
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chiara Bruckmann | + 2294 word(s) | 2294 | 2021-10-19 04:51:03 | | | |
| 2 | Chiara Bruckmann | -3 word(s) | 2291 | 2021-10-26 16:41:05 | | | | |
| 3 | Jason Zhu | -1247 word(s) | 1044 | 2021-10-27 04:02:59 | | |
Video Upload Options
MEIS1 is highly expressed in the bone marrow, and its predominant and better-known role is in embryonic and adult hematopoiesis. Along withPBX1 and HOXA9, MEIS1 is expressed in hematopoietic stem cells (HSCs) but downregulated during their differentiation.
1. Introduction
Homeobox transcription factor MEIS1 belongs to the three-amino acids loop extension (TALE) sub-family of homeobox proteins. MEIS1 was discovered because a retroviral integration close to its regulatory region induced murine myeloid leukemia[1]. Since then, the roles of MEIS1 in development, cell regulation, and transformation have been extensively investigated. MEIS1 is highly expressed in the bone marrow, and its predominant and better-known role is in embryonic and adult hematopoiesis[2][3][4][5]. Along with PBX1 and HOXA9 , MEIS1 is expressed in hematopoietic stem cells but downregulated during their differentiation[5][6]. MEIS1 regulates growth and differentiation during vertebrate development and is widely expressed in the central nervous system and periphery, both during embryogenesis and after birth [3][4]. During embryo development, Meis1 ablation results in hematopoietic, vascular, and ocular defects. It has been also well defined the role of Meis1 and Meis2 in mouse axial skeleton formation[7][8][9] and in anterior-posterior patterning for limb initiation[10], for which Meis loss-of-function (Meis1 and Meis2 double knock-out) embryos are limbless, with undeveloped appendicular condensations. A direct connection between MEIS1 expression, leukemia, and cancer has been established from the start[1].
2. PREP1 and MEIS1
One of the properties of TALE proteins is to interact with each other. Previous studies have defined that the primary binding partner of MEIS1 is PBX (another TALE protein), of which exist four different genes[11][12]. MEIS1 and PBX form a stable heterodimer that cooperatively binds DNA[13][14]. In particular, the N-terminal domain of MEIS1, the MEINOX domain, also known as the Homology Region (HR), serves as the interaction surface with PBX. There is an additional member of the TALE family, pKNOX1, better known as PREP1 (for PBX-regulating Regulating Protein 1), which also binds DNA as heterodimer with PBX[14][15][16][17][18]. MEIS1-PBX1 and PREP1-PBX1 dimers can bind DNA and form trimeric DNA-binding complexes with HOX proteins. During embryonic stages, trimerization of MEIS1/PREP1 with PBX1 and HOXB1 is necessary to express several HOX cluster genes, playing crucial roles in development and hematopoiesis [5][19][20][22][21]. In human leukemia, the cooperation between MEIS1 and HOXA9 with PBX has been extensively reported[23][24][25][26][27]; specifically, the PBX3 form appears to be the prevalent form involved[28]. The interaction of MEIS1 and PREP1 with PBX may lead to overlapping functions as they both share their interaction and bind to DNA target sequences that are very similar, although not identical[29]. On the other hand, the two proteins may compete for PBX, and hence may have opposite functions. These possibilities have been verified: for example, in zebrafish, prep1.1 and meis1 have partially overlapping roles in hox genes regulation[22][30]. Mixed Lineage Leukemia, a leukemia due to translocation of several genes to the amino terminus of the MLL gene[30][31][32][33], appears to be mainly dependent on the overexpression of MEIS1. In PBX-MEIS1-induced human leukemia and in MEIS1-dependent mouse tumorigenesis [28][34], HOXA9 appears to have an accelerating role[35].
3. The MEIS1-PBX1 and the PREP1-PBX Interaction Surface
Like PREP1, MEIS1 requires dimerization with PBX to reach the nucleus. In MEIS1, researchers have mutated into alanine the potential hydrophobic residues corresponding to those identified in PREP1 being crucial for the interaction with PBX [36][37]: in the HR1 L83/L86/L87/I90 and in the HR2 L149/I152/L156/L159 (see Figure 1 , for a comparison of PREP1 and MEIS1 heptad repeats).
