It has been revealed that high NaCl stress (>60 mmol L−1) induced phenolics accumulation in barley seedlings, with γ-aminobutyric acid (GABA) playing a key role. Interestingly, low NaCl stimulus (20 mmol L−1) enhancing phenolics synthesis and growth of barley seedlings was also reported recently. Hence, exogenous GABA and its bio-synthesis inhibitor 3-mercaptopropionic acid (3-MP) were applied to reveal the mechanism of GABA regulating phenolics metabolism in barley seedlings treated with 20 mmol L−1 NaCl. The contents of total phenolics and flavonoids significantly increased by 11.64% and 14.52% under NaCl, respectively. The addition of GABA further increased phenolics and flavonoids contents, especially for gallic acid, protocatechuic acid, caffeic acid, and quercetin, compared with NaCl treatment. Simultaneously, GABA increased the activities and mRNA levels of phenylalanine ammonia lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumalyl CoA ligase (4CL). The addition of 3-MP suppressed the above effects, except for increasing the protein levels of PAL, C4H, and 4CL. Low concentration of NaCl not only promoted growth, but also stimulated endogenous GABA metabolism to affect key enzymes activities and mRNA levels for phenolics synthesis in barley seedlings.
1. Introduction
Barley grains have the characteristics of high vitamins, high dietary fiber, and high protein contents, which could be stimulated by the accumulation of
γ-aminobutyric acid (GABA), phenolics, and other nutrients by germination
[1]. At present, a variety of barley seedling products have been launched globally, including Japanese “green juice”, compound powder tablets, solid drinks, cookies, and so on. GABA is a four-carbon non-protein amino acid found in prokaryotes and eukaryotes with the functions of lowering blood pressure, sedation, improving kidney and liver function, and promoting alcohol metabolism in the human body. It is mainly synthesized from glutamate catalyzed by glutamate dehydrogenase (GAD) in the cytoplasm of monocotyledonous plants
[2], transported to mitochondria and degraded into succinic semialdehyde (SSA) by GABA aminotransferase (GABA-T). SSA is further oxidized to succinic acid by SSA dehydrogenase (SSADH) from the GABA shunt pathway
[3]. GABA is necessary for carbon and nitrogen metabolism in plant growth and development under abiotic stress, including salt, hypoxia, and so on
[4]. In addition, reports have shown that GABA also plays a role in signal transmission in plants under stress
[5]. The GABA content in soybean increased by 25 times under salt and cold stress
[6]. Mayer et al.
[7] revealed that heat shock significantly enhanced the content of endogenous GABA in
Arabidopsis gallnut cells.
Phenolics, including phenolic acids and flavonoids, are important secondary metabolites with anti-cancer, anti-tumor, anti-aging, and other health-promoting functions, which are mainly formed via the shikimate pathway and malonate pathway in plants
[8]. The shikimic acid pathway is an important biological metabolic pathway connecting carbohydrate metabolism and aromatic compounds biosynthesis
[9]. Phenylalanine ammonia lyase (PAL), cinnamic acid 4-hydroxylase (C4H), 4-coumalyl CoA ligase (4CL), chalcone isomerase (CHI), chalcone synthase (CHS), flavanone 3-hydroxylase (F3H), and anthocyanin synthase (ANS) are included in this pathway
[10]. Among these, PAL is a key bridge connecting primary metabolism and phenylpropane metabolism
[11]. Plants accumulate secondary metabolites such as phenolics in response to salt, low oxygen, low temperature, low pH, mechanical stimulation, heat shock, pathogenic microorganism infection, and so on
[12]. The mechanism of phenolics accumulation in plants has been studied recently, mainly in relation to the activities and gene expressions of key enzymes involved in the phenolics synthesis pathway
[13].
Exogenous GABA induced the synthesis of endogenous GABA and other free amino acids (especially proline) in barley seedlings with an increase of PAL, C4H, and 4CL activity, resulting in the enhancement of total phenolics and antioxidant capacity
[14]. Adel et al.
[15] found that phenolic substances in
Arabidopsis thaliana were abundantly enriched under salt stress. Hela et al.
[16] also found that total phenolics and flavonoids contents increased by 1.3 times and 14.5 times, respectively, in lettuce with 100 mmol L
−1 NaCl treatment for 12 days. Both GABA and phenolics in barley seedlings could be accumulated under salt stress
[14]. GABA has been confirmed to be a signal molecule involved in anabolic regulation under abiotic stress
[17]. Ma et al.
