
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hakan Aldskogius | + 3863 word(s) | 3863 | 2021-08-31 12:28:38 | | | |
| 2 | Camila Xu | -1747 word(s) | 2116 | 2021-10-13 07:50:50 | | |
Video Upload Options
Dorsal root injury results in regional loss of sensorimotor function, and often severe neuropathic pain. Studying pathophysiological mechanisms of dorsal root injury and the development of novel treatments for this condition may contribute to therapeutic progress also for direct injuries to the spinal cord.
1. Introduction
Disorders that damage dorsal roots include compression, (e.g., from herniated disc), infections, autoimmune mechanisms, metabolic disorder, neurotoxic agents, and trauma. Clinically, the most important type of traumatic injury of the dorsal root is spinal root avulsion, a forceful, longitudinal traction of spinal nerve roots, interrupting their connections with the spinal cord. This condition may occur during complicated delivery, from a fall from a high height, or in a traffic accident, and most commonly affects spinal nerve roots innervating the upper extremity, i.e., brachial plexus avulsion.
Root avulsion injuries in experimental animals are typically performed by a surgical approach after exposure of the spinal cord and appropriate root entry regions to achieve standardized and reproducible lesions. However, in clinical root avulsion injuries, the forces that pull the roots from the spinal cord are usually exercised from outside the vertebral canal. The lesion is variable in location and extent of injury, and with possible differences in, for example, the consequences for the regional vascular supply. In terms of location, clinical avulsion injuries can occur distal (postganglionic injury) or proximal (preganglionic injury) to the dorsal root ganglia [1]. The postganglionic avulsion injury has the character of a peripheral nerve injury with the possibility for functional recovery, whereas a preganglionic avulsion injury is, in principle, a longitudinal injury of the spinal cord at the entry of spinal nerve roots, and thus provides similar therapeutic challenges as other spinal cord injuries [2].
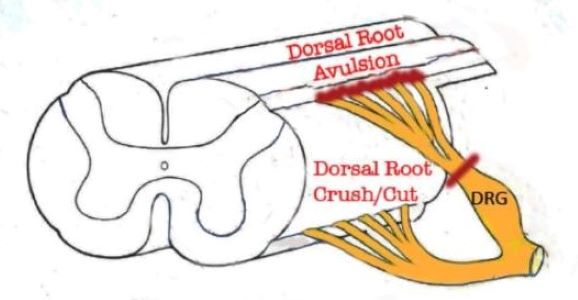
Figure 1. Portion of the spinal cord showing principal locations of dorsal root injuries: Crush/Cut in the peripheral compartment of the dorsal root, and Avulsion, which interrupts dorsal root axons creates a longitudinal injury of the spinal cord itselr.
Spinal root avulsion injuries typically affect ventral and well as dorsal roots, causing paralysis of denervated muscles, loss of sensory and autonomic function, and, most often, neuropathic pain. Cauda equine syndrome, which encompasses avulsion of lumbosacral roots, also leads to weakness or paralysis of muscles controlling micturition, defecation, and sexual functions. Surgical treatment of spinal root avulsion offers a possibility for partial functional restoration of motor function by reinnervation of proximally located muscles, but clinically efficient treatment for restoring sensory function is thus far not available [3]. Thus, dorsal root avulsion exhibits therapeutic challenges specific for this condition but also features clearly relevant for other forms of spinal cord injury. Mechanisms that are implicated in spinal cord pathophysiology and repair are covered in numerous recent reviews, e.g., reference [4].
Axotomy (crushing or cutting) of the dorsal root in its peripheral compartment allows investigation of how axonal extension can be promoted, first along with Schwann cells, and thereafter when encountering central glia, which reacts to the injury at a distance, i.e., without damage to the spinal cord itself. On the other hand, the most centrally located dorsal root injury, dorsal root avulsion, will damage the DREZ, including its central glial component, and thereby constitute a true CNS injury, but isolated to the dorsal root axons and their immediate surroundings [2]. In this brief entry, we describe how DREZ becomes a barrier for axonal ingrowth, give examples of previous and current research exploring how to overcome this barrier in the adult nervous system, and how future spinal cord injury research can benefit from using dorsal root injury models.