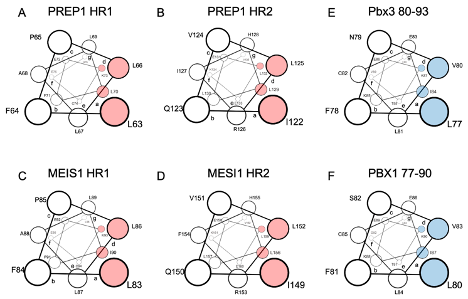
Figure 1. Schematic representation in a top view perspective of the alpha-helices of PREP1 and MEIS1 involved in the leucine zipper with PBX1. For MEIS1 and PREP1, the residues in positions “a” and “d” of the alpha helix, crucial for the interaction with PBX1, are highlighted in red for Pbx3 and PBX1; the important residues for binding MEIS1 and PREP1 are highlighted in blue. Panel (A): PREP1 HR1; panel (B): PREP1 HR2; panel (C): Meis1 HR1; panel (D): Meis1 HR2; panel (E): Pbx3 80-93; panel (F): PBX1 77-90. HR stands for “Homology Region”.
The subcellular localization of the overexpressed wild-type and mutants (L83/L86/L87/I90 and L149/I152/L156/L159) MEIS1 was then determined by immunofluorescence: while wild-type MEIS1 completely localize in the nucleus; as for PREP1, neither of the two MEIS1 quadruple mutants MEIS1 L83A/L86A/L87A/I90A or MEIS1 I149A/L152A/L156A/L159A could efficiently translocate to the nucleus[37].
Previous research reported that the inhibition of the PBX interaction can neutralize MEIS1 oncogenic properties [28][38][39]. It has also been already described that a Meis1 mutated in its HR2 domain could not cooperate with Hoxa9 in colony-forming assays or in in vivo leukemia assays[28]. Likely, this can be explained by the inability of the Meis1 mutant to access the nucleus, as shown for the MEIS1 I149A/L152A/L156A/L159A mutant in. Indeed, pull-down experiments, have confirmed that both MEIS1 L83A/L86A/L87A/I90A and L149/ I152/L156/L159 mutants are unable to bind PBX1, as compared to wild-type MEIS1.
4. Future Perspectives and Challenges: Targeting MEIS1/PBX Interface for AML
Transcription factor MEIS1 drives myeloid leukemogenesis in the context of HOX gene overexpression; however, MEIS1 is still considered a challenging transcription factor to target, for the absence of structural details, besides the homeodomain[40]. Nevertheless, the direct targeting of MEIS1 might provide further avenues for inhibiting the MEIS1/HOX-mediated leukemic transcription program. For example, the recent detailed knowledge of the molecular interaction between TALE oncogenic transcription factors allows the design of novel therapeutics, such as small peptide inhibitors. It would be more convenient to target MEIS1 before it reaches the nucleus, i.e., while or immediately after it has been synthesized. Newly synthesized MEIS1 or PREP1 immediately dimerizes with PBX, forming a stable complex that is then able to enter the nucleus and, in addition, acquire the ability to bind DNA. Therefore, preventing the formation of a MEIS1-PBX dimer would have a dual effect: not only the prevention of DNA binding before but also not allowing the localization of MEIS1-PBX to the nucleus. The MEIS1-PBX interaction domain was identified a long time ago, but only recently, due to its homology to the PREP1-PBX1 interaction domain, were the essential residues identified in detail.
References
- Moskow, J.J.; Bullrich, F.; Huebner, K.; Daar, I.O.; Buchberg, A.M. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol Cell Biol 1995, 15, 5434-5443, doi:10.1128/mcb.15.10.5434.
- Imamura, T.; Morimoto, A.; Takanashi, M.; Hibi, S.; Sugimoto, T.; Ishii, E.; Imashuku, S. Frequent co-expression of HoxA9 and Meis1 genes in infant acute lymphoblastic leukaemia with MLL rearrangement. Br J Haematol 2002, 119, 119-121, doi:10.1046/j.1365-2141.2002.03803.x.
- Azcoitia, V.; Aracil, M.; Martínez, A.C.; Torres, M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol 2005, 280, 307-320, doi:10.1016/j.ydbio.2005.01.004.
- Hisa, T.; Spence, S.E.; Rachel, R.A.; Fujita, M.; Nakamura, T.; Ward, J.M.; Devor‐Henneman, D.E.; Saiki, Y.; Kutsuna, H.; Tessarollo, L.; et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. The EMBO Journal 2004, 23, 450-459, doi:10.1038/sj.emboj.7600038.
- Pineault, N.; Helgason, C.D.; Lawrence, H.J.; Humphries, R.K. Differential expression of Hox, Meis1, and Pbx1 genes in primitive cells throughout murine hematopoietic ontogeny. Experimental hematology 2002, 30, 49-57, doi:10.1016/s0301-472x(01)00757-3.
- Moens, C.B.; Selleri, L. Hox cofactors in vertebrate development. Developmental Biology 2006, 291, 193-206.
