
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christian W. Hönemann | + 2027 word(s) | 2027 | 2021-09-22 11:27:06 | | | |
| 2 | Peter Tang | Meta information modification | 2027 | 2021-10-14 04:11:01 | | |
Video Upload Options
Anemia, iron deficiency and other hematinic deficiencies are a major cause of perioperative transfusion needs and are associated with increased morbidity and mortality. Anemia can be caused either by decreased production of hemoglobin or red blood cells or by increased consumption and blood loss. Decreased production can involve anything from erythropoietin or vitamin B12 insufficiency to absolute or functional lack of iron.
1. Introduction
2. Patient Blood Management in the ERAS Concept
3. Causes of Anemia
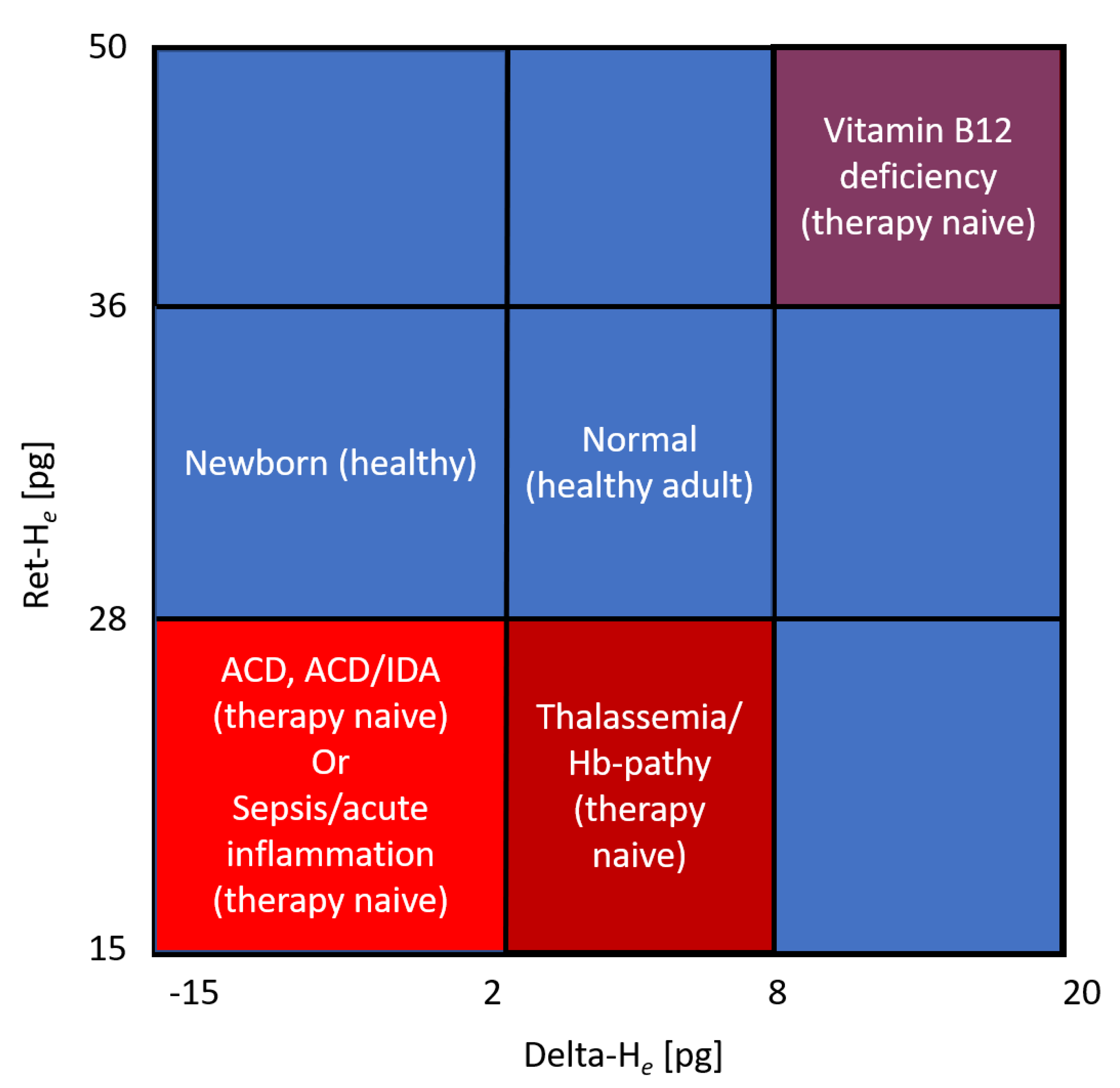
4. Reticulocyte Hemoglobin
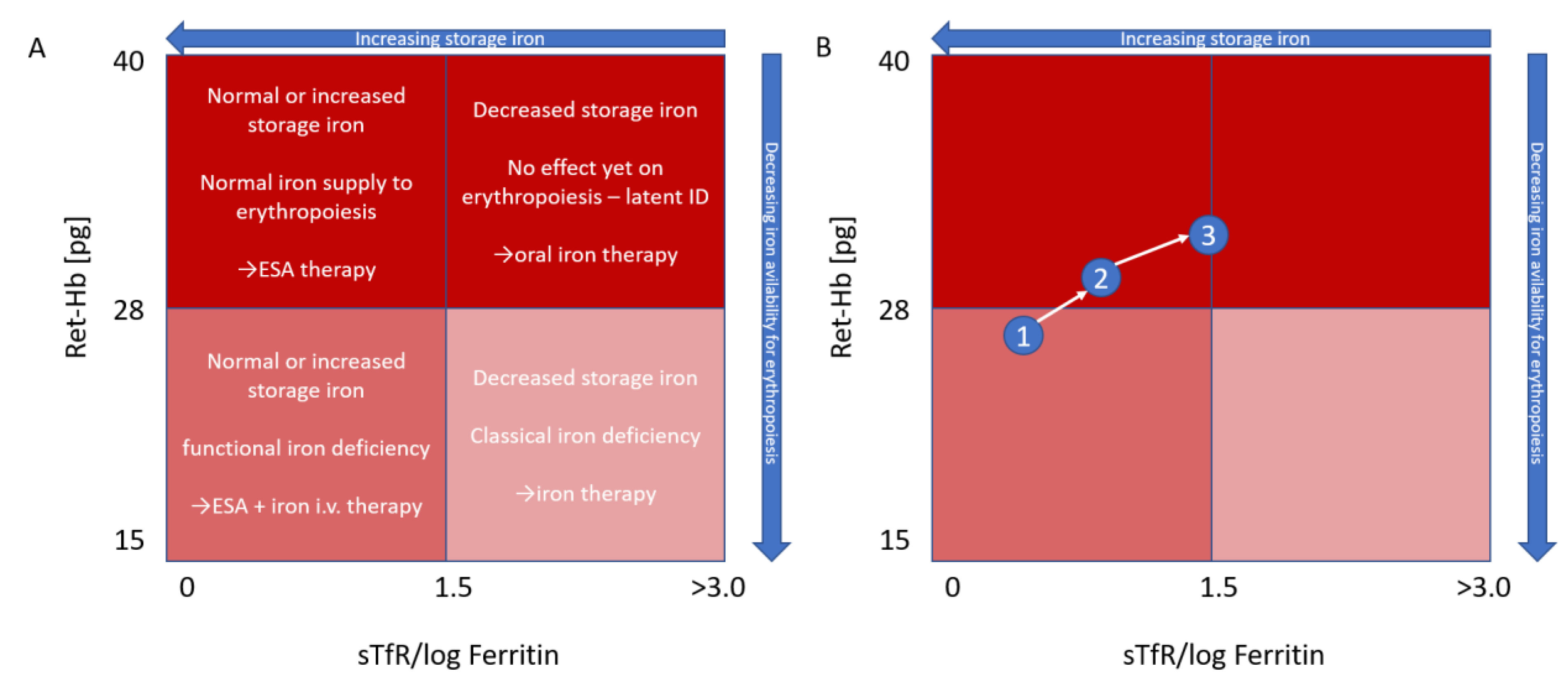
5. Treatment
|
Cellular assessment |
Hb < 11 g/dL RBC indices (MCH, MCHC, MCV) White blood cell and differential count Platelet and reticulocyte count |
|
Iron assessment |
Hypochromic cells % (if sample < 6 h old) Reticulocyte Hb (RET-He) Serum ferritin C-reactive protein |
|
Iron therapy targets |
Hypochromic cells < 6% Reticulocyte Hb (RET-He) > 29 pg Ferritin > 100 µg/L TSAT > 20% |
Hb = mean hemoglobin, RBC = red blood cells, MCH = mean corpuscular hemoglobin, MCHC = mean corpuscular hemoglobin concentration, MCV = mean corpuscular volume, Reticulocyte Hb = Ret-He = mean reticulocyte hemoglobin, TSAT = transferrin saturation.
6. Postoperative Iron Deficiency
7. Patient Blood Management (PBM) and Iron Deficiency
8. Potential Role of Ret-He in Treatment of Iron Deficiency in Septic Patients
References
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet Lond. Engl. 2011, 378, 1396–1407.
- Gómez-Ramírez, S.; Bisbe, E.; Shander, A.; Spahn, D.R.; Muñoz, M. Management of Perioperative Iron Deficiency Anemia. Acta Haematol. 2019, 142, 21–29.