2. Promoting Regenerative Competence after Dorsal Root Injury
An appropriate cell body response, comprising a set of so-called regeneration associated genes (RAGs) [5], combined with the emergence of growth supportive axon-glial interactions at the injury site and during axonal elongation, are fundamental for successful nerve regeneration. Axotomy of dorsal root axons results in a much weaker regenerative response by the sensory neurons than what follows after injury to their peripheral branch. Rodent regenerating dorsal root axons grow with a rate of ca 1 mm/24 h compared to ca 3 mm/24 h for injured peripheral nerve axons [6] and fail to display the molecular modifications that is typical after peripheral nerve injury [7][8][9]. Furthermore, injured dorsal root axons have limited ability to grow for long distances even in a peripheral nerve environment [10][11] unless actions to enhance their intrinsic regenerative potential are taken.
Interventions, e.g., the use of growth factors or cell-based approaches to promote regeneration of dorsal root axons, are likely to result in differential effects depending on neuronal subtype-specific growth factor receptor pattern and cell-matrix interactions signaling systems.
The potential of DRG neurons to mount an efficient regenerative response after dorsal root injury is shown by a so-called conditioning lesion, i.e., an injury to the peripheral sensory axons. This intervention, which is typically performed one to two weeks prior to injury to the central processes of the same sensory neurons induces RAGs in DRG neurons and stimulates growth supportive interactions between dorsal root axons and associated non-neuronal cells, thereby promoting elongation of injured dorsal root axons [12]. The conditioning lesion paradigm has made a significant contribution to our understanding of regeneration failure after injury to primary sensory axons.
Growth factor-mediated growth of dorsal root axons appears to act on intracellular pathways, which at least in part share those implicated in the conditioning lesion response [13], although simultaneous effects on non-neuronal cells at the DREZ may also occur [14]. Importantly, there appears to be a limited time window for neurotrophin-mediated entry of regenerating dorsal root axons since just a short delay of treatment fails to support spinal cord ingrowth [15]. This emphasizes the desire to initiate growth supportive therapy as soon as possible. Dorsal root injury is quite favorable for addressing this aspect.
3. Overcoming Growth Inhibition at the DREZ
Previous studies reported that a conditioning lesion combined with enzymatic degradation of chondroitinase sulfate proteoglycans with chondroitinase ABC [16], their removal through gene deletion [17], blocking Nogo-receptor function [18][19], or integrin activation [20] allow regeneration of injured dorsal root axons into the spinal cord. However, recent experiments indicate that a conditioning lesion in combination with proteoglycan degradation or blocking myelin-associated inhibitors is not sufficient in this context, and that specific growth factor stimulation, in this specific case with GDNF, is required for sensory axon entry and functional recovery [21]. This finding indicates that the mechanisms which prevent spinal cord ingrowth by injured dorsal root axons are still far from clear and need further investigations.
The limited effect on sensory axon spinal cord ingrowth by pharmacological interventions targeting the DREZ may be related to the observation that regenerating sensory axons form synapse-like contacts with non-neuronal cells, possibly NG2-positive glia, rather than traversing the PNS-CNS boundary and enter the spinal cord [22][23][24][25]. This process resembles the appearance of synapse-like connections between injured sensory axons in the dorsal column and NG2 positive glia [26]. Thus, NG2+ glia may provide cues for establishing contacts with regenerating dorsal root axons, perhaps originating from specific sensory neurons. To identify these cues in detail and apply counteracting interventions may contribute to functional dorsal root axon regeneration.
During Wallerian degeneration of peripheral nerves, Schwann cells in cooperation with fibroblasts and immune cells, clear disintegrating axons and myelin in preparation for a growth permissive pathway for axon regeneration. In contrast, myelin debris arising from Wallerian degeneration in the central nervous system remains for long periods of time [27][28]. In humans, residues of degenerating myelin have been observed decades after spinal cord injury. These residues and the associated cellular reactions appear to hamper axonal growth. To speed up myelin clearance in the injured spinal cord is thus a relevant objective for promoting spinal cord injury repair. This issue can be favorably explored after dorsal root injury, which allows examination of myelin degradation and elimination in the spinal cord dorsal column without direct damage to the spinal cord, leaving its vascular supply and tissue architecture essentially unaffected.