- López-Delgado, A.C.; Delgado, I.; Cadenas, V.; Sánchez-Cabo, F.; Torres, M. Axial skeleton anterior-posterior patterning is regulated through feedback regulation between Meis transcription factors and retinoic acid. Development 2021, 148, doi:10.1242/dev.193813.
- Salsi, V.; Vigano, M.A.; Cocchiarella, F.; Mantovani, R.; Zappavigna, V. Hoxd13 binds in vivo and regulates the expression of genes acting in key pathways for early limb and skeletal patterning. Developmental Biology 2008, 317, 497-507, doi: 10.1016/j.ydbio.2008.02.048.
- Mercader, N.; Leonardo, E.; Azpiazu, N.; Serrano, A.; Morata, G.; Martínez, C.; Torres, M. Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature 1999, 402, 425-429, doi:10.1038/46580.
- Delgado, I.; Giovinazzo, G.; Temiño, S.; Gauthier, Y.; Balsalobre, A.; Drouin, J.; Torres, M. Control of mouse limb initiation and antero-posterior patterning by Meis transcription factors. Nat Commun 2021, 12, 3086, doi:10.1038/s41467-021-23373-9.
- Monica, K.; Galili, N.; Nourse, J.; Saltman, D.; Cleary, M.L. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol Cell Biol 1991, 11, 6149-6157, doi:10.1128/mcb.11.12.6149-6157.1991.
- Wagner, K.; Mincheva, A.; Korn, B.; Lichter, P.; Pöpperl, H. Pbx4, a new Pbx family member on mouse chromosome 8, is expressed during spermatogenesis. Mechanisms of development 2001, 103, 127-131, doi:10.1016/s0925-4773(01)00349-5.
- Chang, C.P.; Brocchieri, L.; Shen, W.F.; Largman, C.; Cleary, M.L. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol Cell Biol 1996, 16, 1734-1745, doi:10.1128/mcb.16.4.1734.
- Longobardi, E.; Penkov, D.; Mateos, D.; De Florian, G.; Torres, M.; Blasi, F. Biochemistry of the tale transcription factors PREP, MEIS, and PBX in vertebrates. Developmental Dynamics 2014, 243, 59-75
- Burglin, T.R. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic acids research 1997, 25, 4173-4180.
- Burglin, T.R. The PBC domain contains a MEINOX domain: coevolution of Hox and TALE homeobox genes? Development genes and evolution 1998, 208, 113-116.
- Mukherjee, K.; Burglin, T.R. Comprehensive analysis of animal TALE homeobox genes: new conserved motifs and cases of accelerated evolution. Journal of molecular evolution 2007, 65, 137-153.
- Blasi, F.; Bruckmann, C.; Penkov, D.; Dardaei, L. A tale of TALE, PREP1, PBX1, and MEIS1: Interconnections and competition in cancer. Bioessays 2017, 39, doi:10.1002/bies.201600245.
- Huang, Y.; Sitwala, K.; Bronstein, J.; Sanders, D.; Dandekar, M.; Collins, C.; Robertson, G.; MacDonald, J.; Cezard, T.; Bilenky, M.; et al. Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood 2012, 119, 388-398, doi:10.1182/blood-2011-03-341081.
- Merabet, S.; Mann, R. To be specific or not: the critical relationship between Hox and TALE proteins. Trends in genetics : TIG 2016, 32, 334-347, doi:10.1016/j.tig.2016.03.004.
- Dard, A.; Jia, Y.; Reboulet, J.; Bleicher, F.; Lavau, C.; Merabet, S. The human HOXA9 protein uses paralog-specific residues of the homeodomain to interact with TALE-class cofactors. Scientific reports 2019, 9, 5664, doi:10.1038/s41598-019-42096-y.
- Deflorian, G.; Tiso, N.; Ferretti, E.; Meyer, D.; Blasi, F.; Bortolussi, M.; Argenton, F. Prep1.1 has essential genetic functions in hindbrain development and cranial neural crest cell differentiation. Development 2004, 131, 613-627, doi:10.1242/dev.00948.
- Thorne, R.M.W.; Milne, T.A. Dangerous liaisons: cooperation between Pbx3, Meis1 and Hoxa9 in leukemia. Haematologica 2015, 100, 850-853, doi:10.3324/haematol.2015.129932.
- Shen, W.F.; Rozenfeld, S.; Kwong, A.; Köm ves, L.G.; Lawrence, H.J.; Largman, C. HOXA9 forms triple complexes with PBX2 and MEIS1 in myeloid cells. Mol Cell Biol 1999, 19, 3051-3061, doi:10.1128/mcb.19.4.3051.
- Schnabel, C.A.; Jacobs, Y.; Cleary, M.L. HoxA9-mediated immortalization of myeloid progenitors requires functional interactions with TALE cofactors Pbx and Meis. Oncogene 2000, 19, 608-616, doi:10.1038/sj.onc.1203371.
