Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Farshid Sefat | + 3373 word(s) | 3373 | 2021-10-22 04:44:16 | | | |
| 2 | Amina Yu | -22 word(s) | 3351 | 2021-10-22 11:59:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sefat, F. Regenerative Medicine of Liver. Encyclopedia. Available online: https://encyclopedia.pub/entry/15288 (accessed on 07 February 2026).
Sefat F. Regenerative Medicine of Liver. Encyclopedia. Available at: https://encyclopedia.pub/entry/15288. Accessed February 07, 2026.
Sefat, Farshid. "Regenerative Medicine of Liver" Encyclopedia, https://encyclopedia.pub/entry/15288 (accessed February 07, 2026).
Sefat, F. (2021, October 22). Regenerative Medicine of Liver. In Encyclopedia. https://encyclopedia.pub/entry/15288
Sefat, Farshid. "Regenerative Medicine of Liver." Encyclopedia. Web. 22 October, 2021.
Copy Citation
The liver is a very integral organ in the human body and in healthy adults’ the weight of this organ is approximately 1.4 kg, which makes it the largest gland in the human body. The rib cage protects the liver; therefore, it is unusual to be able to feel the liver from outside the body
liver
scaffold
regenerative medicine
hepatocyte
stem cell
1. Bioengineered Scaffolds
Liver tissue engineering (LTE) holds the potential of restoring in part or total functions of the liver, with the aim of treating acute or chronic liver diseases. The ultimate goal of LTE is to reproduce a completely functional liver to be transplanted into affected patients or be used as an extracorporeal device. Tissue engineering techniques include cell seeding or implanting cells into scaffolds with biodegradable properties and structures capable of maintaining three-dimensional (3D) cell growth. Typically, these scaffolds comprise an artificial matrix or ECM, along with a selection of polymers that offer mechanical support for 3D cell proliferation. LTE technical approaches are dependent on the use of adult hepatocytes since these cells are anchorage-dependent cells. Hepatocytes are also regarded as being very sensitive to the ECM environment for maintenance of their differentiated functions and viability. Therefore, in order to produce an effective hepatocyte cell culture, LTE requires an appropriate ECM environment [1][2].
Scaffold material selection will have a vital effect on the success of the liver tissue engineering technical approach. The scaffold should be capable of providing sufficient support for growing tissue and surface topography for successful cell attachment. Scaffolds should also be designed to provide channels for cell migration. Animal-extracted ECM scaffolds have the advantage of supplying binding sites to enable integrin-mediated cell adhesion. However, Hammond et al., 2006 discussed several problems associated to this particular type of scaffold, which include: low mechanical strength, not being immediately scaleable and experiencing interbatch variability [3]. Thus, biodegradable polymers are becoming increasingly popular within LTE. These polymers have been identified to behave more predictably in vitro, which is why they are more susceptible to being modified to improve cell-surface attachment. Additionally, they have the capability to be constructed into complex micro-scaffolds and degrade to form natural metabolites.
Biodegradable polymers, which include polylactic lactic acid (PLA), poly (L-lactic) acid (PLLA) polyglycolic acid (PGA) and PDMS are frequently used to produce a scaffold (Figure1) that provides a microenvironment comparable to the in vivo environment [4][5]. Li et al., 2013 have reported that this microenvironment includes a high supply of oxygen and nutrients, a 3D ECM, as well as high cell density. These biomaterials have been exhibited to support viable hepatocyte populations, which demonstrates their excellent biocompatibility [6]. Hammond et al., 2006, stated how various surface-modification procedures are available to increase the cell-surface adhesion rate of these biomaterials, whilst not causing any adverse effects to their bulk properties [3].