The long-term lingering of myelin debris after spinal cord injury is probably the result of an insufficient activation of the macrophage/microglial program required for rapid and complete myelin degradation [29][30][31]. Toll-like receptor signaling mediates myelin phagocytosis [32][33], whereas beta-amyloid precursor clearing enzyme (BACE)1 [34][35], and signal regulatory protein-α (SIRPα) [36] delays this process. These findings provide options for approaches for rapid elimination of myelin debris and, hence, possibly minimize one obstacle for sensory axon extension across the DREZ.
4. Bridges for Dorsal Root Injury Repair
Cell-based bridges, acellular bridges, and their combinations are attractive strategies for the repair of the spinal cord and dorsal root injury. The overall purpose of these strategies is to promote functional recovery by (i) survival/growth support for intrinsic cells,(ii) disease modification to facilitate endogenous repair, and (iii) replacement of lost cells or introduction of relay cells. The implementation of these strategies for spinal cord injury is discussed by Guo [37]. Lin et al. analyze how stem cells and biomimetic material for spinal cord repair interact with the human immune system [38], and Rodríguez-Barrera addresses the potential of recruitment from endogenous stem cells of cells for spinal cord injury repair [39]. Here, we discuss bridges aimed to promote functional growth of injured dorsal root axons into the spinal cord.
Early studies aimed to provide a bridge for regenerating dorsal root axons into the spinal cord by exploiting, for example, Schwann cells [40], or activated macrophages [41], both types of cells which cooperate in peripheral nerve regeneration. These attempts were not successful, however; Schwann cells do not readily integrate into a CNS environment [42][43][44], and activated macrophages in a CNS environment tend to induce axonal retraction rather than extension [45].
Olfactory ensheathing cells (OECs) are CNS glia, ensheathing non-myelinated olfactory nerve axons in the intact nervous system, and able to serve as a cellular substrate for regenerating axons. OEC implants readily integrate into non-olfactory CNS regions and have been shown to be able to provide a pathway for sensory axon growth into the spinal cord and restore sensorimotor function after spinal root avulsion [46].
Stem cell-based transplants at the DREZ could facilitate entry of regenerating dorsal root axons, or as neuronal relays to interconnect dorsal root axons in the PNS compartment with spinal cord neurons (see Figure 2). Human neural progenitor transplants were shown to support sensory axon ingrowth and partial recovery of sensory function after dorsal root avulsion, seemingly by creating channels, which facilitated growth across the DREZ [47].
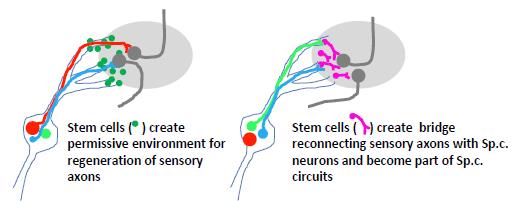
Figure 2. Two possible alternative to implant stem cells at the DREZ. Left: stem cells implanted to faciliate ingrowth of injured sensory axons. Right: stem cells are used to create a neuronal relay where sensory axons are making synaptic contact with implanted stem cells, which, in turn, project their axons into the spinal cord.
In addition, to provide growth-supporting substrate, OEC or stem cell implants are likely to release growth supportive agents and presumably modify immune responses in a way that does not antagonize sensory axon growth across the DREZ. Clarifying the mechanisms underlying these approaches and explore the way to improve their efficiency is thus likely to contribute to the exploitation of cell-based therapies in direct spinal cord injury, e.g., by the administration of exosomes containing defined growth supporting and/or disease-modifying components[37][48].
3D bioprinting has rapidly developed as a highly attractive technology for biomimetic implants that is able to support neuronal survival, counteract disease driving processes and support functional repair following spinal cord injury[37]. Based on detailed information on the underlying pathophysiological processes and conditions of the patient, 3D bioprinted implants may be designed to match patient specific conditions[49]. Recent studies have demonstrated 3D bioprinted constructs that support neurite outgrowth from dorsal root ganglion neurons in vitro[50][51][52].