- Collins, C.T.; Hess, J.L. Deregulation of the HOXA9/MEIS1 Axis in Acute Leukemia. Current opinion in hematology 2016, 23, 354-361, doi:10.1097/MOH.0000000000000245.
- Krivtsov, A.V.; Twomey, D.; Feng, Z.; Stubbs, M.C.; Wang, Y.; Faber, J.; Levine, J.E.; Wang, J.; Hahn, W.C.; Gilliland, D.G.; et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 2006, 442, 818-822, doi:10.1038/nature04980.
- Garcia-Cuellar, M.-P.; Steger, J.; Füller, E.; Hetzner, K.; Slany, R.K. Pbx3 and Meis1 cooperate through multiple mechanisms to support Hox-induced murine leukemia. Haematologica 2015, 100, 905-913, doi:10.3324/haematol.2015.124032.
- Penkov, D.; Mateos San Martin, D.; Fernandez-Diaz, L.C.; Rossello, C.A.; Torroja, C.; Sanchez-Cabo, F.; Warnatz, H.J.; Sultan, M.; Yaspo, M.L.; Gabrieli, A.; et al. Analysis of the DNA-binding profile and function of TALE homeoproteins reveals their specialization and specific interactions with Hox genes/proteins. Cell Rep 2013, 3, 1321-1333, doi:10.1016/j.celrep.2013.03.029.
- Ladam, F.; Stanney, W.; Donaldson, I.J.; Yildiz, O.; Bobola, N.; Sagerström, C.G. TALE factors use two distinct functional modes to control an essential zebrafish gene expression program. Elife 2018, 7, doi:10.7554/eLife.36144.
- Slany, R.K. The molecular biology of mixed lineage leukemia. Haematologica 2009, 94, 984-993, doi:10.3324/haematol.2008.002436.
- Muntean, A.G.; Hess, J.L. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol 2012, 7, 283-301, doi:10.1146/annurev-pathol-011811-132434.
- Meyer, C.; Burmeister, T.; Gröger, D.; Tsaur, G.; Fechina, L.; Renneville, A.; Sutton, R.; Venn, N.C.; Emerenciano, M.; Pombo-de-Oliveira, M.S.; et al. The MLL recombinome of acute leukemias in 2017. Leukemia 2018, 32, 273-284, doi:10.1038/leu.2017.213.
- Dardaei, L.; Modica, L.; Iotti, G.; Blasi, F. The deficiency of tumor suppressor prep1 accelerates the onset of meis1- hoxa9 leukemogenesis. PloS one 2014, 9, e96711, doi:10.1371/journal.pone.0096711.
- Kroon, E.; Krosl, J.; Thorsteinsdottir, U.; Baban, S.; Buchberg, A.M.; Sauvageau, G. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. The EMBO Journal 1998, 17, 3714-3725, doi:10.1093/emboj/17.13.3714.
- Bruckmann, C.; Tamburri, S.; De Lorenzi, V.; Doti, N.; Monti, A.; Mathiasen, L.; Cattaneo, A.; Ruvo, M.; Bachi, A.; Blasi, F. Mapping the native interaction surfaces of PREP1 with PBX1 by cross-linking mass-spectrometry and mutagenesis. Scientific reports 2020, 10, 16809, doi:10.1038/s41598-020-74032-w.
- Blasi, F.; Bruckmann, C. MEIS1 in Hematopoiesis and Cancer. How MEIS1-PBX Interaction Can Be Used in Therapy. Journal of Developmental Biology 2021, 9, 44.
- Dardaei, L.; Longobardi, E.; Blasi, F. Prep1 and Meis1 competition for Pbx1 binding regulates protein stability and tumorigenesis. Proc Natl Acad Sci U S A 2014, 111, E896-905, doi:10.1073/pnas.1321200111.
- Mamo, A.; Krosl, J.; Kroon, E.; Bijl, J.; Thompson, A.; Mayotte, N.; Girard, S.; Bisaillon, R.; Beslu, N.; Featherstone, M.; et al. Molecular dissection of Meis1 reveals 2 domains required for leukemia induction and a key role for Hoxa gene activation. Blood 2006, 108, 622-629, doi:10.1182/blood-2005-06-2244.
- Jolma, A.; Yin, Y.; Nitta, K.R.; Dave, K.; Popov, A.; Taipale, M.; Enge, M.; Kivioja, T.; Morgunova, E.; Taipale, J. DNA-dependent formation of transcription factor pairs alters their binding specificity. Nature2015, 527, 384-388, doi:10.1038/nature15518.