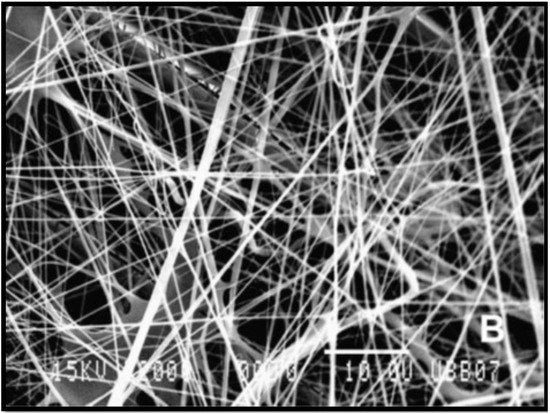
Figure 1. Scanning electron microscopy (SEM) micrograph (2000×) of poly (L-lactic) acid (PLLA) fibres produced from electrospinning [5].
Jain et al., 2013 have assessed different hydrophilic polymers which have been used in the production of bioengineered scaffolds for liver reconstruction [7]. Chitosan has been identified to have a likeness to glycosaminoglycan, thereby causing it to be a popular matrix for hepatocyte culture. Chitosan scaffolds manufactured as composites, nanofibers, and hydrogels are commonly used for the maintenance of hepatocytes in vitro. Being a hydrophilic polymer, chitosan has the ability to promote spheroid formation within hepatocytes. Hybrid scaffolds comprised of chitosan with collagen have been used effectively for hepatocyte differentiation and spheroid formation. Scaffolds constructed from alginate, as seen in Figure 2, have been utilised to microencapsulate or cultivate hepatocytes to develop implantable constructs [5][8]. Additionally, alginate is also a hydrophilic polymer, and as a result it stimulates spheroid formation. This consequentially increases cellular interactions, along with hepatocyte function. In order to create favourable growth conditions for hepatocyte culture, porous alginate scaffolds are produced to contain approximately 90% porosity and a pore size of 100 µm. They are capable of encouraging spheroid formation due to low cell adherence to the substrate.
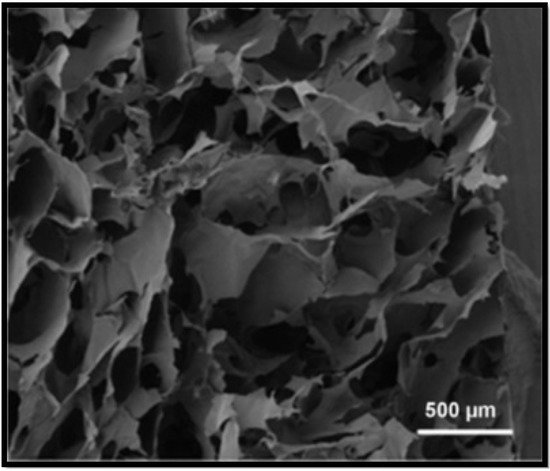
Figure 2. SEM of the cross–section of alginate scaffold [8].
Recent studies have demonstrated structure optimisation of porous and mesh scaffolds manufactured from biomaterials that retain low cell-adhesion strength. Altering the surface features of the selected biomaterial enables the possibility to further improve function of the scaffold. Hepatocytes exhibit different behaviour on a monolayer of a polymer, in contrast to a porous or mesh configuration on the same polymer. Research conducted by Edgar et al., 2016 demonstrated that porous scaffolds comprise interconnected micropores with hydrophilic characteristics and exceptional fluid absorption, resulting from their large surface area to volume ratio [9]. Moreover, their mechanically poor architecture, flexibility, and degradability, allow porous scaffolds to be a useful application for wound repair. Mesh scaffolds have become relatively popular within the TE community because of their ability to show a structural architecture similar to that of natural soft tissue. Mesh scaffolds are produced via electrospinning nanofibers that are composed of the desired biomaterial. These nanofibers are woven to form a 3D structure capable of supporting an environment for cellular adhesion and proliferation [3].