5. Conclusions
Dorsal root injury offers attractive experimental models to determine key mechanisms in regeneration failure after spinal cord injury, and how to develop novel therapeutic strategies for spinal cord repair, Dorsal root avulsion constitutes a longitudinal spinal cord injury, and is therefore of particular relevance in the broader context of spinal cord injury. A successful strategy to tackle dorsal root avulsion injury most likely requires a combinatorial approach, applied in an appropriate spatial and temporal manner, might include the following actions. i) Cell-based approaches, e.g. via exosomes derived from primed stem cells, or as bioprinted constructs, may be suitable for promoting tissue repair at the injury site., as well as for creating pathways across the DREZ. ii) Intrathecal administration of agents that specifically counteract non-neuronal axon growth inhibitors at the DREZ, as well as along ascending sensory pathways are likely to be needed as additive measures. iii) Application of gene regulatory tools to initiate and maintain axonal growth by dorsal root ganglion neurons. The dorsal root-spinal cord unit provides a favorable platform for assessing the outcome of these interventions as single or combined therapies.
References
- Wade, R.G.; Takwoingi, Y.; Wormald, J.C.R.; Ridgway, J.P.; Tanner, S.; Rankine, J.J.; Bourke, G. Magnetic resonance imaging for detecting root avulsions in traumatic adult brachial plexus injuries: protocol for a systematic review of diagnostic accuracy. Syst. Rev. 2018, 7, 76. doi: 10.1186/s13643-018-0737-2.
- Carlstedt, T.; Havton. L. The longitudinal spinal cord injury: lessons from intraspinal plexus, cauda equina and medullary conus lesions. Handbook Clin. Neurol. 2012, 109, 337-354. doi: 10.1016/B978-0-444-52137- 8.00021-8.
- Carlstedt, T. New Treatments for Spinal Nerve Root Avulsion Injury. Front. Neurol. 2016, 7, 135. doi: 10.3389/fneur.2016.00135. Erratum in: Front. Neurol. 2017, 8, 326.
- Hachem, L.D.; Fehlings, M.G. Pathophysiology of Spinal Cord Injury. Neurosurg Clin N Am. 2021, 32, 305-313. doi: 10.1016/j.nec.2021.03.002.
- Mahar, M.; Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323-337. doi: 10.1038/s41583-018-0001-8.
- Richardson, P.M.; Verge, V.M. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 1987, 4, 11, 406-408. doi: 10.1016/0006-8993(87)91096-1.
- Broude, E.; McAtee, M.; Kelley, M.S.; Bregman, B.S. c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp. Neurol. 1997, 148, 367-377. doi: 10.1006/exnr.1997.6665.
- Schwaiger, F.W.; Hager, G.; Schmitt, A.B.; Horvat, A.; Hager, G.; Streif, R.; Spitzer, C.; Gamal, S.; Breuer, S.; Brook, G.A,; Nacimiento, W.; Kreutzberg, G.W. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur. J. Neurosci. 2000, 12, 1165-1176. doi: 10.1046/j.1460-9568.2000.00005.x.
- Kong, G.; Zhou, L.; Serger, E.; Palmisano, I.; De Virgiliis, F.; Hutson, T.H.; Mclachlan, E.; Freiwald, A.; La Montanara, P.; Shkura, K.; Puttagunta, R.; Di Giovanni, S. AMPK controls the axonal regenerative ability of dorsal root ganglia sensory neurons after spinal cord injury. Nat. Metab. 2020, 2, 918-933. doi: 10.1038/s42255-020-0252-3.
- Richardson, P.M.; Issa, V.M. Peripheral injury enhances central regeneration of primary sensory neurones. Nature 1984, 309, 791-793. doi: 10.1038/309791a0.
- Richardson, P.M.; Verge, V.M. The induction of a regenerative propensity in sensory neurons following peripheral axonal injury. J. Neurocytol. 1986,15, 585-594. doi: 10.1007/BF01611859.
