in 4 Dogs with Muscle-Invasive Transitional Cell Carcinoma of the Urinary Tract. Can. Vet. J. 2012, 53, 875–879.
- Alhalabi, O.; Shah, A.Y.; Lemke, E.A.; Gao, J. Current and Future Landscape of Immune Checkpoint Inhibitors in Urothelial Cancer. Oncol. Williston Park N 2019, 33, 11–18.
- Choi, J.W.; Withers, S.S.; Chang, H.; Spanier, J.A.; Trinidad, V.L.D.L.; Panesar, H.; Fife, B.T.; Sciammas, R.; Sparger, E.E.; Moore, P.F.; et al. Development of Canine PD-1/PD-L1 Specific Monoclonal Antibodies and Amplification of Canine T Cell Function. PLoS ONE 2020, 15, e0235518.
- Coy, J.; Caldwell, A.; Chow, L.; Guth, A.; Dow, S. PD-1 Expression by Canine T Cells and Functional Effects of PD-1 Blockade. Vet. Comp. Oncol. 2017, 15, 1487–1502.
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H.; Kim, S.; Okagawa, T.; Izumi, Y.; et al. PD-L1 Immunohistochemistry for Canine Cancers and Clinical Benefit of Anti-PD-L1 Antibody in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Npj Precis. Oncol. 2021, 5, 1–9.
- Maeda, S.; Murakami, K.; Inoue, A.; Yonezawa, T.; Matsuki, N. CCR4 Blockade Depletes Regulatory T Cells and Prolongs Survival in a Canine Model of Bladder Cancer. Cancer Immunol. Res. 2019, 7, 1175–1187.
- Knapp, D.W.; Dhawan, D.; Ramos-Vara, J.A.; Ratliff, T.L.; Cresswell, G.M.; Utturkar, S.; Sommer, B.C.; Fulkerson, C.M.; Hahn, N.M. Naturally-Occurring Invasive Urothelial Carcinoma in Dogs, a Unique Model to Drive Advances in Managing Muscle Invasive Bladder Cancer in Humans. Front. Oncol. 2020, 9, 1493.
- Sommer, B.C.; Dhawan, D.; Ratliff, T.L.; Knapp, D.W. Naturally-Occurring Canine Invasive Urothelial Carcinoma: A Model for Emerging Therapies. Bladder Cancer Amst. Neth. 2018, 4, 149–159.
- Fulkerson, C.M.; Dhawan, D.; Ratliff, T.L.; Hahn, N.M.; Knapp, D.W. Naturally Occurring Canine Invasive Urinary Bladder Cancer: A Complementary Animal Model to Improve the Success Rate in Human Clinical Trials of New Cancer Drugs. Int. J. Genom. 2017, 2017, 6589529.
- Schiffman, J.D.; Breen, M. Comparative Oncology: What Dogs and Other Species Can Teach Us about Humans with Cancer. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20140231.
- Dow, S. A Role for Dogs in Advancing Cancer Immunotherapy Research. Front. Immunol. 2020, 10.
- John, B.A.; Said, N. Insights from Animal Models of Bladder Cancer: Recent Advances, Challenges, and Opportunities. Oncotarget 2017, 8, 57766–57781.
- Sciarra, A.; De Matteis, A.; Mariotti, G.; Voria, G.; Lucera, R.; Di Silverio, F. Histopathological Aspects of Transitional Cell Carcinoma of the Bladder: Analysis of 20 Years Experience. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2004, 11, 467–475.
- De Brot, S.; Robinson, B.D.; Scase, T.; Grau-Roma, L.; Wilkinson, E.; Boorjian, S.A.; Gardner, D.; Mongan, N.P. The Dog as an Animal Model for Bladder and Urethral Urothelial Carcinoma: Comparative Epidemiology and Histology. Oncol. Lett. 2018, 16, 1641–1649.
- Sun, W.; Wilhelmina Aalders, T.; Oosterwijk, E. Identification of Potential Bladder Progenitor Cells in the Trigone. Dev. Biol. 2014, 393, 84–92.
