| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Isabella Gigante | + 2716 word(s) | 2716 | 2020-07-28 08:46:35 | | | |
| 2 | Bruce Ren | Meta information modification | 2716 | 2020-08-04 04:06:01 | | | | |
| 3 | Bruce Ren | Meta information modification | 2716 | 2020-08-04 04:06:25 | | |
Video Upload Options
Colorectal cancer (CRC) is the third most common cancer worldwide. There is a need for the early diagnosis of CRC for a better prognostic outcome. It is, therefore, crucial to understand the CRC pathogenesis in all its aspects. In many cases, one of the main causes of cancer-related deaths is the presence of metastases. In this context, an often overlooked aspect is the metastatic tropism, since CRC, like other cancers, is more prone to metastasize some organs rather than others. Beyond the liver and lung, and differently from other types of cancers, a not usual site of CRC metastases is the bone. However, it may assume a crucial role in the development and the outcome of the disease. Therefore, this review aims to discuss the complex relations between bone markers and CRC pathogenesis, suggesting the use of these molecules as potential targets for therapeutic purposes. Different osteogenic molecules, some of whom are growth factors and are implicated in the different osteogenic pathways, have been proved to also be involved in CRC progression. Some of them are oncogenes, while others oncosuppressors, and in a future perspective, some of them may represent new potential CRC biomarkers.
1. Introduction
Colorectal cancer (CRC) is widespread across the world; it represents one of the most common cancers, and is among the leading causes of tumor death. Although the etiology of CRC relies on genetic causes, other factors (e.g., family history, inflammatory bowel disease, sex, smoking, folate intake, high intake of fats, alcohol, red and processed meats, sugars) can often actively contribute to its onset [1][2]. Besides, in 70% of CRC cases, it develops from previous neoplasms, such as colorectal bowel adenomatous polyps [3]. The ability of healthy colon epithelial cells to transform into neoplastic cells through the adenoma–carcinoma sequence has been largely described [4]. This sequence is regulated by oncogenes and oncosuppressors, subject to mutations and dysregulations that favor tumor development. High-performance techniques allow for quickly associating phenomena of genomic instability correlated with those processes promoting the development of cancer. These advances point to the possibility of early diagnosis and related therapeutic intervention [5].
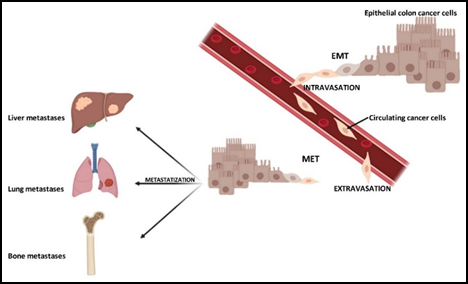
A large part of the mortality rate from cancer, as well as tumor recidivism, is due to the staging and the presence or absence of metastases, where it has metastasized and whether there are micro- or macro-metastases. The process of the formation of metastases is characterized by several steps, all fundamental and essential to each other. These steps are not yet fully understood, as well as the molecular pathways that may regulate their occurrence, which genes are expressed and which are not. It is necessary that epithelial cells, following genetic mutations, become the trigger of what then evolves into carcinoma in situ. Later, some cancer cells detach from the primary mass to spread and place themselves in a distant site in the body where they form a metastasis [6]. This process is possible due to the occurrence of a process known as the “epithelial–mesenchymal transition” (EMT).
In this sequence of events, epithelial cells lose their different types of cell–cell and cell–extracellular matrix (ECM) junctions, and the apical–basal polarity, while acquiring an invasive, migratory capacity and secreting multiple components of the ECM [7]. Consequently, these cells are termed circulating tumor cells (CTCs), which invade the ECM, and enter the vascular system. Thanks to the blood flow, CTCs can reach a more distant site, where inputs of the “mesenchymal–epithelial transition” (MET) allow them to acquire capacities to perform extravasation and spread in the parenchyma. Following the inversion of the EMT process, cancer cells acquire epithelial properties again and, first of all, the high proliferative rate to create metastases [8].
Each tumor has preferential sites in which it produces metastases, the so called metastatic tropism [9]. Cancer formation and progression cannot be detached from cancer stem cells (CSCs). CSCs are fundamental in different aspects of tumorigenesis, such as tumor transformation, progression, therapeutic resistance and in metastatic tropism and, consequently, in the formation of metastases [10][11]. Therefore, a greater understanding of these mechanisms is crucial. The injection of allograft-derived pancreatic cancer tumor stem cells into wild type mice [9] demonstrated the production of metastases only in the liver or lung and liver, depending on whether the cell pool inoculation had been done by intrasplenic injection or in the caudal vein, respectively. It was also shown that the size of the metastatic masses is larger when they form in the liver than the lung. Overall, these findings support how metastatic tropism is affected by the presence of direct blood flow that, starting from the inoculation site, can reach distant organs. Following this event, the implantation of CSCs and the production of metastases is then influenced by the microenvironment of the host organ, which may be more or less suitable.
As for CRC, after total surgical removal of the primary tumor mass, recurrences in the form of metastases can occur preferentially in the liver, lungs, lymph nodes, peritoneum and bone [12] (Figure 1). In this context, the colon and bone tissue, apparently so distant, have something in common. A disease in one of these apparatuses may well affect the physiological state of the other. They are more related than one can imagine. This review aims to describe what is known in the literature, reporting the state of the art on this topic.