[14] found that GABA mediates phenolic compounds accumulation and antioxidant system enhancement in germinated hulless barley under NaCl stress (60 mM). However, higher NaCl concentration inhibited the growth and decreased the biomass of barley seedlings
[13].
Interestingly, our recent study showed that low NaCl (20 mmol L
−1) did not cause the stress effect on barley seedlings, but promoted growth compared with high salt stress, increasing total phenolics content and antioxidant capacity
[18]. In addition, a large number of studies have concentrated on phenolic acids metabolism in cereals
[19]. Flavonoids, as an important component of phenolics, are worthy of attention. Although the function of GABA in phenolic acids synthesis in barley seedlings under NaCl stress has been reported, the effects of GABA metabolism on phenolics accumulation under low NaCl treatment in barley seedlings is worthy of investigation, as it might be a different mode. Hence, the effects of GABA on the synthesis of phenolic acids and flavonoids in barley seedlings treated with a low concentration of NaCl were investigated on the physio-biochemistry and molecular levels.
2. GABA Metabolism of Barley Seedlings
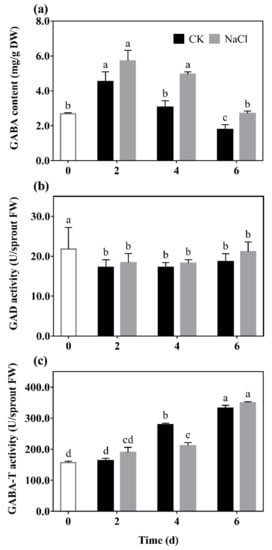
GABA content (a), GAD (b), and GABA-T (c) activity of barley seedlings treated with 20 mM NaCl are shown in Figure 1. It can be seen that the GABA content of barley seedlings increased to its maximum on the 2nd day, which was 1.25 times that of the control. GABA content gradually decreased with the increase of germination days under N treatment, which always higher than the control (Figure 1a).
Figure 1. The effect of NaCl on GABA content (a), GAD activity (b), and GABA-T activity (c) of barley seedlings. Barley seedings were cultured under CK and N treatments, respectively. Sampling was performed on day 0, 2, 4, and 6, respectively. Bars represent standard deviation of means (n = 3), and means with different lower case letters were significantly different (p < 0.05). Two-way ANOVA was used.
The results show that there was a downward trend in the GAD activity of barley seedlings with the increase of germination days (Figure 1b). Among them, GAD activity under N treatment was significantly increased by 14.84% and 36.59% compared with the control on the 2nd and 6th days, respectively. The GABA-T activity of the barley seedlings significantly decreased by 37.82% compared with the control on the 4th day, and there was no significant difference on the other days.
Results indicated that low salt stimulation could significantly promote endogenous GABA synthesis and regulate GABA metabolism in barley seedlings.
3. Physiological Indicators of Barley Seedlings
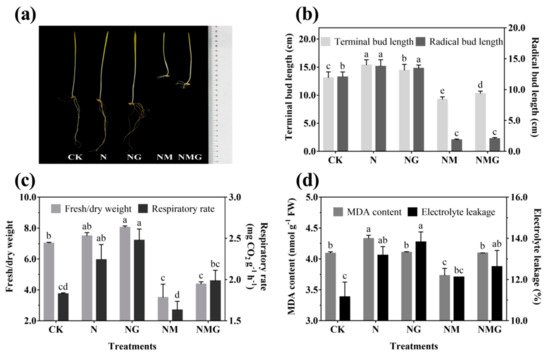
The morphology of 6-day-old barley seedlings under different treatments is shown in Figure 2a. The terminal and radical bud length increased significantly under NaCl (N) and NaCl + GABA (NG) treatments (Figure 2b). The growth of barley seedlings was inhibited by 3-MP treatment (NM), especially the radical length, but the terminal length was improved by the addition of GABA (NMG) (Figure 2a,b). NaCl and GABA not only promoted the growth of barley seedlings, but also increased their ratio of fresh to dry weight and their respiration rate (Figure 2c). The ratio of fresh to dry weight and the respiration rate of barley seedlings treated with GABA were much higher than the others, which were significantly increased by 13.65% and 35.52% compared with the control (CK), respectively. Oxidative damage of barley seedlings was deepened by NaCl (Figure 2d), mainly manifested by the increase of MDA content and electrolyte leakage. However, there was no significant difference in the MDA content of barley seedlings under NG compared with CK, while electrolyte leakage was significantly increased by 23.84%. In short, NaCl and exogenous GABA could stimulate the growth of barley seedlings, increase the permeability of cell membranes, and accelerate cell growth and metabolism.
