
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shih-Hsuan Chan | + 2129 word(s) | 2129 | 2021-10-25 08:06:54 | | | |
| 2 | Nora Tang | Meta information modification | 2129 | 2021-10-27 04:31:58 | | |
Video Upload Options
Hepatocellular carcinoma is the most prevalent form of liver cancer in the world. Annually, HCC affects approximately 900,000 individuals, and over 70% of new cases are diagnosed in Asia. The etiology of HCC is complicated due to the multiple risk factors involved.
1. Introduction
The etiology of HCC is complicated due to the multiple risk factors involved [1]. HCC usually arises from a background of chronic liver disease caused by alcohol abuse, metabolic syndrome, hepatitis B or C infections and/or aflatoxin exposure that eventually scars the liver parenchyma, leading to the irreversible condition of liver cirrhosis and the subsequent development of HCC [2]. Most HCC patients are diagnosed with advanced disease, and the majority of them are unresectable due to the early dissemination of cancer cells inside the liver. HCC tends to grow near blood vessels such as the portal vein or hepatic vein, which makes surgery an impossible task. α-Fetoprotein (AFP) is commonly used as a serum biomarker for early detection of HCC and also for evaluation of the prognosis and monitoring of response to therapy [3][4]. Systemic chemotherapy for many years is ineffective and therefore not an option for HCC, due to the highly resistant nature of HCC. Locoregional therapies including percutaneous ablation, transarterial chemoembolization (TACE) and radioembolization serve as the main alternative therapies, but largely depend on tumor location, burden and other complications [5]. Ablation is the first line option over surgery for unresectable HCC; however, treatment outcome is still disappointing and recurrence is often seen. In 2008, a tyrosine kinase inhibitor (TKIs), sorafenib, was the first approved targeted therapy to be used as a first-line drug to treat advanced HCC [6]. Subsequently, three additional TKIs, lenvatinib, regorafenib and cabozantinib, have been approved and become available for use in first-line and second-line settings and found to provide beneficial effects and prolonged survival [7][8]. Despite of the application of TKI therapy, median survival with advanced HCC remains unsatisfactory at less than two years [5][9]. Recently, ramucirumab, a therapeutic monoclonal antibody drug that acts against vascular endothelial growth factor (VEGF) receptor 2, has shown significant survival benefits in patients with increased AFP (>400 ng/mL) after sorafenib [10]. A VEGF-neutralizing antibody, bevacinumab, has also proven effective in a single-agent phase II trial in HCC patients. However, serious bleeding complications were observed in 11% [11].
Immune surveillance plays an important role in identifying and eliminating normal cells that become malignant. As when fighting invading pathogens, the innate and adaptive immune systems work together to form an anti-tumor army to destroy cancer cells. However, the interplay between cancer cells, stromal cells, and infiltrating immune cells eventually creates an immunosuppressive tumor microenvironment (TME) that leads to immune evasion, which was previously considered impossible to reverse. There are two main aspects explaining the formation of an immunosuppressive TME. First, as HCC progresses, cancer cells recruit immune cells such as myeloid-derived suppressor cells (MDSCs) or M2 tumor-associated macrophages (TAMs) and FoxP3 + regulatory T cells (T reg ), which are known to help the tumor grow better, by secreting chemokines, cytokines and growth factors to form a tumor-promoting niche. As a result, the accumulation of tumor-promoting immunes cells eventually comprises an area of anti-tumor immunity. Second, upregulation of co-inhibitory molecules such as immune checkpoint ligand PDL1 and increased expression of tolerance-related enzymes such as indoleamine 2,3-deoxygenase (IDO) and arginase-1 in cancer cells or tumor-infiltrating immune cells also contribute to the formation of immunosuppressive TME. In addition, downregulation of tumor-associated antigens (TAAs), also known as tumor antigen escape [12][13], and reduced recognition of TAAs by immune cells through alterations in the antigen-processing machinery both play a significant role in the promotion of tumor progression [14]. Therefore, immunotherapies aiming to reverse and overcome the immunosuppressive TME in order to effectively enhance the activity of tumor-killing immune cells point out the future direction of HCC therapy.