- Yang, X.; Liu, R.; Xu, Y.; Ma, X.; Zhou, B. The Mechanisms of Peripheral Nerve Preconditioning Injury on Promoting Axonal Regeneration. Neural Plast. 2021, 2021:6648004. doi: 10.1155/2021/6648004.
- Teng, F.Y.; Tang, B.L. Axonal regeneration in adult CNS neurons—signaling molecules and pathways. J. Neurochem. 2006, 96, 1501-1508. doi: 10.1111/j.1471-4159.2006.03663.x.
- Hanna-Mitchell, A.T;, O'Leary, D.; Mobarak, M.S.; Ramer, M.S.; McMahon, S.B.; Priestley, J.V.; Kozlova, E.N.; Aldskogius, H.; Dockery, P.; Fraher, J.P. The impact of neurotrophin-3 on the dorsal root transitional zone following injury. Spinal Cord 2008, 46, 804-810. doi: 10.1038/sc.2008.57.
- Ramer, M.S.; Duraisingam, I.; Priestley, J.V.; McMahon, S.B, Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J. Neurosci. 2001, 21, 2651-2660. doi: 10.1523/JNEUROSCI.21-08-02651.2001.
- Steinmetz, M.P.; Horn, K.P.; Tom, V.J.; Miller, J.H.; Busch, S.A.; Nair, D.; Silver, D.J.; Silver, J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J. Neurosci. 2005; 25, 8066-8076.
- Quaglia, X.; Beggah, A.T.; Seidenbecher, C.; Zurn, A.D. Delayed priming promotes CNS regeneration post-rhizotomy in Neurocan and Brevican-deficient mice. Brain 2008, 131(Pt 1), 240-249. doi: 10.1093/brain/awm279.
- Harvey, P.A;, Lee, D.H.; Qian, F.; Weinreb, P.H.; Frank, E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J. Neurosci. 2009, 29, 6285-6295. doi: 10.1523/JNEUROSCI.5885-08.2009.
- Peng, X.; Zhou, Z.; Hu, J.; Fink, D.J.; Mata, M. Soluble nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration. J. Biol. Chem. 2010, 285, 2783-2795. doi: 10.1074/jbc.M109.046425.
- Cheah, M.; Andrews, M.R.; Chew, D.J.; Moloney, E.B.; Verhaagen, J.; Fässler, R.; Fawcett, J.W. Expression of an activated integrin promotes Long-Distance sensory axon regeneration in the spinal cord. J. Neurosci. 2016, 36:7283-7297. doi: 10.1523/JNEUROSCI.0901-16.2016
- Zhai, J.; Kim, H.; Han, S.B.; Manire, M.; Yoo, R.; Pang, S.; Smith, G.M.; Son, Y.J. Co-targeting myelin inhibitors and CSPGs markedly enhances regeneration of GDNF-stimulated, but not conditioning-lesioned, sensory axons into the spinal cord. Elife 2021, 10, e63050. doi: 10.7554/eLife.63050.
- Carlstedt, T. Regenerating axons form nerve terminals at astrocytes. Brain Res. 1985, 347, 188-191. doi: 10.1016/0006-8993(85)90911-4.
- Liuzzi, F.J.; Lasek, R.J. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 1987, 237, 642-645. doi: 10.1126/science.3603044.
- Di Maio, A.; Skuba, A.;Himes, B.T.; Bhagat S.L.; Hyun, J.K.; Tessler, A.; Bishop, D.; Son, Y.-J. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J. Neurosci. 2011, 31, 4569-4582. doi: 10.1523/JNEUROSCI.4638-10.2011.
- Han, SB, Kim H, Skuba A, Tessler A, Ferguson T, Son YJ. Sensory Axon Regeneration: A Review from an in vivo Imaging Perspective. Exp Neurobiol. 2012 Sep;21(3):83-93. doi: 10.5607/en.2012.21.3.83.
- Filous, A.R.; Tran, A.; Howell, C.J.; Busch, S.A.; Evans, T.A.; Stallcup, W.B.; Kang, S.H.; Bergles, D.E.; Lee, S.I.; Levine, J.M.; Silver, J. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J. Neurosci. 2014, 34, 16369-16384. doi: 10.1523/JNEUROSCI.1309-14.2014.