Furthermore, one of the most significant challenges to LTE is to manufacture a porous scaffold with high mechanical strength, along with having the ability to maintain vascularisation. Numerous studies emphasise that the function of the scaffold is determined by the following main factors: pore numbers, pore size, and pore connectivity. Size of pores could have a significant influence on cell migration, as extremely large pores may diminish vascularisation. In comparison, pores that are smaller than 100 nm could affect diffusion of nutrients and waste. Inefficient diffusion of nutrients could lead to decreased viability of implanted cells and overall failure of the implanted device. Therefore, the porosity must be suitably balanced with the chosen biomaterial, in addition to their mechanical features and cellular influence [10][11]. The following table (Table 1) summarizes biomaterials utilized for liver tissue engineering scaffolds.
Table 1. Summary of biomaterials used for scaffolds in liver tissue engineering.
| Biomaterials Utilised for Liver Tissue Engineering Scaffolds | |||
|---|---|---|---|
| Biomaterial | Advantages | Disadvantages | Ref |
| Polylactic Acid (PLA) |
|
|
[6][12][13][14] |
| Poly (L-lactide) (PLLA) |
|
|
[15][16] |
| Polyglycolide (PGA) |
|
|
[15][12][13][16] |
| Polydimethylsiloxane (PDMS) |
|
|
[17][18][15] |
| Chitosan |
|
|
[7][12][16][19] |
| Alginate |
|
|
[7][16][20] |
| Animal extracted ECM |
|
|
[3] |
2. Stem Cell Therapy
Kwak et al., 2018 conducted research on stem cell-based therapy (SCBT) used to treat liver cirrhosis and found it to have promising results in both preclinical and clinical trials. It seems SCBT may in the future become a popular alternative to orthotopic liver transplantation, which is currently the only definite treatment for a patient suffering from end-stage liver cirrhosis [21]. There is still much more to understand regarding the precise mechanisms of stem cells. Several stem cells of diverse origin have been utilised for hepatic regeneration and this section will delve further into this matter.
2.1. Hematopoietic Stem Cells (HSA)
Hematopoietic stem cells (HSCs) have been routinely utilised to investigate SCBT [22][23]. HSCs have a CD34 cell surface marker and are the most common type of stem cell within bone marrow [24]. HSCs can transform into progenitor cells via differentiation and can renew themselves [21][25][26]. HSC’s can also be derived from cytokine-mobilized and umbilical blood [22][23]. Blood disorders have been treated using HSCs for an extensive period, and therefore the use of the cells in SCBT is not thought to be a novel approach [23][27].
Jang et al., 2004 carried out a study to see how in vivo/in vitro HSCs behaved towards injury of the liver, since functional and phenotypic changes occur within the liver in this instance. It was found that HSCs have the potential to differentiate into liver cells when they are co-cultured with an injured liver [28]. The in vitro experiment displayed that microenvironmental cues had a hand in the transformation of HSCs into liver cells. This was found through analysis of protein expression, chromosomal studies and tissue-specific genes. A trend was noticed when HSCs were transplanted into mice who had liver injuries, it was noticed that viable hepatocytes were being introduced as the injury progressed. The restoration of liver function occurred between two to seven days after the HSC transplantation occurred. From research it can be concluded that HSCs can differentiate into functional hepatocytes and contribute towards the regeneration of an injured liver. Lagasse et al., 2000 experimental results further support this conclusion. Adult bone marrow cells were intravenously injected into a tyrosinemia type I affected FAH mouse and ultimately only HSC cells gave rise to hepatic regeneration [29].
From the research of Liu et al., 2006, liver regeneration (after partial orthotopic liver transplant) can be induced by mobilizing HSCs with granulocyte-colony stimulating factor (G-CSF). It is less difficult to conduct this treatment in a universal way. Current therapeutic procedures such as this one, have a basic concept of mobilizing, proliferating and directing stem cells to improve recovery from liver injury by a partial liver transplantation. Although stem cells can be linked to restoring liver function, it is not yet possible to define their exact action in the liver regeneration process. Tsolaki et al., 2014 reiterated this uncertainty since they also were unsure if the liver regeneration processes were triggered or mediated by the on-site HSCs and/or by the effect of the G-CSF. This further emphasises the difficulty of characterising exact root causes for hepatic regeneration. Additionally, G-CSF alongside other mobilization agents such as Plerixafor and a G-CSF+Plerixafor combination can potentially aid the reversal of chronic liver injury via unique processes. A clear observation was made that the G-CSF possessed the greatest anti-fibrotic ability when it was used for an extended period. This result further proves the link between G-CSF and its anti-fibrotic outcome [30].