2. Tumor Microenvironment of HCC
The liver is the organ responsible for the detoxification of gut-derived blood and systemic circulation. Therefore, the frequent exposure of liver cells to food antigens and to microbial products generated by gut bacteria shapes the dynamic complexities of a liver microenvironment that fosters immune tolerance [15]. This tolerogenic milieu is maintained by liver antigen-presenting cells (APCs), including resident kuffer cells (KCs), sinusoidal endothelial cells (SECs) and stellate cells (SCs), through the secretion of an array of immunosuppressive cytokines, chemokines and growth factors [16]. Kuffer cells are known to express immunosuppressive cytokine IL-10 and IDO, and prostaglandins to promote T reg activation [17][18]. Myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs) and regulatory T cells also produce IL-10 to attenuate the ability of APCs to stimulate T cells and to promote PDL1 expression in monocytes [19]. TGF-β is a well-known soluble factor that attenuates the anti-tumor response by inhibiting the activation of dendritic cells (DCs) [20] and polarizing macrophages towards the M2 phenotype [21], as well as inducing T reg cell activation [22]. In HCC TME, TGF-β is mainly produced by cancer cells, T reg cells and macrophages [15]. High serum TGF-β has been linked to poor prognosis of patients with HCC after sorafenib treatment [23]. SCs-derived hepatocyte growth factor (HGF) also promotes the infiltration and accumulation of MDSCs and T reg cells inside the TME [24][25]. Vascular endothelial growth factor (VEGF) produced by cancer cells or MDSCs plays an essential role in promoting angiogenesis, leading to the formation of an abnormal tumor vasculature that not only serves as a barrier for cytotoxic T lymphocytes (CTLs) but disables them by expressing PDL1 and Fas ligand [26][27][28]. Aside from its role in inducing abnormal tumor vasculature, Courau, et al. showed that VEGF and TGF-β could cooperatively foster immunotolerant TME by blunting the antigen-presenting functions of DC and generating MDSCs [29].
3. Current and Ongoing Strategies of Immune Checkpoint Blockade for HCC
Co-inhibitory molecules expressed by the effector lymphocytes fall into a category of immune checkpoint which serves the purpose of preventing overactivation of lymphocytes upon the engagement of APCs like DCs or macrophages [30]. To evade immune surveillance, tumor cells exploit this mechanism to express the corresponding ligands of co-inhibitory molecules to blunt effector T cells or macrophages. Program death 1 (PD1), cytotoxic T lymphocyte-associated antigen 4 (CTLA4) , T cell immunoglobulin and mucin domain containing-3 (TIM3), lymphocyte-activation gene 3 (LAG3), and siglec-10 are among the most extensively studied immune checkpoints [31][32]. PD1 has been found to be expressed by activated T cells, nature killer (NK) cells, MDSCs, monocytes and DCs [33][34][35]. Its corresponding ligand, PDL1, has been found in tumor cells, stromal cells, and myeloid lineage cells like macrophages and DCs [35]. CTLA4 is predominantly expressed by T reg cells and only upregulated in activated T cells [36][37]. In addition, the sialic acid-binding immunoglobulin (Ig)-like lectins 10 (siglec-10) has recently been identified as a new class of co-inhibitory molecule expressed by macrophages to interact with its corresponding ligand, CD24, expressed on the tumor surface [32][38].
