Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michele Gottardi | + 3633 word(s) | 3633 | 2021-09-18 18:28:27 | | | |
| 2 | Conner Chen | Meta information modification | 3633 | 2021-10-15 08:13:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gottardi, M. Gemtuzumab Ozogamicin and AML Therapeutic. Encyclopedia. Available online: https://encyclopedia.pub/entry/14950 (accessed on 08 February 2026).
Gottardi M. Gemtuzumab Ozogamicin and AML Therapeutic. Encyclopedia. Available at: https://encyclopedia.pub/entry/14950. Accessed February 08, 2026.
Gottardi, Michele. "Gemtuzumab Ozogamicin and AML Therapeutic" Encyclopedia, https://encyclopedia.pub/entry/14950 (accessed February 08, 2026).
Gottardi, M. (2021, October 11). Gemtuzumab Ozogamicin and AML Therapeutic. In Encyclopedia. https://encyclopedia.pub/entry/14950
Gottardi, Michele. "Gemtuzumab Ozogamicin and AML Therapeutic." Encyclopedia. Web. 11 October, 2021.
Copy Citation
Acute myeloid leukemia (AML) is a complex hematological malignancy characterized by genetic and clinical heterogeneity and high mortality. Gemtuzumab Ozogamicin (GO) is a drug approved for the treatment of acute myeloid leukemia (AML). It targets leukemic cells that express the CD33 molecule on their surface and brings the toxic agent calicheamicin inside the cell to kill it. AML patients can benefit of the addition of GO to chemotherapy during induction regimens, pre- and post-transplantation.
CD33
acute myeloid leukemia
gemtuzumab ozogamicin
1. Introduction
Many patients affected by acute myeloid leukemia (AML) benefit of chemotherapy regimens and hematopoietic stem cell transplant (HSCT). However, progress has been modest in this therapeutic setting and huge challenges remain, mainly related to off-target cytotoxicities and to different chemoresistance mechanisms.
Immunotherapy is an innovative biological cancer therapy that exploits patient natural immune defenses to identify and eradicate cancer cells. Different types of immunotherapy have been developed, including antibody drug conjugates (ADCs) [1][2]. The advances of ADCs is to combine the specificity of a monoclonal antibody with the therapeutically benefits of chemotherapy agents [3]. Indeed, in contrast to conventional chemotherapeutics, ADCs provide superior efficacy and specificity while showing low risk of off-target cytotoxicity. Indeed, the chemotherapy particles associated with ADC remain inactive while passing through blood flow and become active only after ADC internalization. As a consequence, ADCs can increase the therapeutic window by reducing the Minimum Effective Dose (MED) along with enhancing the Maximum Tolerated Dose (MTD) [4][5][6].
Great interest in AML has been raised by the sialic acid-binding immunoglobulin-like lectin (Siglec) CD33 as a therapeutic target. Indeed, CD33 became an ideal target for the development of new ADCs due to the fact that its expression is common on the surface of AML blast cells and almost absent in normal pluripotent hematopoietic stem cells. Gemtuzumab ozogamicin (GO) is a humanized anti-CD33 monoclonal antibody covalently linked to various molecules of the cytotoxic agent N-acetyl gamma calicheamicin.
When GO binds CD33 antigen on the cell surface, the GO-CD33 complex is internalized and calichemicin molecules are released inside the cytoplasm. Active calichemicin particles intercalate DNA, thus inducing DNA damages, which, if left unrepaired, lead to cell cycle arrest and leukemic cell apoptosis. Different clinical trials have highlighted the benefit of GO on patient survival. GO is also the first antibody drug conjugate approved by the U.S. Food and Drug Administration (FDA) and the increasing knowledge of the GO metabolic pathway has improved our understanding on biomarkers of response.