2.2. Mesenchymal Stem Cells (MSC)
Mesenchymal stem cells (MSCs) are being thoroughly investigated for the purposes of regenerative medicine as they possess distinct biological properties [31]. MSCs can be extracted from adipose tissue, umbilical cord blood and bone marrow. Since MSCs can undergo extensive expansion within in vitro conditions, this allows them to swiftly reach the desired quantity when required for in vivo therapy [32][33]. Mesenchymal stem cell (MSC) can be differentiated into hepatocytes, promote liver regeneration, inhibit liver fibrosis and induce liver apoptosis, particularly via paracrine mechanisms [34]. Dominici et al., 2006 states that it is difficult to compare results from research papers investigating MSCs due to the different procedures used for isolation, expansion and characterization of cells, which in turn hinders progress in this field of study [35]. To combat this issue, the International Society for Cellular Therapy has defined a criteria list to characterize the human MSC. Firstly, it is crucial that the MSC displays plastic adherence when stored in a standard cell culture environment. Additionally, the MSC must express (CD90, CD105, CD73) and lack expression of (HLA-DR, CD34, CD14 or CD11b, CD79alpha or CD19 and CD45) certain cell surface molecules. Finally, the MSC must have the differential ability to transform into adipocytes, chondroblasts and osteoblasts in vitro.
Though isolated MSCs all express the MSC marker profile, they are commonly diverse in both differentiation and proliferation. In vitro cultivated MSCs have excellent differentiation ability, immunoregulatory potential and can aid tissue remodelling through the emission of trophic factors. These key properties make MSCs useful in cellular therapy [36][37]. The review paper by Shiota and Itaba 2016 found that MSCs have the potential to improve liver diseases such as fatty liver disease, acute liver failure and liver fibrosis. MSCs have great versatility and, therefore, they can differentiate into hepatic lineages. In one study, HNF3β was expressed when a tetracycline-controlled system was regulated in the UE7T-13 (bone marrow derived) MSCs. This expression caused a ripple effect, since it also increased the expression of alpha-fetoprotein, epithelial cell adhesion molecules, albumin and tyrosine amino transferase genes. When tetracycline treatment was continued over 8 days, it was seen that majority of the MSCs expressed albumin. Albumin is linked to the maturing of hepatocytes. It can be concluded that MSCs are a valuable source to use in SCBT to treat liver disease [33][38].
Gao et al., 2017 carried out an investigation with (adipose-derived) MSCs and found they are useful for suppressing rejection in reduced sized liver allografts. Regulatory T cell production is stimulated by MSCs; this is key in its immunosuppressive effects. MSCs were also found to boost hepatocyte regeneration and hinder hepatocyte apoptosis, which in turn promotes liver regeneration [39]. Liu et al., 2018 explored (bone marrow-derived) MSCs and found evidence that MSC transplantation could potentially aid recovery for patients who have undergone a liver resection. Liver regeneration occurs since MSCs encourage cell proliferation/growth and MSC infusion can potentially improve lipid accumulation within the liver [40].
2.3. Embryonic Stem Cells (ESC)
Embryonic stem cells (ESCs) are categorised as pluripotent stem cells that are extracted from the inner cell mass of blastocyst stage embryos. These stem cells retain the potential to self-renew indefinitely, whilst having the ability to differentiate into all cell types in the human body (as seen in Figure 3) when supplied with the relevant stimulus for differentiation [8][41]. This high degree of differentiation and unlimited potential for self-renewal, allow ESCs to be considered as an effective regenerative therapy for tissue replacement following liver disease. Tsolaki and Yannaki, 2015 discussed that ESCs have the capacity to proficiently differentiate into hepatocyte-like cells in vitro, creating cells that have some similar properties to that of mature hepatocytes [42]. For this reason, ESCs are a valuable option for studying the molecular source of hepatocyte differentiation. A recent study by Tolosa et al., 2015 identified an effective method for the differentiation of ESCs into neonatal hepatocytes that had the ability to reintroduce livers in vivo without instigating tumour development in mice [43].