Figure 1. Colorectal cancer and its metastatic tropism. Primary tumor cells can be subjected to epithelial–mesenchymal transition (EMT), in order to generate mesenchymal cells with more motility and invasiveness. These mesenchymal cells enter the bloodstream, becoming circulating cancer cells (intravasation). Through the blood flow and under cellular signals, these cells reach distant sites where they metastasize. At this point, the circulating cancer cells come out from the blood stream (extravasation), undergo an inverse transformation, namely mesenchymal–epithelial transition (MET). Metastases are formed in preferential sites (metastatic tropism), such as liver, lung or bone.
2. CRC and Bone Metastases
Compared to liver and lung metastases, bone metastases in CRC occur only in 10–15% of cases [13]. In such patients, the five-year prognosis is less than 5% [14]. The diagnostic picture of these patients is very often characterized by skeletal-related events (SREs), which makes the clinical course of the disease worse. SREs can be constituted by the weakening of the bone structure, at both the trabecular and cortical level, and bone pain, as well as a higher probability of fractures. These pathological events worsen the patient’s survival and their quality of life [15][16]. In addition, gender and age are among the factors related to poor survival, Babu et al. presented a clinical study in which CRC patients with bone metastases were male and young. However, whether sex affects the prognosis of these subjects needs to be deeply investigated [17].
Santini et al. [18] collected the clinical data of a cohort of Italian CRC patients with different skeletal problems and bone metastases. According to their findings, the most affected bones by CRC metastases were the spine (65% of cases), hip/pelvis (34% of cases), long bones (26% of cases) and other bone sites (17% of cases). These percentages highlight the need for the early diagnosis of bone problems related to CRC and, therefore, for an equally early intervention to improve and extend the patient’s survival. To perform a timely early diagnosis of bone metastases, a scoring technique has been assessed using different clinical factors, such as tumor localization, lymph node metastases, and, finally, the presence of metachronous lung metastases as a third risk factor. This scoring technique can help clinicians immediately identify CRC patients most at risk for the development of bone metastases and make it possible to intervene directly with suitable therapies and relieve bone metastasis-related SREs [19][20].
Baek et al. [21] reported that only 1.1% of 5479 CRC patients showed CRC-related bone metastases. Most of these patients were at a late stage of cancer at the time of the CRC diagnosis. Bone metastases were already present at diagnosis in half of them, while the other half of the patients developed bone metastases during the course of the disease. As expected and independent from the presence of metastases in other organs, the presence of bone metastases is also associated with the presence of different SREs, and this situation led to painful patient survival.
In CRC, bone metastases usually develop later than those in other organs or tissue, such as liver and lung metastases, and there is a preferential link between bone and lung metastases. The prognosis is more severe in cases in which the metastasis of the tumor involves several sites simultaneously [22]. Bone metastases, perhaps more than others, are highly debilitating because of the various bone-related clinical pictures that they entail. Therefore, it would be helpful to be able to diagnose these metastases in a shorter time compared to their onset. Studies on this topic were performed by evaluating cases of rectal or colon cancer cases individually. Recently, Zhenghong et al. [22] have reported a higher percentage of bone metastases in rectal cancer patients than in CRC patients. Probably, this finding may depend on the broader vascularization in the rectum compared to the colon [23][24].
3. CRC and Bone Marrow
Several studies investigated the interactions between colorectal cancer and bone marrow (BM). Taketo et al. reported that the loss of the oncosuppressor SMAD4 is synonymous with CRC advancement. The authors noted, in both in vitro and in vivo experiments, that the loss of SMAD4 implies the lack of the block of expression of the gene C-C motif chemokine ligand 15 (CCL15) [25][26]. In this circumstance, CCL15 is expressed by cancer cells and induces the recruitment of CCR1+ myeloid cells from BM. The C-C chemokine receptor type 1 (CCR1)+ cells have the characteristic of expressing and secreting matrix metalloproteinase 9 (MMP9), which is involved in tumor invasiveness by promoting tumor–stromal interactions. The analysis of human liver metastases, related to CRC, have shown that CCL15 expression, linked to a higher content of CCR1+ cells, is associated with a lower patient survival with respect to CCL15-negative liver metastases [26][27]. SMAD4 and CCL15 are inversely correlated, since the action of SMAD4 induces a negative regulation of the promoter of CCL15, causing the inhibition of CCL15 gene expression. Moreover, inhibitors of the CCL15–CCR1 axis have been suggested as potential therapeutic agents [26].
The role of BM-derived CCR1+ myeloid cells in CRC pathogenesis was also investigated by others research groups. In this regard, Kiyasu et al. very recently reported that the depletion of CCR1 induced a reduction in CRC growth. In particular, after reconstituting sub-lethally irradiated wild-type mice with the BM of wild-type or CCR1−/− mice, they implanted colorectal cancer cells in these mouse models [28]. They noted that mice with CCR1 cell depletion showed a reduction in tumor growth and liver metastases, with respect to CRC mouse models with wild-type BM. The depletion of CCR1+ myeloid cells, genetically induced or by using an anti-CCR1 antibody, caused a suppression of CRC development, indicating CCR1 as a potential therapeutic target [28].