- Zhang, D.; Wei, G.; Li, P.; Zhou, X.; Zhang, Y. Urine-Derived Stem Cells: A Novel and Versatile Progenitor Source for Cell-Based Therapy and Regenerative Medicine. Genes Dis. 2014, 1, 8–17.
- Katleba, K.; Lombard, A.P.; Tsamouri, M.-M.; Baek, H.B.; Nishida, K.S.; Libertini, S.J.; Platero, A.J.; Ma, A.-H.; Pan, C.-X.; Ghosh, P.M.; et al. Depletion of Androgen Receptor Low Molecular Weight Isoform Reduces Bladder Tumor Cell Viability and Induces Apoptosis. Cancer Lett. 2021, 504, 49–57.
- Mitra, A.P.; Datar, R.H.; Cote, R.J. Molecular Pathways in Invasive Bladder Cancer: New Insights Into Mechanisms, Progression, and Target Identification. J. Clin. Oncol. 2006, 24, 5552–5564.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674.
- Goebell, P.J.; Knowles, M.A. Bladder Cancer or Bladder Cancers? Genetically Distinct Malignant Conditions of the Urothelium. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 409–428.
- Mitra, A.P.; Hansel, D.E.; Cote, R.J. Prognostic Value of Cell-Cycle Regulation Biomarkers in Bladder Cancer. Semin. Oncol. 2012, 39, 524–533.
- Wu, G.; Wang, F.; Li, K.; Li, S.; Zhao, C.; Fan, C.; Wang, J. Significance of TP53 Mutation in Bladder Cancer Disease Progression and Drug Selection. PeerJ 2019, 7, e8261.
- Zhang, J.; Chen, X. P53 Tumor Suppressor and Iron Homeostasis. FEBS J. 2019, 286, 620–629.
- Zhang, J.; Chen, X.; Kent, M.S.; Rodriguez, C.O.; Chen, X. Establishment of a Dog Model for the P53 Family Pathway and Identification of a Novel Isoform of P21 Cyclin-Dependent Kinase Inhibitor. Mol. Cancer Res. MCR 2009, 7, 67–78.
- Suárez-Bonnet, A.; Herráez, P.; Aguirre, M.; Suárez-Bonnet, E.; Andrada, M.; Rodríguez, F.; Espinosa de Los Monteros, A. Expression of Cell Cycle Regulators, 14-3-3σ and P53 Proteins, and Vimentin in Canine Transitional Cell Carcinoma of the Urinary Bladder. Urol. Oncol. 2015, 33, 332.e1–332.e7.
- Hanazono, K.; Nishimori, T.; Fukumoto, S.; Kawamura, Y.; Endo, Y.; Kadosawa, T.; Uchide, T. Immunohistochemical Expression of P63, Ki67 and β-Catenin in Canine Transitional Cell Carcinoma and Polypoid Cystitis of the Urinary Bladder. Vet. Comp. Oncol. 2016, 14, 263–269.
- Maeda, S.; Tomiyasu, H.; Tsuboi, M.; Inoue, A.; Ishihara, G.; Uchikai, T.; Chambers, J.K.; Uchida, K.; Yonezawa, T.; Matsuki, N. Comprehensive Gene Expression Analysis of Canine Invasive Urothelial Bladder Carcinoma by RNA-Seq. BMC Cancer 2018, 18, 472.
- Li, Y.; Liu, D.; Zhou, Y.; Li, Y.; Xie, J.; Lee, R.J.; Cai, Y.; Teng, L. Silencing of Survivin Expression Leads to Reduced Proliferation and Cell Cycle Arrest in Cancer Cells. J. Cancer 2015, 6, 1187–1194.
- Suzuki, A.; Hayashida, M.; Ito, T.; Kawano, H.; Nakano, T.; Miura, M.; Akahane, K.; Shiraki, K. Survivin Initiates Cell Cycle Entry by the Competitive Interaction with Cdk4/P16 INK4a and Cdk2/Cyclin E Complex Activation. Oncogene 2000, 19, 3225–3234.
- Pennati, M.; Folini, M.; Zaffaroni, N. Targeting Survivin in Cancer Therapy: Fulfilled Promises and Open Questions. Carcinogenesis 2007, 28, 1133–1139.