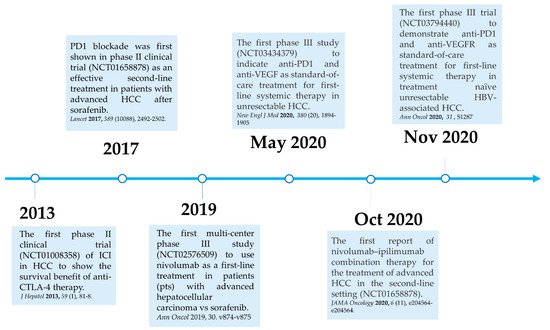
Here, we summarize the current milestones regarding recent clinical trials testing ICIs as a potential systemic treatment for advanced HCC ( Figure 1 ). The first immune checkpoint inhibitor (ICI), ipilimumab, the anti-CTLA-4 monoclonal antibody (mAb), was approved by the U.S. Food and Drug Administration (FDA) in March 2011 for the treatment of patients with advanced melanoma [39]. In 2013, a pilot clinical trial involving 20 patients with advanced HCC and a background of chronic hepatitis C virus (HCV) infection who received tremelimumab treatment, another anti-CTLA-4 mAb, showed promising results in terms of safety, antitumor and antiviral activity [40]. This encouraging result has led to the approval of another ICI, the PD1 inhibitor nivolumab, for the treatment of patients after sorafenib failure in the CheckMate 040 phase I/II clinical trial. In the CheckMate 040 trial ( n = 262), durable objective response rate (ORR), defined as the sum of complete (CR) and partial (PR) response rates, was observed in 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase, The median overall survival (OS) in sorafenib-experienced patients was 16.5 months. Further, the two-year survival rate among the responders was over 80% [41]. Soon after, nivolumab was approved by the FDA as a second-line systemic therapy for advanced HCC after sorafenib. Another PD1 inhibitor, pembrolizumab. was also shown effective and tolerable in patients with advanced HCC after sorafenib in a non-randomized, open-label phase II trial. ORR was recorded in 17% 95% CI 11–26) of patients previously treated with sorafenib [42]. Given the consistent results in terms of anti-tumor activity and safety from different PD1 mAb, a randomized, double-blind, phase III trial (KEYNOTE 240) ( n = 413) testing pembrolizumab versus placebo after sorafenib failure was launched in a second-line setting. Median OS was 13.9 months (95% CI 11.6–16) in the pembrolizumab treatment group and 10.36 months (95% CI 8.3–13.5) in the placebo group, and a statistically significant survival benefit was observed (Hazard ration HR 0.78; p = 0.0238) in the final analysis [43]. Although, OS and progression-free survival (PFS) did not reach the prespecified criteria, the results were in line with KEYNOTE 224, indicating a favorable risk-to-benefit ratio in the pembrolizumab group. Table 1 summarizes current important phase III trials involving ICI therapy as a major treatment modality.
| Trial Identifier | ICI/Isotype | Drug | Treatment Arm | Eligible Patient/Setting | Endpoint | Ref. |
|---|---|---|---|---|---|---|
| Monotherapies | ||||||
| NCT02702401 | PD1/IgG4 | Pembrolizumab | 1. Placebo 2. Pembrolizumab |
Advanced HCC/2L | Dec. 2022 | [43] |
| NCT02576509 | PD1/IgG4 | Nivolumab Sorafenib |
1. Sorafenib 2. Nivolumab |
Advanced HCC/1L | June 2021 | [44] |
| NCT03755739 | PD1/IgG4 | Pembrolizumab | 1. Peripheral in fusion | Advanced HCC/1L | Nov. 2021 | Ongoing |
| 2. Artery infusion | ||||||
| 3. Intra-tumor infusion | ||||||
| NCT03062358 | PD1/IgG4 | Pembrolizumab | 1. Placebo 2. Pembrolizumab |
Advanced HCC/2L | Jan. 2022 | Ongoing |
| NCT03412773 | PD1/IgG4 | Tislelizumab | 1. Placebo 2. Tislelizumab |
Unresectable HCC/1L | May 2022 | [45] |
| Combination therapies of ICI and TKI or anti-angiogenic agents | ||||||
| NCT03298451 | PD1 CTLA-4 (IgG4) |
Durvalumab Tremelimumab |
1. Sorafenib 2. Tremelimumab 3. Tremelimumab plus durvalumab |
HCC BCLC stage B not eligible for locoregional therapy/1L | June 2021 | [46] |
| NCT03794440 | PD1/IgG4 VEGF/IgG1 |
Sintilimab Bevacizumab biosimilar |
1. Sorafenib 2. Sintilimab plus Bevacizumab biosimilar |
Advanced HCC/1L | Dec. 2022 | [47] |