BM metastases, although rare, characterize CRC tumorigenesis due to their high vascularization. Furthermore, the formation of BM metastases is promoted by the slowness of the bone marrow bloodstream, which helps the deposition of the metastatic cells, and the presence of several growth factors, secreted following interactions between tumor cells and BM stroma [29]. The occurrence of these conditions creates the right conditions for tumor development. Metastases in BM often go unnoticed if they are mild, because they are not yet detectable with the most common imaging techniques, or they are detected late when they are well extended and cause severe pain or osteolytic fractures [29]. BM metastases are commonly observed in different solid tumors, such as breast, lung, prostate and, rarely, in CRC patients [30]. Chuwa et al. very recently described a case report of a CRC patient with BM metastases [31]. In particular, this patient presented disseminated carcinomatosis of bone marrow (DCBM). DCBM was diagnosticated by analysis of a BM biopsy, since the patient presented a persistent pancytopenia. BM biopsy analysis showed the infiltration of non-hematopoietic malignant cells and BM necrosis, pivotal features of DCBM [31]. The micro-metastasis of BM is related to poor prognosis [31][32].
An important role in CRC tumorigenesis is played by mesenchymal stem cells (MSCs). These cells, derived from BM, secrete growth factors, cytokines and chemokines into the stroma of developing tumors [33]. Nishikawa G. et al. reported that MSCs promote CRC progression through C-C chemokine receptor type 5 (CCR5) ligands, such as C-C motif chemokine ligand 3 (CCL3), CCL4 and CCL5. These ligands bind the receptor, CCR5, expressed by CRC cells [34]. The authors also observed that high serum levels of CCR5 ligands are related to a poor prognosis in CRC patients, therefore, CCR5 ligands could have value as predictive biomarkers. As previously reported by other groups, it was noted that an inhibition of CCR5, and consequently a reduction in the MSC–CRC cell interactions, corresponds to a reduction in tumor growth [34][35][36].
Although, to date, there are numerous studies indicating that CRC can present a bone-related symptomatology (i.e., osteolytic lesions, skeletal related events, etc.) due to bone metastases, it remains not fully clarified how CRC cells interact with bone cells. The result of this interaction is an imbalance between functional cells within the bone, i.e., osteoblasts and osteoclasts, usually in favor of the former, resulting in the formation of osteolytic metastases, due to a preeminent osteoclastogenesis [37]. In this process, chemokines play a relevant role, leading to the interaction between cancer and host cells. Different chemokines are implicated in the CRC cells’ chemoattraction to bone tissue, promoting cancer cell metastasis. The metastatic tropism is due to the interactions between ligands present on cancer cells and their specific receptors present on the cells of certain organs, or vice versa. Gong ZC et al. [38] very recently showed the relevance of CCL3, expressed by BM-derived monocytes, in osteoclastogenesis in CRC bone metastases. The authors reported that CRC cell-derived Epidermal Growth Factor (EGF) activates BM-derived monocytes and stimulates their high CCL3 expression. CCL3 promotes osteoclast maturation and, consequently, osteoclastogenesis [38]. Another interaction implicated in cancer cell recruitment has been demonstrated between CXCR4, expressed in CRC cells, and CXCL12, located in BM-derived cells. Furthermore, Itatani Y. et al. [12] described in detail other interactions existing between CRC cells and other myeloid cells.
Several proteins are differently involved in the relations between bone tissue and CRC by promoting tumor cell invasion and increasing the activity of other molecules with possible interferences in osteoinductive processes. A series of molecules, which are involved in these processes to varying degrees, is addressed below (Table 1).
Table 1. Molecular factors and their mechanisms of action in bone tissue and in Colorectal Cancer (CRC).
|
Molecular Factor |
Mechanism of Action in Bone Tissue |
Mechanism of Action in CRC |
References |
|
BMP9 |
Stimulation of the production of bone tissue |
Antitumoral, pro-apoptotic |
|
|
BMP5 |
Stimulation of the production of bone tissue |
Antitumoral, pro-apoptotic |
|
|
OPG |
Protection of bone tissue from the erosive action of osteoclasts |
Oncogene/oncosuppressor |
|
|
OPN |
Involvement in bone remodeling/bone turnover |
Promotion of tumorigenesis |
|
|
BSP |
Involvement in both bone formation and bone erosion |
Protumoral biomarker |
|
|
TRAP |
Osteoclast maturation, bone erosion |
Antitumoral biomarker |
|
|
RUNX2 |
Involvement in osteoblastogenesis |
Protumoral, anti-apoptotic |
|
|
TGFβ1 |
Regulation of the proliferation and differentiation of osteoprogenitor cells |
Antitumoral |
4. Conclusions
Colorectal cancer metastasis is a complex process with many molecular components that act as oncogenes and oncosuppressors. Numerous clinic studies have clarified that bone biomarkers are important players in CRC, as some of them correlate with cancer development and prognosis.
After elucidating the molecular mechanisms that support the bone biomarkers’ actions in CRC pathogenesis, new bone molecule-based therapies may be realized. Interestingly, the manipulation of endogenous bone biomarkers by administering siRNA inhibitors could be useful in modulating the expression of downstream pathways. To date, no therapies targeting these molecules have been developed to treat CRC in human clinical trials. Despite this, the use of these bone molecular factors as therapeutic targets is very promising since they are able to regulate the course of the neoplasm.