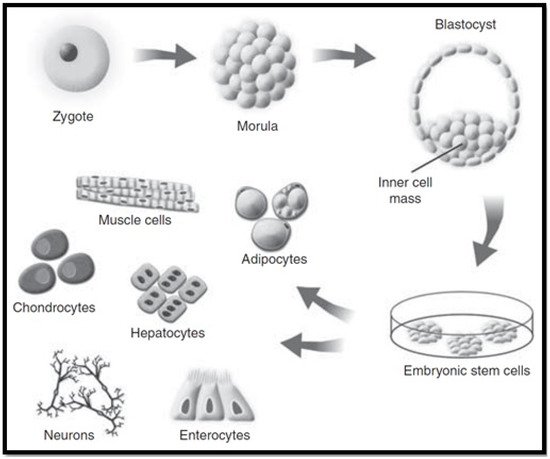
Figure 3. Embryonic stem cell cultivation [41].
However, there are some limitations to the use of ESCs in cellular therapies. The fact that the derivation of ESCs requires the destruction of blastocyst stage embryos raises ethical issues that have slowed the advancement of ESC research. Tsolaki and Yannaki, 2015 emphasise that regardless of the significant progress and development of complex differentiation methods mimicking embryonic development, ESCs extracted from hepatocyte-like cells are unable to completely function as mature adult hepatocytes [42]. Furthermore, the concern of immunological incompatibility of the transplanted cells, hinder their application as a cell replacement therapy [44].
2.4. Induced Pluripotent Stem Cells (iPSC)
Induced pluripotent stem cells (iPSCs) have properties comparable to those of ESCs, such as self-renewal and pluripotency, while avoiding the main issues that are associated with the use of ESCs. iPSCs can be reprogrammed from somatic cells through the use of pluripotency factors. Forbes et al., 2015 established that they do not require embryonic material (as seen in Figure 4) [41], thereby obviating ethical controversies [45]. In addition, iPSCs hold the potential to be clinically beneficial since they can be formed from autologous stem cells, which provides the opportunity for autologous use and avoiding the condition for immunosuppression [46]. In the event that iPSCs are used for the derivation of hepatocyte-like cells for treatment of a genetic liver disorder, then the autologous source would need some type of “gene surgery” before use. Nicolas et al., 2016 stated that the use of iPSCs as a cellular therapy date back to 2006, and has drastically grown in popularity as a favourable alternative to ESCs [44]. However, before considering their clinical applications, several issues need to be resolved.
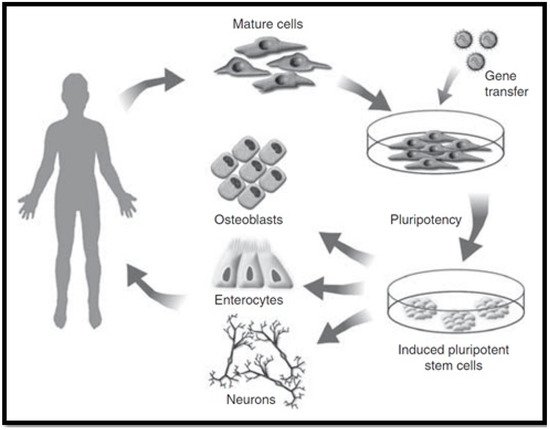
Figure 4. Induced pluripotent stem cell cultivation [41].