| NCT03847428 | PDL1/IgG1 VEGF/IgG1 |
Durvalumab Bevacizumab |
1. Combination with resection/MWA 2. Resection/MWA alone |
HCC eligible for curative resection/MWA/2L | June 2023 | [48] |
| NCT03713593 | PD1/IgG4 VEGFR |
Pembrolizumab Lenvatinib | 1. Lenvatinib 2. Pembrolizumab |
Advanced HCC/1L | July 2022 | [49] |
| NCT03764293 | PD1/IgG4 TKI |
Camrelizumab Apatinib |
1. Apatinib | Advanced HCC/1L | Jan. 2022 | Ongoing |
| NCT03434379 | PDL1/IgG1 VEGF/IgG1 |
Atezolizumab Bevacizumab |
1. Sorafenib 2. Atezolizumab plus Bevacizumab |
Advanced HCC/1L | June 2022 | [50] |
Aside from PD1/PDL1 and CTLA-4, here we discuss other important co-inhibitory molecules expressed by T cells which have been identified as potential immune checkpoints that modulate T cell activation. T-cell immunoglobulin and mucin-domain containing-3 (TIM3) is a membrane-bound protein that is originally expressed in CD8 + cytotoxic T cells and interferon-γ-producing CD4 + T helper 1 (Th1) cells [51]. Initially, the function of TIM3 was associated with autoimmune disease. Blocking of TIM3 with TIM3-specific antibody or administration of TIM-3 immunoglobulin (Ig) fusion protein resulted in Th1 cell and macrophage hyperactivation in an experimental autoimmune encephalomyelitis (EAE) mouse model [52]. Later, TIM3 expression was found in several immune cells, including T reg cells [53] , myeloid cells [54], natural killer (NK) cells [55] and dendritic cells (DCs) [51]. Notably, co-expression of TIM3 and PD1 has been observed in dysfunctional Th1 cells that express low levels of IL-2 and IFN-γ in the preclinical model [56]. A synergistic anti-tumor effect of combined anti-PD1 and anti-TIM3 immunotherapy has been observed in solid tumors in mouse models [56][57]. Therefore, combination treatment of TIM3 and PD1 or CTLA-4 inhibitor could serve as an attractive ICI regimen for HCC. Recently, a phase II trial assessing the efficacy and safety of anti-PD1 and anti-TIM3 combination therapy (NCT03680508) has been launched; the results are still being awaited.
Another important aspect to examine is that compensatory upregulation of TIM3 and LAG3 may confer resistance to anti-PD1/PDL1 treatment. In a preclinical mouse model, Oweida et al. demonstrated that TIM3 upregulation was observed in tumor-infiltrating CD4 and CD8 T cells in murine head and neck squamous cell carcinoma (HNSCC) tumors treated with radiotherapy (RT) and anti-PDL1 therapy. Combined treatment with anti-TIM3, anti-PDL1 and RT led to the promotion of T cell cytotoxicity, decreased T reg and significant tumor regression as compared with anti-PDL1 plus RT treatment [58]. In an effort to address the resistance mechanisms of PD1 blockade, Limagne et al. showed that accumulation of galetin-9-expressing monocytic MDSCs (mMDSCs) and TIM3-expressing CD8 T cells was found in lung cancer patients with resistance to anti-PD1 therapy, and may play a crucial role in resistance to PD1 blockade. They demonstrated that anti-TIM3 antibody in vitro could reverse resistance to anti-PD1 in peripheral blood mononuclear cells (PBMCs) isolated from lung cancer patients. Moreover, galetin-9-expressing mMDSCs could impede TIM3 + CD8 T cell activity to reduce anti-PD1 treatment efficacy [59]. In addition, Jikova et al. analyzed fresh HCC biopsies and peripheral blood samples from 21 HCC patients treated with sorafenib or PD1/PDL1 blockade therapy to show that non-responders tended to have TIM3 and LAG3 upregulation on circulating T cells compared with responders [60].
References
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710.
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236.
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380.