In conclusion, although the intestinal tract and bone tissue seem to be so far from each other in terms of anatomy, embryology and physiology, they are more related than one can imagine. Several relations have been demonstrated between these two organs, implicating different molecules. The study of their molecular relations opens new horizons for diagnosis and therapies for CRC patients.
References
- Ahmadi, A.; Hashemi Nazari, S.S.; Mobasheri, M; Does ethnicity affect survival following colorectal cancer? A prospective, cohort study using Iranian cancer registry.. Medical journal of the Islamic Republic of Iran 2014, 28, 83.
- Lori S. Tillmans; Robert A. Vierkant; Alice H. Wang; N. Jewel Samadder; Charles F. Lynch; Kristin E. Anderson; Amy J. French; Robert W. Haile; Lisa J. Harnack; John D Potter; et al.Susan L. SlagerThomas C. SmyrkStephen N. ThibodeauJames R. CerhanPaul J. Limburg Associations between cigarette smoking, hormone therapy, and folate intake with incident colorectal cancer by TP53 protein expression level in a population-based cohort of older women.. Cancer Epidemiology Biomarkers & Prevention 2013, 23, 350-5, 10.1158/1055-9965.EPI-13-0780.
- Robert G. Hardy; Stephen J Meltzer; Janusz Jankowski; ABC of colorectal cancer: Molecular basis for risk factors. BMJ 2000, 321, 886-889, 10.1136/bmj.321.7265.886.
- Kenneth W Kinzler; Bert Vogelstein; Lessons from Hereditary Colorectal Cancer. Cell 1996, 87, 159-170, 10.1016/s0092-8674(00)81333-1.
- Bruce M. Brenner; Daniel Rosenberg; High-throughput SNP/CGH approaches for the analysis of genomic instability in colorectal cancer. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2010, 693, 46-52, 10.1016/j.mrfmmm.2010.04.002.
- Isaiah J. Fidler; The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Reviews Cancer 2003, 3, 453-458, 10.1038/nrc1098.
- Brigitte Boyer; Jean Paul Thiery; Epithelium-mesenchyme interconversion as example of epithelial plasticity. APMIS 1993, 101, 257-268, 10.1111/j.1699-0463.1993.tb00109.x.
- Jean Paul Thiery; Hervé Acloque; Ruby Y.J. Huang; M. Angela Nieto; Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871-890, 10.1016/j.cell.2009.11.007.
- Ryo Okuda; Keisuke Sekine; Daisuke Hisamatsu; Yasuharu Ueno; Takanori Takebe; Yun-Wen Zheng; H. Taniguchi; Tropism of cancer stem cells to a specific distant organ.. In Vivo 2014, 28, 361-365.
- Feng Li; Benjamin Tiede; Joan Massagué; Yibin Kang; Beyond tumorigenesis: cancer stem cells in metastasis. Cell Research 2007, 17, 3-14, 10.1038/sj.cr.7310118.
- Fariba Behbod; Jeffrey M. Rosen; Will cancer stem cells provide new therapeutic targets?. Carcinogenesis 2005, 26, 703-711, 10.1093/carcin/bgh293.
- Yoshiro Itatani; Kenji Kawada; Susumu Inamoto; Takamasa Yamamoto; Ryotaro Ogawa; M. Taketo; Yoshiharu Sakai; The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis. International Journal of Molecular Sciences 2016, 17, 643, 10.3390/ijms17050643.
- Mark L. Sundermeyer; Neal J. Meropol; Andre Rogatko; Hao Wang; Steven J. Cohen; Changing Patterns of Bone and Brain Metastases in Patients with Colorectal Cancer. Clinical Colorectal Cancer 2005, 5, 108-113, 10.3816/ccc.2005.n.022.
- Muhammad A. Khattak; Hilary L. Martin; Carol Beeke; Timothy Price; Scott Carruthers; Susan Kim; Robert Padbury; Christos S. Karapetis; Survival Differences in Patients With Metastatic Colorectal Cancer and With Single Site Metastatic Disease at Initial Presentation: Results From South Australian Clinical Registry for Advanced Colorectal Cancer. Clinical Colorectal Cancer 2012, 11, 247-254, 10.1016/j.clcc.2012.06.004.
- Robert E. Coleman; Skeletal complications of malignancy. Cancer 1997, 80, 1588-1594, 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z.
- Hidetaka Kawamura; Tatsuro Yamaguchi; Yuuta Yano; Takahiro Hozumi; Yasunobu Takaki; Hiroshi Matsumoto; Daisuke Nakano; Keiichi Takahashi; Characteristics and Prognostic Factors of Bone Metastasis in Patients With Colorectal Cancer. Diseases of the Colon & Rectum 2018, 61, 673-678, 10.1097/dcr.0000000000001071.
- Sunny Garg; Mc Suresh Babu; Kc Lakshmaiah; K Govind Babu; Rekha V Kumar; D Loknatha; Linu Jacob Abraham; Lk Rajeev; Kn Lokesh; Ah Rudresha; et al.Suparna Ajit Rao Colorectal cancer presenting as bone metastasis. Journal of Cancer Research and Therapeutics 2017, 13, 80-83, 10.4103/0973-1482.181177.