iPSCs have increased expectations for regenerative medicine due to their potential to deliver personalised treatment, while their derivation from patient-specific cells overcomes the risk of rejection. Although iPSCs are an interesting alternative to ESCs, there are some issues that need to be addressed before this new form of cellular therapy can move from proof of concept to the clinic. Yu et al., 2014 suggests that an in-depth preclinical evaluation of iPSCs in appropriate large animal models is essential to make sure that treatment with iPSC-derived cells is safe and efficacious before human trials [47]. Hence, important concerns need to be addressed, which include effectiveness of the iPSC-based treatments, and long-lasting safety tolerability. It is vital that the optimal reprograming method is established by using clinically applicable standardised protocols. Cost-efficient manufacturing techniques need to also ensure that they can support the development of rapid differentiation methodologies for culturing mature cell types from iPSCs. Despite these restrictions, iPSC-derived hepatocytes still promise innovative solutions for liver cell therapy, and donor grafts extracted from iPSCs could potentially provide suitable organs for liver transplants [42].
2.5. Endothelial Progenitor Cells (EPCs)
Endothelial progenitor cells are categorised as immature endothelial cells that are located within bone marrow and peripheral blood vessels. These cells manifest from hemangioblasts and are responsible for the neovascularisation of damaged tissue in the body. However, Kwak et al., 2018 emphasises that EPCs are likely to be derived from several cell lineages, which is proven by their various surface markers [21]. According to an animal study conducted by Nakumura et al., 2007, transplantation of EPCs resulted in the intervention of liver fibrosis by successfully suppressing the activation of HSCs. These EPCs were also exhibited to stimulate hepatocyte proliferation and greater matrix metalloproteinase activity, in addition to increased secretion of growth factors [48]. Rautou, 2012 states that, in vitro, EPCs are identified by their capability to produce adherent cell populations that differentiate and multiply into endothelial lineage. When in vivo, EPCs contribute to the dynamic process of angiogenesis in ischemic sites or assist with vascular repair following damage to vessel walls [49]. Accumulating data display that these characteristics of EPCs detect a heterogeneous cluster of cells with respect to their origin, differentiation and functional properties.
SCBT has been deemed an alternative treatment for liver diseases since promising results have been found via clinical and experimental research. A range of stem cells (including HSCs, MSCs, iPSCs, EPCs, ESCs) have been explored to investigate their clinical potential and viability. Extensive research has been carried out with MSCs, which results in a broader understanding of the potential regenerative liver treatments available. Long-term efficacy is still unproven and experimental trials do not have standardized protocols, which is disadvantageous. However innovative technologies of the future are likely to resolve the current issues associated with SCBT [21]. Table 2 summarizes the derivation of stem cells that have been derived from their respective locations and additional information.
Table 2. Summary of derivations and key facts for; stem cells including hematopoietic stem cell therapy, mesenchymal stem cells, embryonic stem cells, induced pluripotent stem cells and endothelial progenitor cells.
| Derivation of Stem of Stem Cells | ||
|---|---|---|
| Stem Cell Type | Derivation | Key Facts |
| HSC |
|
|
| MSC |
|
|
| ESC |
|
|
| iPSC |
|
|
| EPC |
|
|
References
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol. Commun. 2017, 2, 131–141.
- Rad, A.T.; Ali, N.; Kotturi, H.S.R.; Yazdimamaghani, M.; Smay, J.; Vashaee, D.; Tayebi, L. Conducting scaffolds for liver tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4169–4181.
- Hammond, J.S.; Beckingham, I.J.; Shakesheff, K.M. Scaffolds for liver tissue engineering. Expert Rev. Med Devices 2006, 3, 21–27.
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/Body-on-a-Chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48.
- François, S.; Chakfé, N.; Durand, B.; Laroche, G. A poly(l-lactic acid) nanofibre mesh scaffold for endothelial cells on vascular prostheses. Acta Biomater. 2009, 5, 2418–2428.
- Li, Y.-S.; Harn, H.-J.; Hsieh, D.-K.; Wen, T.-C.; Subeq, Y.-M.; Sun, L.-Y.; Lin, S.-Z.; Chiou, T.-W. Cells and Materials for Liver Tissue Engineering. Cell Transplant. 2013, 22, 685–700.