Figure 2. The effect of GABA on the growing status (
a), terminal/radical bud length (
b), ratio of fresh to dry weight and respiratory rate (
c), and MDA content and electrolyte leakage (
d) of 6-day-old barley seedlings under 20 mmol L
−1 NaCl. The lower case letters for each index indicate significant differences at
p < 0.05 among different treatments. One-way ANOVA was used for data analysis. The data were presented as mean ± SD,
n ≥ 4.
4. Discussion
There have been a large number of studies on the metabolism and accumulation of GABA under salt stress. It has been found that high concentrations of salt increased GABA content, but inhibited the growth of plants at the same time
[17]. However, this study showed that low NaCl concentration (20 mmol L
−1) did not inhibit the growth of barley seedlings, but promoted the growth status (
Figure 2). Simultaneously, the endogenous GABA level significantly increased (
Figure 1a), due to a higher level of GAD activity compared with CK (
Figure 1b). GABA, as a signal molecule, has been widely studied in plant stress signal transmission
[2]. This study found that total phenolics and flavonoids contents were significantly increased by GABA under N treatment, and decreased by 3-MP. The further added GABA had a certain recovery effect on total phenolics and flavonoids contents (
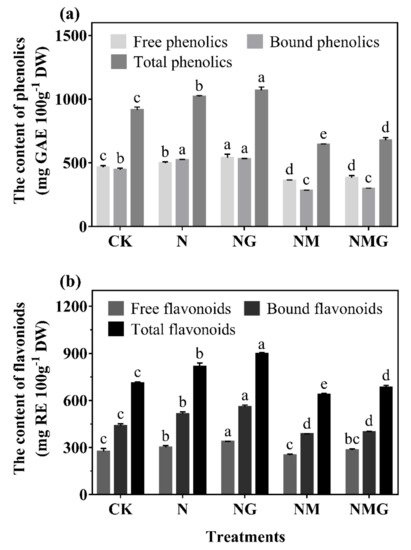
Figure 3), indicating that GABA participated in the synthesis of phenolics under N treatment.
Phenolics are mainly distributed in the cortex of plants, combined with cellulose, hemicellulose, and other non-starch polysaccharides
[12], which play a critical role in resisting adversity and protecting plants from abiotic and biotic stress
[13]. The synthesis of phenolics is closely related to the growth of plants. NaCl (20 mmol L
−1) significantly promoted the growth of barley seedlings and increased phenolics content. GABA further promoted the above effects under N treatment (
Figure 2 and
Figure 3), indicating that GABA promoting the accumulation of phenolics was primarily related to the promotion of growth of barley seedlings. Total phenolics and flavonoids contents of barley seedlings increased significantly by 11.64% and 14.52% under N treatment, respectively (
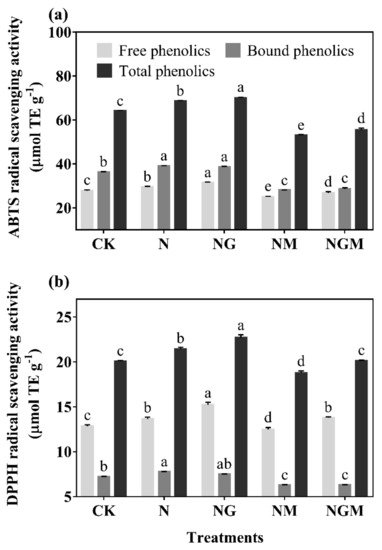
Figure 3), and led to an increase of ABTS and DPPH free radical scavenging ability (
Figure 4). As a signal molecule, GABA plays a key role in the response of plants to external stimuli. Under salt stress, GABA accumulated in germinated soybean
[4], faba bean
[20], and brown rice
[21]. At the same time, GABA promoted the synthesis of phenolics under NaCl stress (60 mmol L
−1) in barley seedlings
[13]. Although low concentrations of NaCl promoted the growth and biomass accumulation of barley seedlings in the present study, MDA content and electrical leakage also increased significantly (
Figure 2d), indicating that low concentration of NaCl also slightly caused damage to the tissue and cells of seedlings. Simultaneously, the synthesis of endogenous GABA was also activated (
Figure 1), and GABA participated in phenolics synthesis of barley seedlings stimulated by low concentration of NaCl (20 mmol L
−1).