- Colagrande, S.; Inghilesi, A.L.; Aburas, S.; Taliani, G.G.; Nardi, C.; Marra, F. Challenges of advanced hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7645–7659.
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Personeni, N.; Pressiani, T.; Santoro, A.; Rimassa, L. Regorafenib in hepatocellular carcinoma: Latest evidence and clinical implications. Drugs Context 2018, 7, 212533.
- Kudo, M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers 2018, 10, 412.
- Abou-Alfa, G.K.; Huitzil-Melendez, F.D.; O’Reilly, E.M.; Saltz, L.B. Current management of advanced hepatocellular carcinoma. Gastrointest. Cancer Res. 2008, 2, 64–70.
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296.
- Siegel, A.B.; Cohen, E.I.; Ocean, A.; Lehrer, D.; Goldenberg, A.; Knox, J.J.; Chen, H.; Clark-Garvey, S.; Weinberg, A.; Mandeli, J.; et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J. Clin. Oncol. 2008, 26, 2992–2998.
- Ledererova, A.; Dostalova, L.; Kozlova, V.; Peschelova, H.; Ladungova, A.; Culen, M.; Loja, T.; Verner, J.; Pospisilova, S.; Smida, M.; et al. Hypermethylation of CD19 promoter enables antigen-negative escape to CART-19 in vivo and in vitro. J. Immunother. Cancer 2021, 9, 8.
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; Lanauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295.
- Cai, L.; Michelakos, T.; Yamada, T.; Fan, S.; Wang, X.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol. Immunother. 2018, 67, 999–1009.
- Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009, 27, 147–163.
- Sangro, B.; Sarobe, P.; Hervas-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543.
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321.
- Ormandy, L.A.; Hillemann, T.; Wedemeyer, H.; Manns, M.P.; Greten, T.F.; Korangy, F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005, 65, 2457–2464.
- Kuang, D.M.; Zhao, Q.; Peng, C.; Xu, J.; Zhang, J.P.; Wu, C.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009, 206, 1327–1337.
- Zhong, M.; Zhong, C.; Cui, W.; Wang, G.; Zheng, G.; Li, L.; Zhang, J.; Ren, R.; Gao, H.; Wang, T.; et al. Induction of tolerogenic dendritic cells by activated TGF-beta/Akt/Smad2 signaling in RIG-I-deficient stemness-high human liver cancer cells. BMC Cancer 2019, 19, 439.
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306.
- Yamagiwa, S.; Gray, J.D.; Hashimoto, S.; Horwitz, D.A. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 2001, 166, 7282–7289.
- Lin, T.H.; Shao, Y.Y.; Chan, S.Y.; Huang, C.Y.; Hsu, C.H.; Cheng, A.L. High Serum Transforming Growth Factor-beta1 Levels Predict Outcome in Hepatocellular Carcinoma Patients Treated with Sorafenib. Clin. Cancer Res. 2015, 21, 3678–3684.
- Hochst, B.; Schildberg, F.A.; Sauerborn, P.; Gabel, Y.A.; Gevensleben, H.; Goltz, D.; Heukamp, L.C.; Turler, A.; Ballmaier, M.; Gieseke, F.; et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J. Hepatol. 2013, 59, 528–535.
- Dunham, R.M.; Thapa, M.; Velazquez, V.M.; Elrod, E.J.; Denning, T.L.; Pulendran, B.; Grakoui, A. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. 2013, 190, 2009–2016.
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119.
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264.
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485.
- Courau, T.; Nehar-Belaid, D.; Florez, L.; Levacher, B.; Vazquez, T.; Brimaud, F.; Bellier, B.; Klatzmann, D. TGF-beta and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight 2016, 1, e85974.
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086.
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669.
- Yin, S.S.; Gao, F.H. Molecular Mechanism of Tumor Cell Immune Escape Mediated by CD24/Siglec-10. Front. Immunol. 2020, 11, 1324.
- Nguyen, L.T.; Ohashi, P.S. Clinical blockade of PD1 and LAG3—Potential mechanisms of action. Nat. Rev. Immunol. 2015, 15, 45–56.