- Bignami, A.; Dahl, D.; Nguyen, B.T.; Crosby, C.J. The fate of axonal debris in Wallerian degeneration of rat optic and sciatic nerves. Electron microscopy and immunofluorescence studies with neurofilament antisera. J. Neuropathol. Exp. Neurol. 1981, 40, 537-550. doi: 10.1097/00005072-198109000-00005.
- George, R.; Griffin, J.W. Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp. Neurol. 1994, 129, 225-236. doi: 10.1006/exnr.1994.1164.
- Liu, L.; Persson, J.K.; Svensson, M.; Aldskogius, H. Glial cell responses, complement, and clusterin in the central nervous system following dorsal root transection. Glia 1998, 23, 221-238.
- Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J. Neuroinflammation 2011, 8, 109. doi: 10.1186/1742-2094-8-109
- McPhail, L.T.; Borisoff, J.F.; Tsang, B.; Hwi, L.P.; Kwiecien, J.M.; Ramer, M.S. Protracted myelin clearance hinders central primary afferent regeneration following dorsal rhizotomy and delayed neurotrophin-3 treatment. Neurosci Lett. 2007, 411, 206-211. doi: 10.1111/j.1460-9568.2004.03837.x.
- Hosmane, S.; Tegenge, M.A.; Rajbhandari, L.; Uapinyoying, P.; Kumar, N.G; Thakor, N.; Venkatesan, A. Toll/Interleukin-1 Receptor Domain-Containing Adapter Inducing Interferon-β Mediates Microglial Phagocytosis of Degenerating Axons. J. Neurosci. 2012, 32, 7745-7757. doi: 10.1523/JNEUROSCI.0203-12.2012.
- Rajbhandari, L.; Tegenge. M.A.; Shrestha, S.; Ganesh Kumar, N.; Malik, A.; Mithal, A.; Hosmane, S.; Venkatesan, A. Toll-like receptor 4 deficiency impairs microglial phagocytosis of degenerating axons. Glia 2014, 62, 1982-1991. doi: 10.1002/glia.22719.
- Farah, M.H.; Pan, B.H.; Hoffman, P.N.; Ferraris, D.; Tsukamoto, T.; Nguyen, T.; Wong, P.C.; Price, D.L, Slusher, B.S.; Griffin, J.W. Reduced BACE (beta-amyloid precursor cleaving enzyme) 1 activity enhances clearance of myelin debris and regeneration of axons in the injured peripheral nervous system. J. Neurosci. 2011, 31, 5744-5754. doi: 10.1523/JNEUROSCI.6810-10.2011;
- Tallon, C.; Rockenstein, E.; Masliah, E.; Farah, M.H. Increased BACE1 activity inhibits peripheral nerve regeneration after injury. Neurobiol Dis. 2017, 106:147-157. doi: 10.1016/j.nbd.2017.07.003.
- Elberg., G.; Liraz-Zaltsman, S.; Reichert, F.; Matozaki, T.; Tal, M.; Rotshenker, S. Deletion of SIRPα (signal regulatory protein-α) promotes phagocytic clearance of myelin debris in Wallerian degeneration, axon regeneration, and recovery from nerve injury. J. Neuroinflammation 2019, 16, 277. doi: 10.1186/s12974-019-167.
- Guo, S.; Redenski, I.; Levenberg, S. Spinal Cord Repair: From Cells and Tissue Engineering to Extracellular Vesicles. Cells 2021, 10(8), 1872. doi.org/10.3390/cells10081872.
- Lin, C.; Ekblad-Nordberg, Å.; Michaëlsson, J.; Götherström, C.; Hsu,C.-C.; Ye, H.; Johansson, J.; Rising, A.; Sundström, E.; Åkesson, E. In Vitro Study of Human Immune Responses to Hyaluronic Acid Hydrogels, Recombinant Spidroins and Human Neural Progenitor Cells of Relevance to Spinal Cord Injury Repair. Cells 2021, 10(7), 1713; doi.org/10.3390/cells10071713
- Rodríguez-Barrera, R; Rivas-González, M.; García-Sánchez, J.; Mojica-Torres, D.; Ibarra, A. Neurogenesis after Spinal Cord Injury: State of the Art. Cells 2021, 10, 1499; doi.org/10.3390/cells10061499 -
- Sims, T.J.; Gilmore, S.A. Regeneration of dorsal root axons into experimentally altered glial environments in the rat spinal cord. Exp. Brain Res. 1994, 99, 25-33. doi: 10.1007/BF00241409.