Figure 3. The effect of GABA on phenolics (a) and flavonoids (b) contents in 6-day-old barley seedlings under 20 mmol L−1 NaCl. The lower case letters indicate significant differences at p < 0.05 among different treatments. One-way ANOVA was used. The data are presented as mean ± SD, n ≥ 3.
Figure 4. The effect of GABA on the ABTS (a) and DPPH (b) radical scavenging capacities in 6-day-old barley seedlings under 20 mmol L−1 NaCl. The lower case letters indicate significant differences at p < 0.05 among different treatments. One-way ANOVA was used. The data are presented as mean ± SD, n = 3.
As the main phenolics in barley seedlings, the composition and contents of phenolic acids and flavonoids were affected by GABA. GABA mainly increased the contents of bound phenolic acids and flavonoids under N treatment, especially for caffeic acid, ferulic acid (
Table 1), fisetin, myricetin, and quercetin (
Table 2). It showed that GABA had different effects on the existence and contents of various phenolic acids and flavonoids. However, 3-MP significantly decreased the contents of bound phenolic acids and flavonoids, especially bound protocatechuic acid,
p-hydroxybenzoic acid, and catechinic acid, illustrating that endogenous GABA had an important effect on the synthesis of phenolics in barley seedlings treated with NaCl, which improved the resistance to abiotic stress of plants
[2]. In this study, there was no stress under 20 mmol L
−1 NaCl, so the mechanism of GABA promoting phenolics and enhancing antioxidant capacity was different. GABA promoted the growth of barley seedlings, increasing the metabolism rate of cells and demand for material energy, resulting in the increased demand for plant cell wall components, including phenolic acids and flavonoids, leading to the synthesis of large amounts of phenolics.
Table 1. The individual phenolic acid content in barley seedlings.
| Type |
Treatments |
Content (µg g−1 DW) |
| Free |
Bound |
Total |
| Gallic acid |
CK |
12.40 ± 0.20 e |
ND |
12.40 ± 0.20 e |
| N |
258.52 ± 2.95 c |
ND |
258.52 ± 2.95 c |
| NG |
361.63 ± 1.04 a |
ND |
361.63 ± 1.04 a |
| NM |
212.65 ± 3.38 d |
ND |
212.65 ± 3.38 d |
| NMG |
281.46 ± 0.65 b |
ND |
281.46 ± 0.65 b |
| Protocatechuic acid |
CK |
320.70 ± 22.57 c |
5.81 ± 0.21 a |
326.50 ± 22.79 c |
| N |
ND |
5.37 ± 0.09 b |
5.37 ± 0.09 d |
| NG |
622.01 ± 12.53 b |
5.90 ± 0.06 a |
627.90 ± 12.58 b |
| NM |
597.17 ± 40.89 b |
ND |
597.17 ± 40.89 b |
| NMG |
692.15 ± 5.09 a |
ND |
692.15 ± 5.09 a |
| p-Hydroxybenzoic acid |
CK |
6.10 ± 0.15 b |
3.56 ± 0.05 c |