2. CD33 Structure and Expression: Rationale for a Targeted Therapy in AML
CD33 is a 67 kDa glycosylated transmembrane protein belonging to the Siglec family [7]. The downstream pathway and biological functions of CD33 are still poorly understood, however Siglecs family members may regulate cytokine production, dampen inflammatory and immune responses, modulate intracellular calcium mobilization, cell adhesion, apoptosis of leukemic cells and myeloid cell maturation [8][9]. From a structural point of view, CD33 cytoplasmic tail contains two conserved immunoreceptor tyrosine-based inhibition motifs (ITIM and ITIM-like motifs) which, upon phosphorylation, promote the recruitment and activation of Src homology 2 domain-containing phosphatases 1 and 2 (SHP-1 and SHP-2). These activated SHPs further dephosphorylate various signaling molecules and suppress cellular activation [8]. The suppressor of cytokine signaling 3 (SOCS3) kinase competes with SHP-1 or SHP-2 for binding to the ITIMs. After the interaction with ITIM motifs, SOCS3 promotes the proteasome-dependent degradation of CD33, resulting in myeloid cell activation and proliferation [9].
Recently, different isoforms of CD33 gene have been identified [10]. Among them, the one missing exon 2 (CD33∆E2) has been predicted to have a clinical impact [11][12][13]. Briefly, this isoform lacks of the V-set domain containing the immune-dominant epitopes that represent the binding site of most CD33 antibodies, including GO.
In the healthy population, binding of antibodies recognizing the V-set domain of CD33 showed that CD33 is displayed on the surface of cells committed to the myeloid lineage, from myeloblasts to monocytes and also myelocytes and is down-regulated as the normal cells mature towards terminally-differentiated granulocytes, while it is retained on macrophages and dendritic cells [14][15]. However, in AML myeloid cells fail to differentiate, thus indicating that a high number of binding sites for CD33-specific agents are preserved and can be therapeutically exploited.
3. Gemtuzumab Ozogamicin: Mechanism of Cytotoxicity
Exploratory clinical studies indicated that the pharmacological inhibition of CD33 using unconjugated anti-CD33 antibodies have limited activity against AML cells.
From a structural point of view, GO is a recombinant humanized immunoglobulin G4 (IgG4) kappa (named P67.6) which specifically targets the CD33 antigen, linked to N-acetyl gamma calicheamicin, via the acid-labile hybrid 4-(4’-acetylphenoxy) butanoic acid linker. To improve the clinical applicability, researchers grafted the complementary-determining regions of P67.6 into a human immunoglubluin G4 (IgG4) kappa framework (hP67.6). Moreover, to stabilize the drug and to prevent Fab-arm exchange with endogenous human IgG4, a core-hinge mutation (S228P) was introduce in the IgG4 sequence [16][17]. Unconjugated P67.6 and hP67.6 antibodies per se lack substantial anti-leukemic activity, but they are useful to deliver a chemotherapy (e.g., calicheamicin derivatives) to CD33+ cells.
As already mentioned, after binding to the CD33 antigen, the GO-CD33 complex is rapidly internalized (Figure 1). The 4-(4’-acetylphenoxy)butanoic acid linker that connects the antibody portion to calicheamicin is rapidly hydrolyzed in acid environment, such as the lysosomes of the myeloblast. Following the hydrolyzation, calicheamicin dimethyl hydrazide is released and reduced by the glutathione into highly reactive species, which then bind to the DNA in the minor groove. The addition of calicheamicin to the DNA structure causes site-specific damages and, in particular, double-stranded breaks which are extremely toxic for proliferating cells. Downstream, the activation of the DNA repair pathway is mediated by the ataxia-telangiectasia mutated (ATM)/ataxia-telangiectasia and Rad3-related (ATR) protein kinases [18][19] (Figure 1). In turn, ATM and ATR kinases phosphorylate CHK1 and CHK2 proteins, which induce G2/M cell cycle arrest. The DNA-dependent pathway (DNA-PK) kinase participates in the response to DNA damages by phosphorylating H2AX histones and consequently promoting the recruitment of other DNA repair mediators on the site of damage. Finally, the DNA damage is repaired throughout the homologous recombination (HR) repair or the non-homologous end joining (NHEJ) repair mechanisms. Hence, it has been showed that cancer cells defective of different DNA damage response genes (e.s. ATM or DNA-PK) are hypersensitive to calicheamicin [20][21]. If the DNA damage is not repaired, ATM/ATR kinases trigger apoptosis through the phosphorylation of two BCL2 family proteins (BAX and BAK), which release the cytochrome-c and activate caspase 9 and 3 [22][23] (Figure 1). Data from a phase II trial suggest that the inhibition of BCL2 functionality using a specific antisense (Oblimersen sodium) may enhance the induction of leukemic cell apoptosis in patients subjected to a concomitant treatment with GO [24].