- Prewitt, C.M.; Niesman, I.R.; Kane, C.J.; Houlé, J.D. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp. Neurol. 1997, 148, 433-443. doi: 10.1006/exnr.1997.6694;].
- Grimpe, B.; Pressman, Y.; Lupa, M.D.; Horn, K.P.; Bunge, M.B.; Silver, J. The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol. Cell. Neurosci. 2005, 28, 18-29. doi: 10.1016/j.mcn.2004.06.010.
- Afshari, F.T.; Kwok, J.C.; Fawcett, J.W. Astrocyte-produced ephrins inhibit schwann cell migration via VAV2 signaling. J Neurosci. 2010, 30, 4246-4255. doi: 10.1523/JNEUROSCI.3351-09.2010.
- Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. Glia 2010, 58, 857-869. doi: 10.1002/glia.20970.
- Horn, K.P.; Busch, S.A.; Hawthorne, A.L.; van Rooijen, N.; Silver, J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 2008, 28, 9330-9341. doi: 10.1523/JNEUROSCI.2488-08.2008.
- Minkelyte, K.; Collins, A.; Liadi, M.; Ibrahim, A.; Li, D.; Li, Y. High-Yield Mucosal Olfactory Ensheathing Cells Restore Loss of Function in Rat Dorsal Root Injury. Cells 2021, 10, 1186. doi: 10.3390/cells10051186.
- Hoeber, J.; Trolle, C.; König, N.; Du, Z.; Gallo, A.; Hermans, E.; Aldskogius, H.; Shortland, P.; Zhang, S.C.; Deumens, R.; Kozlova, E.N. Human Embryonic Stem Cell-Derived Progenitors Assist Functional Sensory Axon Regeneration after Dorsal Root Avulsion Injury. Sci. Rep. 2015, 5, 10666. doi: 10.1038/srep10666.
- Andjus, P.; Kosanović, M.; Milićević, K.; Gautam, M.; Vainio, S.J.; Jagečić, D.; Kozlova, E.N.; Pivoriūnas, A.; Chachques, J.C.; Sakaj, M.; Brunello, G.; Mitrecic, D.; Zavan, B. Extracellular Vesicles as Innovative Tool for Diagnosis, Regeneration and Protection against Neurological Damage. Int. J. Mol. Sci. 2020, 21, 6859. doi: 10.3390/ijms21186859.
- Selim, O.; Lakhani, S.; Midha, S.; Mosahebi, A.; Kalaskar, D.M. Three-Dimensional Engineered Peripheral Nerve: Toward a New Era of Patient-Specific Nerve Repair Solutions. Tissue Eng. Part B Rev. 2021, doi: 10.1089/ten.TEB.2020.0355.
- Behbehani, M.; Glen, A.; Taylor, C.S.; Schuhmacher, A.; Claeyssens, F.; Haycock, J.W. Pre-clinical evaluation of advanced nerve guide conduits using a novel 3D in vitro testing model. Int. J. Bioprint. 2017, 4, 123. doi: 10.18063/IJB.v4i1.123.
- Jeffries GDM, Xu S, Lobovkina T, Kirejev V, Tusseau F, Gyllensten C, Singh AK, Karila P, Moll L, Orwar O. 3D micro-organisation printing of mammalian cells to generate biological tissues. Sci Rep. 2020 Nov 10;10(1):19529. doi:10.1038/s41598-020-74191-w.
- Ngo TB, Spearman BS, Hlavac N, Schmidt CE. Three-Dimensional Bioprinted Hyaluronic Acid Hydrogel Test Beds for Assessing Neural Cell Responses to Competitive Growth Stimuli. ACS Biomater Sci Eng. 2020 Dec 14;6(12):6819-6830. doi: 10.1021/acsbiomaterials.0c00940.