9.66 ± 0.20 c |
| N |
11.08 ± 0.48 a |
4.56 ± 0.01 b |
15.64 ± 0.47 b |
| NG |
11.42 ± 0.17 a |
5.45 ± 0.04 a |
16.87 ± 0.21 a |
| NM |
5.39 ± 0.03 c |
ND |
5.39 ± 0.03 d |
| NMG |
5.83 ± 0.05 bc |
ND |
5.83 ± 0.05 d |
| Vanillic acid |
CK |
23.27 ± 0.12 a |
11.33 ± 0.10 c |
34.61 ± 0.02 a |
| N |
9.64 ± 0.62 b |
13.04 ± 0.13 b |
22.68 ± 0.75 b |
| NG |
5.26 ± 0.50 c |
15.20 ± 0.12 a |
20.46 ± 0.37 c |
| NM |
ND |
6.51 ± 0.03 d |
6.51 ± 0.40 e |
| NMG |
3.92 ± 0.19 d |
5.87 ± 0.40 e |
9.79 ± 0.22 d |
| Caffeic acid |
CK |
9.75 ± 0.01 c |
13.16 ± 0.06 b |
22.90 ± 0.07 c |
| N |
13.75 ± 0.02 b |
13.18 ± 0.25 b |
26.92 ± 0.27 b |
| NG |
15.20 ± 0.07 a |
104.20 ± 1.42 a |
119.40 ± 1.49 a |
| NM |
9.04 ± 0.02 d |
7.32 d |
16.36 ± 0.02 e |
| NMG |
8.54 ± 0.08 e |
10.13 ± 0.08 c |
18.67 d |
| Syringic acid |
CK |
1.08 ± 0.01 c |
ND |
1.08 ± 0.01 c |
| N |
2.51 ± 0.02 a |
ND |
2.51 ± 0.02 a |
| NG |
2.34 ± 0.04 b |
ND |
2.34 ± 0.04 b |
| NM |
ND |
ND |
ND |
| NMG |
ND |
ND |
ND |
| p-Coumaric acid |
CK |
ND |
508.30 ± 0.97 b |
508.30 ± 0.97 b |
| N |
ND |
521.87 ± 0.42 a |
521.87 ± 0.42 a |
| NG |
2.47 ± 0.08 a |
470.17 ± 1.75 c |
472.64 ± 1.83 c |
| NM |
ND |
69.02 ± 0.82d e |
69.02 ± 0.82 e |
| NMG |
ND |
74.88 ± 1.00 d |
74.88 ± 1.00 d |
| Ferulic acid |
CK |
ND |
2463.52 ± 2.59 b |
2463.52 ± 2.59 b |
| N |
ND |
3129.04 ± 6.19 a |
3129.04 ± 6.19 a |
| NG |
2.48 ± 0.01 a |
3144.07 ± 9.93 a |
3146.55 ± 9.93 a |
| NM |
ND |
1448.45 ± 14.51 d |
1448.45 ± 14.51 d |
| NMG |
ND |
1632.20 ± 13.02 c |
1632.20 ± 13.02 c |
| Sinapinic acid |
CK |
30.17 ± 0.02 c |
30.11 ± 0.17 b |
60.28 ± 0.19 c |
| N |
39.03 ± 0.42 a |
34.47 ± 0.60 a |
73.50 ± 1.02 a |
| NG |
37.20 ± 0.39 b |
34.39 ± 0.17 a |
71.58 ± 0.21 b |
| NM |
ND |
30.25 ± 0.09 b |
30.25 ± 0.09 d |
| NMG |
ND |
28.53 ± 0.68 c |
28.53 ± 0.68 e |
Table 2. The individual flavonoid content in barley seedlings.
| Type |
Treatments |
Content (µg g−1 DW) |
| Free |
Bound |
Total |
| Catechinic acid |
CK |
22.90 ± 0.87 b |
23.43 b |
46.33 ± 0.86 b |
| N |
24.17 ± 0.36 b |
24.49 ± 0.13 a |
48.66 ± 0.23 b |
| NG |
49.06 ± 1.96 a |
21.71 ± 0.32 c |
70.77 ± 2.29 a |
| NM |
19.98 ± 0.24 c |
ND |
19.98 ± 0.24 c |
| NMG |
19.85 ± 0.37 c |
ND |
19.85 ± 0.37 c |
| Fisetin |
CK |
159.78 ± 0.33 d |
326.62 ± 10.99 c |
486.40 ± 10.67 c |
| N |
166.63 ± 1.42 c |
593.28 ± 10.52 b |
759.92 ± 11.94 b |
| NG |
201.63 ± 2.61 a |
657.37 ± 6.68 a |
859.00 ± 9.29 a |
| NM |
179.71 ± 2.21 b |
196.24 ± 0.83 d |
375.95 ± 1.38 d |
| NMG |
156.69 ± 0.39 d |
203.00 ± 3.37 d |
359.69 ± 3.76 d |
| Myricetin |
CK |
44.68 ± 0.69 b |