- Daniele Santini; M. Tampellini; Bruno Vincenzi; Toni Ibrahim; C. Ortega; V. Virzi; N. Silvestris; Rossana Berardi; Cristina Masini; N. Calipari; et al.D. OttavianiV. CatalanoG. BadalamentiR. GiannicolaF. FabbriO. VendittiM. E. FrattoC. MazzaraT. P. LatianoF. BertoliniF. PetrelliA. OttoneC. CarotiL. SalvatoreAlfredo FalconeP. GiordaniRaffaele AddeoM. AgliettaS. CascinuSandro BarniE. MaielloGiuseppe Tonini Natural history of bone metastasis in colorectal cancer: final results of a large Italian bone metastases study. Annals of Oncology 2012, 23, 2072-2077, 10.1093/annonc/mdr572.
- Chang Sun; Yuan Deng; Haiyang Zhou; Zhi Qian Hu; Risk factors for the development of metachronous bone metastasis in colorectal cancer patients after curative resection. International Journal of Surgery 2015, 21, 145-149, 10.1016/j.ijsu.2015.07.706.
- Ang Li; Lukas Käsmann; Dirk Rades; Chuangang Fu; A Scoring System to Predict the Development of Bone Metastasis After Radical Resection of Colorectal Cancer. Anticancer Research 2017, 37, 5169-5172, 10.21873/anticanres.11938.
- Se Jin Baek; Hyuk Hur; Byung-Soh Min; Seung-Hyuk Baik; Kang-Young Lee; Nam Kyu Kim; The Characteristics of Bone Metastasis in Patients with Colorectal Cancer: A Long-Term Report from a Single Institution. World Journal of Surgery 2016, 40, 982-986, 10.1007/s00268-015-3296-x.
- Zhenghong; Zihua Zhu; Guoweijian; Zhangning; Caiyunyun; Yingjiangshan; Xiaomi; Retrospective study of predictors of bone metastasis in colorectal cancer patients.. Journal of Bone Oncology 2017, 9, 25-28, 10.1016/j.jbo.2017.10.003.
- R. Kanthan; J. Loewy; S. C. Kanthan; Skeletal metastases in colorectal carcinomas. Diseases of the Colon & Rectum 1999, 42, 1592-1597, 10.1007/bf02236213.
- Hinganu, D.; Eva, I.; Stan, C.I.; Hinganu, M.V.; Morphological aspects of the rectal neovascularization in colorectal cancer - anatomical-surgical and imaging implications. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie 2016, 57, 161-165.
- R Salovaara; S Roth; A Loukola; V Launonen; P Sistonen; E Avizienyte; P Kristo; H Järvinen; S Souchelnytskyi; M Sarlomo-Rikala; et al.Lauri Aaltonen Frequent loss of SMAD4/DPC4 protein in colorectal cancers. Gut 2002, 51, 56-59, 10.1136/gut.51.1.56.
- Yoshiro Itatani; Kenji Kawada; Teruaki Fujishita; Fumihiko Kakizaki; Hideyo Hirai; Takuya Matsumoto; Masayoshi Iwamoto; Susumu Inamoto; Etsuro Hatano; Suguru Hasegawa; et al.Taira MaekawaShinji UemotoYoshiharu SakaiMakoto Mark Taketo Loss of SMAD4 From Colorectal Cancer Cells Promotes CCL15 Expression to Recruit CCR1+ Myeloid Cells and Facilitate Liver Metastasis. Gastroenterology 2013, 145, 1064-1075.e11, 10.1053/j.gastro.2013.07.033.
- L. Losi; Hanifa Bouzourene; Jean Benhattar; Loss of Smad4 expression predicts liver metastasis in human colorectal cancer.. Oncology Reports 2007, 17, 1095-1099, 10.3892/or.17.5.1095.
- Kiyasu, Y.; Kawada, K.; Hirai, H.; Ogawa, R.; Hanada, K.; Masui, H.; Nishikawa, G.; Yamamoto, T.; Mizuno, R.; Itatani, Y., et al.; et al. Disruption of CCR1-mediated myeloid cell accumulation suppresses colorectal cancer progression in mice. Cancer letters 2020, 487, 53-62.
- Rita Assi; Deborah Mukherji; Ali Haydar; Maya Saroufim; Sally Temraz; Ali Shamseddine; Metastatic colorectal cancer presenting with bone marrow metastasis: a case series and review of literature. Journal of Gastrointestinal Oncology 2016, 7, 284-297, 10.3978/j.issn.2078-6891.2015.092.
- Rolf M. Anner; Benjamin Drewinko; Frequency and significance of bone marrow involvement by metastatic solid tumors. Cancer 1977, 39, 1337-1344, 10.1002/1097-0142(197703)39:3<1337::aid-cncr2820390349>3.0.co;2-x.
- Harrison Chuwa; Nadeem M Kassam; Casmir Wambura; Omar A Sherman; Salim Surani; Disseminated Carcinomatosis of Bone Marrow in an African Man with Metastatic Descending Colon Carcinoma. Cureus 2020, 12, e7593, 10.7759/cureus.7593.
- Carsten T. Viehl; Benjamin Weixler; Ulrich Guller; Salome Dell-Kuster; Rachel Rosenthal; Michaela Ramser; Vanessa Banz; Igor Langer; Luigi Terracciano; Guido Sauter; et al.Daniel OertliMarkus Zuber Presence of bone marrow micro‐metastases in stage I‐III colon cancer patients is associated with worse disease‐free and overall survival. Cancer Medicine 2017, 6, 918-927, 10.1002/cam4.1056.