- Fowler, A.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324.
- Vincent, J.L.; Baron, J.-F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D. ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507.
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431.
- Spahn, D.R. Anemia and patient blood management in hip and knee surgery: A systematic review of the literature. Anesthesiology 2010, 113, 482–495.
- Markowitz, M.A.; Waters, J.H.; Ness, P.M. Patient blood management: A primary theme in transfusion medicine. Transfusion 2014, 54, 2587.
- Ferraris, V.A.; Davenport, D.L.; Saha, S.P.; Austin, P.; Zwischenberger, J.B. Surgical Outcomes and Transfusion of Minimal Amounts of Blood in the Operating Room. Arch. Surg. Chic. III 2012, 147, 49–55.
- Muñoz, M.; Shander, A.; Rijhwani, T.; Dyga, R.; Waters, J. Postoperative blood management strategies. In Patient Blood Management: Multidisciplinary Approaches to Optimizing Care; AABB Press: Bethesda, MD, USA, 2016; pp. 233–258.
- Kurniali, P.C.; Curry, S.; Brennan, K.W.; Velletri, K.; Shaik, M.; Schwartz, K.A.; McCormack, E. A Retrospective Study Investigating the Incidence and Predisposing Factors of Hospital-Acquired Anemia. Anemia 2014, 2014, 634582.
- Koch, C.G.; Li, L.; Sun, Z.; Hixson, E.D.; Tang, A.; Phillips, S.C.; Blackstone, E.H.; Henderson, J.M. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J. Hosp. Med. 2013, 8, 506–512.
- Salisbury, A.C.; Alexander, K.P.; Reid, K.J.; Masoudi, F.A.; Rathore, S.S.; Wang, T.Y.; Bach, R.G.; Marso, S.P.; Spertus, J.A.; Kosiborod, M. Incidence, Correlates, and Outcomes of Acute, Hospital-Acquired Anemia in Patients with Acute Myocardial Infarction. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 337–346.
- Salisbury, A.C.; Amin, A.P.; Reid, K.J.; Wang, T.Y.; Masoudi, F.A.; Chan, P.S.; Alexander, K.P.; Bach, R.G.; Spertus, J.A.; Kosiborod, M. Hospital-acquired anemia and in-hospital mortality in patients with acute myocardial infarction. Am. Heart J. 2011, 162, 300–309.e3.
- Meroño, O.; Cladellas, M.; Recasens, L.; García-García, C.; Ribas, N.; Bazan, V.; Farré, N.; Sainz, Á.; Comin, J.; Bruguera, J. In-hospital Acquired Anemia in Acute Coronary Syndrome. Predictors, In-hospital Prognosis and One-year Mortality. Rev. Española Cardiol. 2012, 65, 742–748.
- Salisbury, A.C.; Reid, K.J.; Alexander, K.P.; Masoudi, F.A.; Lai, S.-M.; Chan, P.S.; Bach, R.G.; Wang, T.Y.; Spertus, J.A.; Kosiborod, M. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch. Intern. Med. 2011, 171, 1646–1653.
- Thavendiranathan, P.; Bagai, A.; Ebidia, A.; Detsky, A.S.; Choudhry, N.K. Do blood tests cause anemia in hospitalized patients? J. Gen. Intern. Med. 2005, 20, 520–524.
- Rennke, S.; Fang, M.C. Hazards of hospitalization: More than just “never events”. Arch. Intern. Med. 2011, 171, 1653–1654.
- Choi, J.S.; Kim, Y.A.; Kang, Y.U.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Ahn, Y.-K.; Jeong, M.H.; Kim, S.W. Clinical Impact of Hospital-Acquired Anemia in Association with Acute Kidney Injury and Chronic Kidney Disease in Patients with Acute Myocardial Infarction. PLoS ONE 2013, 8, e75583.
- Salisbury, A.C.; Kosiborod, M.; Amin, A.P.; Reid, K.J.; Alexander, K.P.; Spertus, J.A.; Masoudi, F.A. Recovery From Hospital-Acquired Anemia After Acute Myocardial Infarction and Effect on Outcomes. Am. J. Cardiol. 2011, 108, 949–954.
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. Available online: http://www.who.int/vmnis/indicators/haemoglobin/en/ (accessed on 15 November 2020).
- Brugnara, C.; Schiller, B.; Moran, J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin. Lab. Haematol. 2006, 28, 303–308.
- Wirawan, R.; Tedja, A.T.; Henrika, F.; Lydia, A. Concordance between Reticulocyte Hemoglobin Equivalent and Reticulocyte Hemoglobin Content in CKD Patients Undergoing Hemodialysis. Acta Med. Indones. 2017, 49, 34–40.