- Jain, E.; Damania, A.; Kumar, A. Biomaterials for liver tissue engineering. Hepatol. Int. 2013, 8, 185–197.
- Guillaume, O.; Daly, A.; Lennon, K.; Gansau, J.; Buckley, S.F.; Buckley, C.T. Shape-Memory porous alginate scaffolds for regeneration of the annulus fibrosus: Effect of TGF-β3 supplementation and oxygen culture conditions. Acta Biomater. 2014, 10, 1985–1995.
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332.
- Abbott, R.D.; Kaplan, D.L. Engineering Biomaterials for Enhanced Tissue Regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146.
- Shick, T.M.; Kadir, A.Z.A.; Ngadiman, N.H.A.; Ma’Aram, A. A review of biomaterials scaffold fabrication in additive manufacturing for tissue engineering. J. Bioact. Compat. Polym. 2019, 34, 415–435.
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 1–21.
- Kazemnejad, S. Hepatic Tissue Engineering Using Scaffolds: State of the Art. Avicenna J. Med. Biotechnol. 2009, 1, 135–145.
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212.
- Lee, S.-A.; No, D.Y.; Kang, E.; Ju, J.; Kim, D.-S.; Lee, S.-H. Spheroid-Based three-dimensional liver-on-a-chip to investigate hepatocyte–hepatic stellate cell interactions and flow effects. Lab Chip 2013, 13, 3529–3537.
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95.
- Wei, S.; Yu-Qing, C.; Guo-An, L.; Zhang, M.; Zhang, H.-Y.; Yue-Rong, W.; Ping, H. Organs-on-Chips and Its Applications. Chin. J. Anal. Chem. 2016, 44, 533–541.
- Mauriac, H.; Pannetier, C.; Casquillas, G.V. Organs on chip review. Elveflow 2020.
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Kwak, K.-A.; Cho, H.-J.; Yang, J.-Y.; Park, Y.-S. Current Perspectives Regarding Stem Cell-Based Therapy for Liver Cirrhosis. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–19.
- Almeida-Porada, G.; Zanjani, E.D.; Porada, C.D. Bone marrow stem cells and liver regeneration. Exp. Hematol. 2010, 38, 574–580.
- Irfan, A.; Ahmed, I. Could stem cell therapy be the cure in liver cirrhosis? J. Clin. Exp. Hepatol. 2015, 5, 142–146.
- Lee, J.Y.; Hong, S.-H. Hematopoietic Stem Cells and Their Roles in Tissue Regeneration. Int. J. Stem Cells 2020, 13, 1–12.
- Hombach-Klonisch, S.; Panigrahi, S.; Rashedi, I.; Seifert, A.; Alberti, E.; Pocar, P.; Kurpisz, M.; Schulze-Osthoff, K.; Mackiewicz, A.; Los, M.J. Adult stem cells and their trans-differentiation potential—perspectives and therapeutic applications. J. Mol. Med. 2008, 86, 1301–1314.
- Menichella, G.; Lai, M.; Serafini, R.; Pierelli, L.; Vittori, M.; Ciarli, M.; Rumi, C.; Puggioni, P.; Scambia, G.; Sica, S.; et al. Large Volume Leukapheresis for Collecting Hemopoietic Progenitors: Role of CD 34+ Precount in Predicting Successful Collection. Int. J. Artif. Organs 1999, 22, 334–341.
- Bryder, D.; Rossi, D.J.; Weissman, I.L. Hematopoietic Stem Cells: The Paradigmatic Tissue-Specific Stem Cell. Am. J. Pathol. 2006, 169, 338–346.
- Jang, Y.-Y.; Collector, M.I.; Baylin, S.B.; Diehl, A.M.; Sharkis, S.J. Hematopoietic stem cells convert into liver cells within days without fusion. Nature 2004, 6, 532–539.
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Reitsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000, 6, 1229–1234.