- Anja Torsvik; Rolf Bjerkvig; Mesenchymal stem cell signaling in cancer progression. Cancer Treatment Reviews 2013, 39, 180-188, 10.1016/j.ctrv.2012.03.005.
- Gen Nishikawa; Kenji Kawada; Jun Nakagawa; Kosuke Toda; Ryotaro Ogawa; Susumu Inamoto; Rei Mizuno; Yoshiro Itatani; Yoshiharu Sakai; Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death & Disease 2019, 10, 264, 10.1038/s41419-019-1508-2.
- Yamato Tanabe; Soichiro Sasaki; N Mukaida; Tomohisa Baba; Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancerassociated fibroblast accumulation. Oncotarget 2016, 7, 48335-48345, 10.18632/oncotarget.10227.
- N. Halama; Inka Zoernig; Anna Berthel; Christoph Kahlert; F. Klupp; Meggy Suarez-Carmona; Thomas Suetterlin; Karsten Brand; Juergen Krauss; Felix Lasitschka; et al.Tina LerchlClaudia Luckner-MindenAlexis UlrichMoritz KochJuergen WeitzMartin SchneiderMarkus W. BuechlerLaurence ZitvogelThomas HerrmannAxel BennerChristina KunzStephan LueckeChristoph SpringfeldNiels GrabeChristine S. FalkDirk Jaeger Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587-601, 10.1016/j.ccell.2016.03.005.
- Meng-Yu Wu; Chia-Jung Li; Giou-Teng Yiang; Yeung-Leung Cheng; Andy P. Tsai; Yueh-Tseng Hou; Yu-Chieh Ho; Ming‐Feng Hou; Pei-Yi Chu; Molecular Regulation of Bone Metastasis Pathogenesis. Cellular Physiology and Biochemistry 2018, 46, 1423-1438, 10.1159/000489184.
- Gong Zi-Chen; Qian Jin; Zhang Yi-Na; Wang Wei; Kang Xia; Xu Wei; Wu Juan; Zheng Wei; Zi-Chen Gong; Jin Qian; et al.Yi-Na ZhangWei WangXia KangWei XuJuan WuWei Zheng Colorectal cancer cells promote osteoclastogenesis and bone destruction through regulating EGF/ERK/CCL3 pathway. Bioscience Reports 2020, 40, 1175, 10.1042/bsr20201175.
- Ana Cláudia Carreira; G. G. Alves; Willian F. Zambuzzi; Mari Cleide Sogayar; Jose M. Granjeiro; Bone Morphogenetic Proteins: Structure, biological function and therapeutic applications. Archives of Biochemistry and Biophysics 2014, 561, 64-73, 10.1016/j.abb.2014.07.011.
- Joseph D Lamplot; Jiaqiang Qin; Guoxin Nan; Jinhua Wang; Xing Liu; Liangjun Yin; Justin Tomal; Ruidong Li; Wei Shui; Hongyu Zhang; et al.Stephanie H KimWenwen ZhangJiye ZhangYuhan KongSahitya DenduluriMary Rose RogersAbdullah PrattRex C HaydonHue H LuuJovito AngelesLewis L ShiTong-Chuan He BMP9 signaling in stem cell differentiation and osteogenesis. American journal of stem cells 2013, 2, 1-21.
- Byeong-Joo Noh; Youn Wha Kim; Yong Koo Park; A Rare Colon Cancer with Ossification: Pathogenetic Analysis of Bone Formation.. Annals of clinical and laboratory science 2016, 46, 428-32.
- Gonzalo Sánchez-Duffhues; Christian Hiepen; Petra Knaus; Peter Ten Dijke; Bone morphogenetic protein signaling in bone homeostasis. Bone 2015, 80, 43-59, 10.1016/j.bone.2015.05.025.
- Nancy E. Epstein; Basic science and spine literature document bone morphogenetic protein increases cancer risk. Surgical Neurology International 2014, 5, S552-S560, 10.4103/2152-7806.148039.
- Jin-Hua Wang; Ying-Zi Liu; Liang-Jun Yin; Liang Chen; Jun Huang; Yang Liu; Ran-Xi Zhang; Long-Yang Zhou; Qiu-Jun Yang; Jinyong Luo; et al.Guo-Wei ZuoZhongliang DengBai-Cheng He BMP9 and COX-2 form an important regulatory loop in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Bone 2013, 57, 311-321, 10.1016/j.bone.2013.08.015.
- Yingze Zhao; Tao Song; Wenjuan Wang; Jin Wang; Juanwen He; Ningning Wu; Min Tang; Baicheng He; Jinyong Luo; P38 and ERK1/2 MAPKs Act in Opposition to Regulate BMP9-Induced Osteogenic Differentiation of Mesenchymal Progenitor Cells. PLOS ONE 2012, 7, e43383, 10.1371/journal.pone.0043383.
- Ana Cuadrado; Angel R. Nebreda; Mechanisms and functions of p38 MAPK signalling. Biochemical Journal 2010, 429, 403-417, 10.1042/bj20100323.
- Xiaoji Luo; Jin Chen; Wen-Xin Song; Ni Tang; Jinyong Luo; Zhong-Liang Deng; Katie A Sharff; Gary He; Yang Bi; Bai-Cheng He; et al.Erwin BennettJiayi HuangQuan KangWei JiangYuxi SuGao-Hui ZhuHong YinYun HeYi WangJeffrey S SourisLiang ChenGuo-Wei ZuoAnthony G MontagRussell R ReidRex C HaydonHue H. LuuTong-Chuan He Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Laboratory Investigation 2008, 88, 1264-1277, 10.1038/labinvest.2008.98.