- Tiwari, A.K.; Bhardwaj, G.; Arora, D.; Aggarwal, G.; Pabbi, S.; Dara, R.C.; Sachdev, R.; Raizada, A.; Sethi, M. Applying newer parameter Ret-He (reticulocyte haemoglobin equivalent) to assess latent iron deficiency (LID) in blood donors-study at a tertiary care hospital in India. Vox Sang. 2018, 113, 639–646.
- Toki, Y.; Ikuta, K.; Kawahara, Y.; Niizeki, N.; Kon, M.; Enomoto, M.; Tada, Y.; Hatayama, M.; Yamamoto, M.; Ito, S.; et al. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron defi-ciency. Int. J. Hematol. 2017, 106, 116–125.
- Thomas, C.; Thomas, L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin. Chem. 2002, 48, 1066–1076.
- Thomas, L.; Franck, S.; Messinger, M.; Linssen, J.; Thomé, M.; Thomas, C. Reticulocyte hemoglobin measurement—Comparison of two methods in the diagnosis of iron-restricted erythropoiesis. Clin. Chem. Lab. Med. 2005, 43, 1193–1202.
- Van Wyck, D.B.; Alcorn, H.; Gupta, R. Analytical and biological variation in measures of anemia and iron status in patients treated with maintenance hemodialysis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found 2010, 56, 540–546.
- Maconi, M.; Cavalca, L.; Danise, P.; Cardarelli, F.; Brini, M. Erythrocyte and reticulocyte indices in iron deficiency in chronic kidney disease: Comparison of two methods. Scand. J. Clin. Lab. Investig. 2009, 69, 365–370.
- Fernandez, R.; Tubau, I.; Masip, J.; Muñoz, L.; Roig, I.; Artigas, A. Low reticulocyte hemoglobin content is associated with a higher blood transfusion rate in critically ill patients: A cohort study. Anesthesiology 2010, 112, 1211–1215.
- Chapter 1: Diagnosis and evaluation of anemia in CKD. Kidney Int. Suppl. 2012, 2, 288–291.
- Mikhail, A.; Brown, C.; Williams, J.A.; Mathrani, V.; Shrivastava, R.; Evans, J.; Isaac, H.; Bhandari, S. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017, 18, 1–29. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5709852/ (accessed on 4 November 2020).
- Chapter 2: Use of iron to treat anemia in CKD. Kidney Int. Suppl. 2012, 2, 292–298.
- Muñoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J. Clin. Pathol. 2011, 64, 281–286.
- Spahn, D.R.; Schoenrath, F.; Spahn, G.H.; Seifert, B.; Stein, P.; Theusinger, O.M.; Kaserer, A.; Hegemann, I.; Hofmann, A.; Maisano, F.; et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: A prospective randomised trial. Lancet 2019, 393, 2201–2212.
- Lee, B.; Kim, E.J.; Song, J.; Jung, Y.-S.; Koo, B.-N. A randomised trial evaluating the effect of intraoperative iron administration. Sci. Rep. 2020, 10, 1–8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7522208/ (accessed on 25 November 2020).
- Drabinski, T.; Zacharowski, K.; Meybohm, P.; Rüger, A.M.; De Arellano, A.R. Estimating the Epidemiological and Economic Impact of Implementing Preoperative Anaemia Measures in the German Healthcare System: The Health Economic Footprint of Patient Blood Management. Adv. Ther. 2020, 37, 3515–3536.
- Manzini, P.M.; Dall’Omo, A.M.; D’Antico, S.; Valfrè, A.; Pendry, K.; Wikman, A.; Fischer, D.; Borg-Aquilina, D.; LaSpina, S.; Van Pampus, E.C.M.; et al. Patient blood management knowledge and practice among clinicians from seven European university hospitals: A multicentre survey. Vox Sang. 2017, 113, 60–71.
- Wojciechowska, M.; Wisniewski, O.W.; Kolodziejski, P.; Krauss, H. Role of hepcidin in physiology and pathophysiology. Emerging experimental and clinical evidence. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72.
- Symeonidis, A.; Marangos, M. Iron and Microbial Growth. In Insight and Control of Infectious Disease in Global Scenario; Priti, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 289–330. Available online: https://cdn.intechopen.com/pdfs/33040/InTech-Iron_and_microbial_growth.pdf (accessed on 18 September 2021).
- Weimann, A.; Cremer, M.; Hernáiz-Driever, P.; Zimmermann, M. Delta-He, Ret-He and a New Diagnostic Plot for Differential Diagnosis and Therapy Monitoring of Patients Suffering from Various Disease-Specific Types of Anemia. Clin. Lab. 2016, 62, 667–677.