- Hato, T.; Goyal, L.; Greten, T.F.; Duda, D.G.; Zhu, A.X. Immune checkpoint blockade in hepatocellular carcinoma: Current progress and future directions. Hepatology 2014, 60, 1776–1782.
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029.
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275.
- Han, Y.; Chen, Z.; Yang, Y.; Jiang, Z.; Gu, Y.; Liu, Y.; Lin, C.; Pan, Z.; Yu, Y.; Jiang, M.; et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014, 59, 567–579.
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396.
- Lipson, E.J.; Drake, C.G. Ipilimumab: An anti-CTLA-4 antibody for metastatic melanoma. Clin. Cancer Res. 2011, 17, 6958–6962.
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Inarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88.
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502.
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952.
- Kudo, M.; Lim, H.Y.; Cheng, A.L.; Chao, Y.; Yau, T.; Ogasawara, S.; Kurosaki, M.; Morimoto, N.; Ohkawa, K.; Yamashita, T.; et al. Pembrolizumab as Second-Line Therapy for Advanced Hepatocellular Carcinoma: A Subgroup Analysis of Asian Patients in the Phase 3 KEYNOTE-240 Trial. Liver Cancer 2021, 10, 275–284.
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564.
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, M.; Mercade, T.M.; Tomasello, G.; Boisserie, F.; Hou, J.; et al. A phase 3, randomized, open-label, multicenter study to compare the efficacy and safety of tislelizumab, an anti-PD-1 antibody, versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2018, 36, TPS3110.
- Abou-Alfa, G.K.; Chan, S.L.; Furuse, J.; Galle, P.R.; Kelley, R.K.; Qin, S.; Armstrong, J.; Darilay, A.; Vlahovic, G.; Negro, A.; et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J. Clin. Oncol. 2018, 36, TPS4144.
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990.
- Knox, J.; Cheng, A.; Cleary, S.; Galle, P.; Kokudo, N.; Lencioni, R.; Park, J.; Zhou, J.; Mann, H.; Morgan, S.; et al. A phase 3 study of durvalumab with or without bevacizumab as adjuvant therapy in patients with hepatocellular carcinoma at high risk of recurrence after curative hepatic resection or ablation: EMERALD-2. Ann. Oncol. 2019, 30, iv59–iv60.
- Llovet, J.M.; Kudo, M.; Cheng, A.-L.; Finn, R.S.; Galle, P.R.; Kaneko, S.; Meyer, T.; Qin, S.; Dutcus, C.E.; Chen, E.; et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study. J. Clin. Oncol. 2019, 37, TPS4152.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185.
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541.
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE 2012, 7, e30676.
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 2007, 318, 1141–1143.
- Ndhlovu, L.C.; Lopez-Verges, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734.
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194.
- Fourcade, J.; Sun, Z.; Pagliano, O.; Chauvin, J.M.; Sander, C.; Janjic, B.; Tarhini, A.A.; Tawbi, H.A.; Kirkwood, J.M.; Moschos, S.; et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res. 2014, 74, 1045–1055.
- Oweida, A.; Hararah, M.K.; Phan, A.; Binder, D.; Bhatia, S.; Lennon, S.; Bukkapatnam, S.; Van Court, B.; Uyanga, N.; Darragh, L.; et al. Resistance to Radiotherapy and PD-L1 Blockade Is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin. Cancer Res. 2018, 24, 5368–5380.
- Limagne, E.; Richard, C.; Thibaudin, M.; Fumet, J.D.; Truntzer, C.; Lagrange, A.; Favier, L.; Coudert, B.; Ghiringhelli, F. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. Oncoimmunology 2019, 8, e1564505.
- Macek Jilkova, Z.; Aspord, C.; Kurma, K.; Granon, A.; Sengel, C.; Sturm, N.; Marche, P.N.; Decaens, T. Immunologic Features of Patients With Advanced Hepatocellular Carcinoma Before and During Sorafenib or Anti-programmed Death-1/Programmed Death-L1 Treatment. Clin. Transl. Gastroenterol. 2019, 10, e00058.