- Blanca Herrera; Maarten Van Dinther; Peter Ten Dijke; Gareth J. Inman; Autocrine Bone Morphogenetic Protein-9 signals via Activin Receptor Like Kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Research 2009, 69, 9254-9262, 10.1158/0008-5472.CAN-09-2912.
- Liang Duan; Liwei Ye; Rui Wu; Haiyan Wang; Xueru Li; Huan Li; Shimei Yuan; He Zha; Hui Sun; Jin-Yu Zhang; et al.Xian ChenYan ZhangLan Zhou Inactivation of the Phosphatidylinositol 3-Kinase/Akt Pathway is Involved in BMP9-mediated Tumor-suppressive Effects in Gastric Cancer Cells. Journal of Cellular Biochemistry 2015, 116, 1080-1089, 10.1002/jcb.25063.
- Ke Wang; Honglei Feng; Wei Ren; Xiaoxiao Sun; Jinyong Luo; Min Tang; Lan Zhou; Yaguang Weng; Tong-Chuan He; Yan Zhang; et al. BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. Journal of Cancer Research and Clinical Oncology 2011, 137, 1687-1696, 10.1007/s00432-011-1047-4.
- Shuang-Xue Yuan; Dong-Xu Wang; Qiu-Xiang Wu; Chun-Mei Ren; Yang Li; Qian-Zhao Chen; Yu-Hua Zeng; Ying Shao; Jun-Qin Yang; Yan Bai; et al.Pu ZhangYu YuKe WuWen-Juan SunBai-Cheng He BMP9/p38 MAPK is essential for the antiproliferative effect of resveratrol on human colon cancer. Oncology Reports 2015, 35, 939-947, 10.3892/or.2015.4407.
- Fu‑Shu Li; Jun Huang; Mao‑Zhi Cui; Jin‑Ru Zeng; Pei‑Pei Li; Ling Li; Yan Deng; Ying Hu; Bai-Cheng He; De‑Zhong Shu; et al. BMP9 mediates the anticancer activity of evodiamine through HIF‑1α/p53 in human colon cancer cells. Oncology Reports 2019, 43, 415-426, 10.3892/or.2019.7427.
- Erfei Chen; Fangfang Yang; Hongjuan He; QiQi Li; Wei Zhang; Jinliang Xing; Ziqing Zhu; Jingjing Jiang; Hua Wang; Xiaojuan Zhao; et al.Ruitao LiuLei LeiJing DongYuchen PeiYing YangJunqiang PanPan ZhangShuzhen LiuLe DuYuan ZengLei Lei Alteration of tumor suppressor BMP5 in sporadic colorectal cancer: a genomic and transcriptomic profiling based study. Molecular Cancer 2018, 17, 176, 10.1186/s12943-018-0925-7.
- Mathilde Romagnoli; Karine Belguise; Ziyang Yu; Xiaobo Wang; Esther Landesman-Bollag; David C. Seldin; Dany Chalbos; Sophie Barillé-Nion; Pascal Jézéquel; Margaret L. Seldin; et al.Gail E. Sonenshein Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by Blimp-1-dependent repression of BMP-5.. Cancer Research 2012, 72, 6268-78, 10.1158/0008-5472.CAN-12-2270.
- Aaron Sarver; Amy J French; Pedro M. Borralho; Venugopal Thayanithy; Ann L. Oberg; Kevin A.T. Silverstein; Bruce W Morlan; Shaun M Riska; Lisa A. Boardman; Julie M. Cunningham; et al.Subbaya SubramanianLiang WangThomas C. SmyrkCecília M. P. RodriguesStephen N. ThibodeauClifford J. Steer Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC Cancer 2009, 9, 401-401, 10.1186/1471-2407-9-401.
- Erfei Chen; QiQi Li; Hua Wang; Pan Zhang; Xiaojuan Zhao; Fangfang Yang; Lei Lei; MiR-32 promotes tumorigenesis of colorectal cancer by targeting BMP5. Biomedicine & Pharmacotherapy 2018, 106, 1046-1051, 10.1016/j.biopha.2018.07.050.
- D.L Lacey; E Timms; H.-L Tan; M.J Kelley; Colin R. Dunstan; T Burgess; R Elliott; A Colombero; G Elliott; S Scully; et al.H HsuJ SullivanN HawkinsE DavyC CapparelliA EliY.-X QianS KaufmanI SarosiV ShalhoubG SenaldiJ GuoJ DelaneyW.J Boyle Osteoprotegerin Ligand Is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell 1998, 93, 165-176, 10.1016/s0092-8674(00)81569-x.
- Hisataka Yasuda; Nobuyuki Shima; Nobuaki Nakagawa; Kyoji Yamaguchi; Masahiko Kinosaki; Shin-Ichi Mochizuki; Akihiro Tomoyasu; Kazuki Yano; Masaaki Goto; Akihiko Murakami; et al.Eisuke TsudaTomonori MorinagaKanji HigashioNobuyuki UdagawaNaoyuki Takahashi Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences 1998, 95, 3597-3602, 10.1073/pnas.95.7.3597.