- Makboul, R.; Refaiy, A.E.-R.M.; Badary, F.A.M.; Abdelkawi, I.F.; Merseburger, A.S.; Mohammed, R.A.A. Expression of Survivin in Squamous Cell Carcinoma and Transitional Cell Carcinoma of the Urinary Bladder: A Comparative Immunohistochemical Study. Korean J. Urol. 2015, 56, 31–40.
- Swana, H.S.; Grossman, D.; Anthony, J.N.; Weiss, R.M.; Altieri, D.C. Tumor Content of the Antiapoptosis Molecule Survivin and Recurrence of Bladder Cancer. N. Engl. J. Med. 1999, 341, 452–453.
- Chen, H.-A.; Su, C.-M.; Hsieh, H.-Y.; Tung, C.-L.; Hsu, C.-D.; Wang, Y.-H.; Shen, C.-H. Clinical Significance of Survivin Expression in Patients with Urothelial Carcinoma. Dis. Markers 2014, 2014, 574985.
- Jeon, C.; Kim, M.; Kwak, C.; Kim, H.H.; Ku, J.H. Prognostic Role of Survivin in Bladder Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8.
- Skagias, L.; Politi, E.; Karameris, A.; Sambaziotis, D.; Archondakis, A.; Ntinis, A.; Moreas, I.; Vasou, O.; Koutselini, H.; Patsouris, E. Survivin Expression as a Strong Indicator of Recurrence in Urothelial Bladder Cancer. Predictive Value of Nuclear versus Cytoplasmic Staining. Anticancer Res. 2009, 29, 4163–4167.
- Xu, X.; Li, P.; Fu, D.; Wei, Z.; Xu, S.; Xu, F.; Tian, F.; Ge, J.; Zhang, Z.; Cheng, W. Combined Use of Urinary Survivin Detection and Liquid-Based Cytology for the Early Diagnosis of Bladder Urothelial Carcinoma. Oncol. Lett. 2018, 15, 7739–7743.
- Smith, S.D.; Wheeler, M.A.; Plescia, J.; Colberg, J.W.; Weiss, R.M.; Altieri, D.C. Urine Detection of Survivin and Diagnosis of Bladder Cancer. JAMA 2001, 285, 324–328.
- Uchide, T.; Takatsu, N.; Fujimori, Y.; Fukushima, U.; Itoh, H. Expression of Survivin MRNA in Dog Tumors. DNA Seq. J. DNA Seq. Mapp. 2005, 16, 329–334.
- Rankin, W.V.; Henry, C.J.; Turnquist, S.E.; Turk, J.R.; Beissenherz, M.E.; Tyler, J.W.; Rucker, E.B.; Knapp, D.W.; Rodriguez, C.O.; Green, J.A. Identification of Survivin, an Inhibitor of Apoptosis, in Canine Urinary Bladder Transitional Cell Carcinoma*. Vet. Comp. Oncol. 2008, 6, 141–150.
- Rankin, W.V.; Henry, C.J.; Turnquist, S.E.; Turk, J.R.; Beissenherz, M.E.; Tyler, J.W.; Green, J.A. Comparison of Distributions of Survivin among Tissues from Urinary Bladders of Dogs with Cystitis, Transitional Cell Carcinoma, or Histologically Normal Urinary Bladders. Am. J. Vet. Res. 2008, 69, 1073–1078.
- Lehner, R.; Lucia, M.S.; Jarboe, E.A.; Orlicky, D.; Shroyer, A.L.; McGregor, J.A.; Shroyer, K.R. Immunohistochemical Localization of the IAP Protein Survivin in Bladder Mucosa and Transitional Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. AIMM 2002, 10, 134–138.
- Kavya, N.; Rao, S.; Sathyanarayana, M.L.; Narayanaswamy, H.D.; Byregowda, S.M.; Ranganath, L.; Kamaran, A.; Purushotham, K.M.; Kishore, T.K. Survivin Expression in Canine Spontaneous Cutaneous and Subcutaneous Tumors and Its Prognostic Importance. Vet. World 2017, 10, 1286–1291.