- Tsolaki, E.; Athanasiou, E.; Gounari, E.; Zogas, N.; Siotou, E.; Yiangou, M.; Anagnostopoulos, A.; Yannaki, E. Hematopoietic stem cells and liver regeneration: Differentially acting hematopoietic stem cell mobilization agents reverse induced chronic liver injury. Blood Cells Mol. Dis. 2014, 53, 124–132.
- Shammaa, R.; El-Kadiry, A.E.-H.; Abusarah, J.; Rafei, M. Mesenchymal Stem Cells Beyond Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 72.
- Anker, P.S.I.; Scherjon, S.A.; der Keur, C.K.-V.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345.
- Shiota, G.; Itaba, N. Progress in stem cell-based therapy for liver disease. Hepatol. Res. 2016, 47, 127–141.
- Zhang, S.; Yang, Y.; Fan, L.; Zhang, F.; Li, L. The clinical application of mesenchymal stem cells in liver disease: The current situation and potential future. Ann. Transl. Med. 2020, 8, 565.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by Mesenchymal Stem Cells: Biological Aspects and Clinical Applications. J. Immunol. Res. 2015, 2015, 1–20.
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2013, 21, 216–225.
- Ishii, K.; Yoshida, Y.; Akechi, Y.; Sakabe, T.; Nishio, R.; Ikeda, R.; Terabayashi, K.; Matsumi, Y.; Gonda, K.; Okamoto, H.; et al. Hepatic differentiation of human bone marrow-derived mesenchymal stem cells by tetracycline-regulated hepatocyte nuclear factor 3β. Hepatology 2008, 48, 597–606.
- Gao, W.; Zhang, L.; Zhang, Y.; Sun, C.; Chen, X.; Wang, Y. Adipose-derived mesenchymal stem cells promote liver regeneration and suppress rejection in small-for-size liver allograft. Transpl. Immunol. 2017, 45, 1–7.
- Liu, Y.; Yang, F.; Li, J.; Wang, J.; Wang, X.; Zhang, Y.; Yuan, X.; Zhu, W.; Shi, X. Mesenchymal Stem Cells Enhance Liver Regeneration via Improving Lipid Accumulation and Hippo Signaling. Stem Cells Int. 2018, 2018, 1–11.
- Chagastelles, P.C.; Nardi, N.B. Biology of stem cells: An overview. Kidney Int. Suppl. 2011, 1, 63–67.
- Tsolaki, E.; Yannaki, E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World J. Gastroenterol. 2015, 21, 12334.
- Tolosa, L.; Caron, J.; Hannoun, Z.; Antoni, M.; López, S.; Burks, D.; Castell, J.V.; Weber, A.; Gomez-Lechon, M.-J.; Dubart-Kupperschmitt, A. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res. Ther. 2015, 6, 1–17.
- Nicolas, C.; Wang, Y.; Luebke-Wheeler, J.; Nyberg, S.L. Stem Cell Therapies for Treatment of Liver Disease. Biomedicines 2016, 4, 2.
- Forbes, S.J.; Gupta, S.; Dhawan, A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015, 62, S157–S169.
- Yao, J.; Yu, Y.; Nyberg, S.L. Induced Pluripotent Stem Cells for the Treatment of Liver Diseases: Novel Concepts. Cells Tissues Organs 2020, 1–17.
- Yu, Y.; Wang, X.; Nyberg, S.L. Potential and Challenges of Induced Pluripotent Stem Cells in Liver Diseases Treatment. J. Clin. Med. 2014, 3, 997–1017.
- Nakamura, T.; Torimura, T.; Sakamoto, M.; Hashimoto, O.; Taniguchi, E.; Inoue, K.; Sakata, R.; Kumashiro, R.; Murohara, T.; Ueno, T.; et al. Significance and Therapeutic Potential of Endothelial Progenitor Cell Transplantation in a Cirrhotic Liver Rat Model. Gastroenterology 2007, 133, 91–107.
- Rautou, P.-E. Endothelial progenitor cells in cirrhosis: The more, the merrier? J. Hepatol. 2012, 57, 1163–1165.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
939
Revisions:
2 times
(View History)
Update Date:
26 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