- Ingvild Pettersen; Wenche Bakkelund; Bård Smedsrød; Baldur Sveinbjørnsson; Osteoprotegerin is expressed in colon carcinoma cells.. Anticancer Research 2005, 25, 3809-16.
- John G. Emery; Peter McDonnell; Michael Brigham Burke; Keith C. Deen; Sally Lyn; Carol Silverman; Edward Dul; Edward R. Appelbaum; Chris Eichman; Rocco DiPrinzio; et al.Robert A. DoddsI.E. JamesMartin RosenbergJohn C. LeeP R Young Osteoprotegerin Is a Receptor for the Cytotoxic Ligand TRAIL. Journal of Biological Chemistry 1998, 273, 14363-14367, 10.1074/jbc.273.23.14363.
- Ingunn Holen; Claire M. Shipman; Role of osteoprotegerin (OPG) in cancer. Clinical Science 2006, 110, 279-291, 10.1042/cs20050175.
- E. N. De Toni; S. E. Thieme; Andreas Herbst; A. Behrens; P. Stieber; A. Jung; H. Blum; Burkhard Göke; F. T. Kolligs; OPG Is Regulated by -Catenin and Mediates Resistance to TRAIL-Induced Apoptosis in Colon Cancer. Clinical Cancer Research 2008, 14, 4713-4718, 10.1158/1078-0432.ccr-07-5019.
- Enrico N De Toni; Rothea Nagel; Alexander Philipp; Andreas Herbst; Isabel Thalhammer; Julia Mayerle; Helga-Paula Török; Lydia Brandl; Frank T. Kolligs; Correlation Between Baseline Osteoprotegerin Serum Levels and Prognosis of Advanced-Stage Colorectal Cancer Patients. Cellular Physiology and Biochemistry 2018, 45, 605-613, 10.1159/000487101.
- Helgi Birgisson; Kostas Tsimogiannis; Eva Freyhult; Masood Kamali-Moghaddam; Plasma Protein Profiling Reveal Osteoprotegerin as a Marker of Prognostic Impact for Colorectal Cancer. Translational Oncology 2018, 11, 1034-1043, 10.1016/j.tranon.2018.05.012.
- E.W. Duiker; C.H. Mom; S. De Jong; P.H.B. Willemse; Jourik A Gietema; A.G.J. Van Der Zee; E. G. E. De Vries; The clinical trail of TRAIL. European Journal of Cancer 2006, 42, 2233-2240, 10.1016/j.ejca.2006.03.018.
- S. Tsukamoto; T. Ishikawa; S. Iida; M. Ishiguro; Kaoru Mogushi; H. Mizushima; H. Uetake; H. Tanaka; K. Sugihara; Clinical Significance of Osteoprotegerin Expression in Human Colorectal Cancer. Clinical Cancer Research 2011, 17, 2444-2450, 10.1158/1078-0432.ccr-10-2884.
- Hyun-Soo Kim; Gun Yoon; Sung-Im Do; Sung-Joo Kim; Youn-Wha Kim; Down-regulation of osteoprotegerin expression as a novel biomarker for colorectal carcinoma. Oncotarget 2016, 7, 15187-15199, 10.18632/oncotarget.7885.
- J. Šodek; B. Ganss; M.D. McKee; Osteopontin. Critical Reviews in Oral Biology & Medicine 2000, 11, 279-303, 10.1177/10454411000110030101.
- David T. Denhardt; Masaki Noda; Osteopontin expression and function: Role in bone remodeling. Journal of Cellular Biochemistry 1998, 72, 92-102, 10.1002/(sici)1097-4644(1998)72:30/31+<92::aid-jcb13>3.0.co;2-a.
- Wang Likui; Wang Hong; Zhang Shuwen; Clinical Significance of the Upregulated Osteopontin mRNA Expression in Human Colorectal Cancer. Journal of Gastrointestinal Surgery 2009, 14, 74-81, 10.1007/s11605-009-1035-z.
- Deepak Agrawal; Tingan Chen; Rosalyn Irby; John Quackenbush; Ann F. Chambers; Marianna Szabo; Alan Cantor; Menico Coppola; T.J. Yeatman; Osteopontin identified as colon cancer tumor progression marker.. Comptes Rendus Biologies 2004, 326, 1041-1043, 10.1016/j.crvi.2003.09.007.
- Philip Y. Wai; Zhiyong Mi; Hongtao Guo; Shiva Sarraf-Yazdi; Chengjiang Gao; Junping Wei; Carlos E. Marroquin; Bryan Clary; Paul C. Kuo; Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis 2005, 26, 741-751, 10.1093/carcin/bgi027.
- Wang Likui; Wang Hong; Zhang Shuwen; You Yuangang; Wen Yan; The Potential of Osteopontin as a Therapeutic Target for Human Colorectal Cancer. Journal of Gastrointestinal Surgery 2011, 15, 652-659, 10.1007/s11605-011-1445-6.
- Xin-Lin Wu; Kai-Jin Lin; Ai-Ping Bai; Wan-Xiang Wang; Xing-Kai Meng; Xiu-Lan Su; Ming-Xing Hou; Pei-De Dong; Jun-Jing Zhang; Zhao-Yang Wang; et al.Lin Shi Osteopontin knockdown suppresses the growth and angiogenesis of colon cancer cells. World Journal of Gastroenterology 2014, 20, 10440-10448, 10.3748/wjg.v20.i30.10440.