- Thamm, D.H.; Joseph, J.K.; Rose, B.J.; Meuten, T.K.; Weishaar, K.M. Phase-I Trial of Survivin Inhibition with EZN-3042 in Dogs with Spontaneous Lymphoma. BMC Vet. Res. 2020, 16, 97.
- Ghaffari, A.; Li, Y.; Kilani, R.T.; Ghahary, A. 14-3-3σ Associates with Cell Surface Aminopeptidase N in the Regulation of Matrix Metalloproteinase-1. J. Cell Sci. 2010, 123, 2996–3005.
- Moreira, J.M.A.; Gromov, P.; Celis, J.E. Expression of the Tumor Suppressor Protein 14-3-3σ Is down-Regulated in Invasive Transitional Cell Carcinomas of the Urinary Bladder Undergoing Epithelial-to-Mesenchymal Transition. Mol. Cell. Proteomics 2004, 3, 410–419.
- Lewis, S.A. Everything You Wanted to Know about the Bladder Epithelium but Were Afraid to Ask. Am. J. Physiol. Renal Physiol. 2000, 278, F867–F874.
- Bolla, S.R.; Odeluga, N.; Jetti, R. Histology, Bladder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Wu, X.-R.; Kong, X.-P.; Pellicer, A.; Kreibich, G.; Sun, T.-T. Uroplakins in Urothelial Biology, Function, and Disease. Kidney Int. 2009, 75, 1153–1165.
- Olsburgh, J.; Harnden, P.; Weeks, R.; Smith, B.; Joyce, A.; Hall, G.; Poulsom, R.; Selby, P.; Southgate, J. Uroplakin Gene Expression in Normal Human Tissues and Locally Advanced Bladder Cancer. J. Pathol. 2003, 199, 41–49.
- Gruver, A.M.; Amin, M.B.; Luthringer, D.J.; Westfall, D.; Arora, K.; Farver, C.F.; Osunkoya, A.O.; McKenney, J.K.; Hansel, D.E. Selective Immunohistochemical Markers to Distinguish Between Metastatic High-Grade Urothelial Carcinoma and Primary Poorly Differentiated Invasive Squamous Cell Carcinoma of the Lung. Arch. Pathol. Lab. Med. 2012, 136, 1339–1346.
- Ramos-Vara, J.A.; Miller, M.A.; Boucher, M.; Roudabush, A.; Johnson, G.C. Immunohistochemical Detection of Uroplakin III, Cytokeratin 7, and Cytokeratin 20 in Canine Urothelial Tumors. Vet. Pathol. 2003, 40, 55–62.
- Reed, L.T.; Knapp, D.W.; Miller, M.A. Cutaneous Metastasis of Transitional Cell Carcinoma in 12 Dogs. Vet. Pathol. 2013, 50, 676–681.
- Higuchi, T.; Burcham, G.N.; Childress, M.O.; Rohleder, J.J.; Bonney, P.L.; Ramos-Vara, J.A.; Knapp, D.W. Characterization and Treatment of Transitional Cell Carcinoma of the Abdominal Wall in Dogs: 24 Cases (1985–2010). J. Am. Vet. Med. Assoc. 2013, 242, 499–506.
- Matsumoto, K.; Satoh, T.; Irie, A.; Ishii, J.; Kuwao, S.; Iwamura, M.; Baba, S. Loss Expression of Uroplakin III Is Associated with Clinicopathologic Features of Aggressive Bladder Cancer. Urology 2008, 72, 444–449.
- Lai, Y.; Ye, J.; Chen, J.; Zhang, L.; Wasi, L.; He, Z.; Zhou, L.; Li, H.; Yan, Q.; Gui, Y.; et al. UPK3A: A Promising Novel Urinary Marker for the Detection of Bladder Cancer. Urology 2010, 76, 514.e6–514.e11.
- Sledge, D.G.; Patrick, D.J.; Fitzgerald, S.D.; Xie, Y.; Kiupel, M. Differences in Expression of Uroplakin III, Cytokeratin 7, and Cyclooxygenase-2 in Canine Proliferative Urothelial Lesions of the Urinary Bladder. Vet. Pathol. 2015, 52, 74–82.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
27 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