321.30 ± 0.80 b |
365.99 ± 1.50 c |
| N |
44.60 ± 0.83 b |
415.11 ± 12.03 a |
459.71 ± 12.86 b |
| NG |
53.26 ± 3.47 a |
425.13 ± 3.39 a |
478.39 ± 6.85 a |
| NM |
33.83 ± 0.36 c |
ND |
33.83 ± 0.36 e |
| NMG |
ND |
154.85 ± 6.71 c |
154.85 ± 6.71 d |
| Quercetin |
CK |
ND |
496.19 ± 11.89 b |
496.19 ± 11.89 b |
| N |
ND |
452.24 ± 10.48 c |
452.24 ± 10.48 c |
| NG |
ND |
541.64 ± 9.71 a |
541.64 ± 9.71 a |
| NM |
ND |
164.75 ± 5.84 d |
164.75 ± 5.84 d |
| NMG |
ND |
158.24 ± 4.78 d |
158.24 ± 4.78 d |
| Apigenin |
CK |
0.057 ± 0.001 d |
0.707 ± 0.049 c |
0.764 ± 0.048 c |
| N |
0.096 ± 0.002 b |
0.997 ± 0.025 b |
1.093 ± 0.027 b |
| NG |
0.110 ± 0.006 a |
1.099 ± 0.005 a |
1.209 ± 0.001 a |
| NM |
0.069 ± 0.002 c |
0.270 ± 0.009 e |
0.340 ± 0.007 d |
| NMG |
ND |
0.368 ± 0.002 d |
0.368 ± 0.002 d |
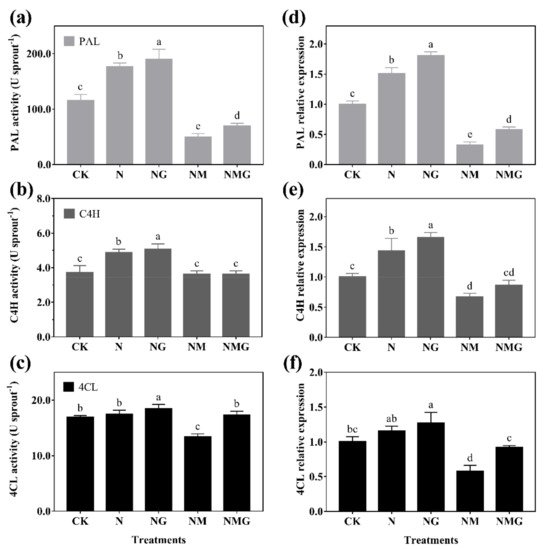
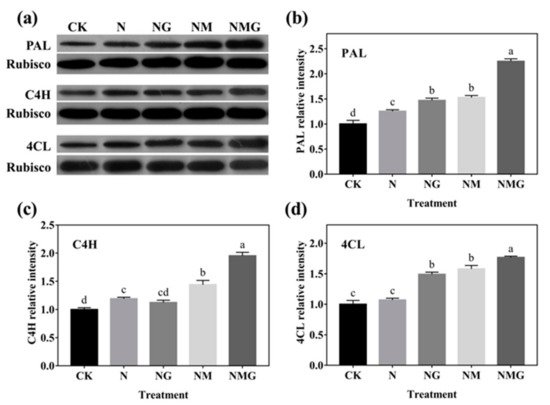
In addition, PAL, C4H, and 4CL are three key rate-limiting enzymes of the phenylpropane metabolism pathway. Their activity and expression determine the synthesis of phenolics. In this study, PAL and C4H activity were enhanced significantly by GABA in barley seedlings under N treatment, which was basically consistent with their gene and protein expression (Figure 5 and Figure 6). Interestingly, 3-MP suppressed the activities of PAL, C4H, and 4CL, while increasing their protein expression, because 3-MP caused damage to cells, which broke the balance of osmotic pressure and inhibited the growth of seedlings. Simultaneously, the activities and mRNA levels of PAL, C4H, and 4CL decreased significantly. More antioxidants were needed because the biosynthesis of phenolics synthesis was inhibited by 3-MP, which might be induced by NaCl.
Figure 5. The effect of GABA on PAL activity (a), C4H activity (b), and 4CL activity (c), and their gene expression (d–f), for 6-day-old barley seedlings under 20 mmol L−1 NaCl. The lower case letters indicate significant differences at p < 0.05 among different treatments. One-way ANOVA was used. The data are presented as mean ± SD, n = 4.
Figure 6. The effect of GABA on PAL, C4H, and 4CL protein expression of 6-day-old barley seedlings under 20 mmol L−1 NaCl. Panels show representative bands (a). Histograms represent relative protein levels of PAL (b), C4H (c) and 4CL (d) of 6-day-old barley seedlings normalized to the corresponding rubisco in different treatments. The lower case letters indicate significant differences at p < 0.05 among different treatments. One-way ANOVA was used. The data are presented as mean ± SD, n = 3.