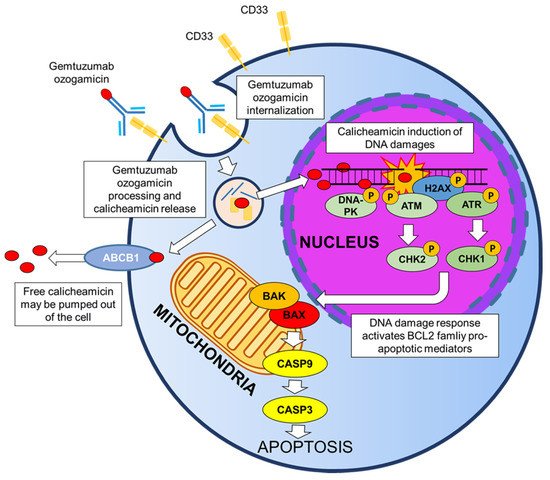
Figure 1. Mechanisms of action of GO.
Cytotoxicity analyses in the HL-60 human leukemia cell line showed a 2000-fold higher effect of the drug compared with the unconjugated calicheamicin alone [25]. In the cytoplasm, free calicheamicin can be pumped out through ATP-binding cassette (ABC) transmembrane transporters (ABCB1 and ABCC1), thus reducing its cytotoxic effect [26]. Independently from the action of transmembrane transporters, the sensitivity to calicheamicin varies significantly among AML patients [27], thus emphasizing the importance of defining the factors that regulate the anti-leukemia activity of GO. The reasons why calicheamicin sensitivity changes among patients are still unknown.
4. Clinical Trials and Clinical Experience of GO in AML
4.1. GO as Monotherapy for Newly Diagnosed or Relapsed/Refractory Adult AML
Three phase II trials (0903B1-201-US/CA [NCT00003131], 0903B1-202-EU, 0903B1-203-US/EU [NCT00003673]) assessed the safety and efficacy of GO administered as a monotherapy at 9 mg/mq on day 1 and day 14 in adult AML patients at first relapse: the results of these studies showed that GO induces a complete remission (CR) or CR with incomplete platelet recovery (CRp) in up to 25–35% of patients [26][28][29][30][31][32][33].
Based on these results on single-drug activity, on May 2000, FDA accelerated the regulatory approval of GO for CD33+ AML patients older than 60 years, in first relapse and unfit for intensive treatment [34].
Phase I studies identified a dose and schedule for GO (9 mg/mq every 2 weeks), able to reach complete or near complete CD33-binding site saturation while lacking dose-limiting non-hematological toxicity [26][35]. This drug schedule is however limited to the fact that the expression of new CD33 molecules is constantly displayed on blasts cell surface and thus the antigen levels, down-modulated by GO exposure, return to baseline after 72 h [35]. Fractionated GO dosing (3 mg/mq every 3 days) may therefore enhance intracellular calicheamicin delivery compared with higher dose schedules, which may be supra-saturating in some patients.
The sequential phase II/III EORTC-GIMEMA AML-19 trial first determined the best GO induction regimen. In the phase II trial GO was administered as monotherapy, 6 mg/mq on day 1 plus 3 mg/mq on day 8 versus (vs.) GO 3 mg/mq on day 1, 3 and 5, while the phase III trial compared GO to best supportive care in patients ≥61 years that were unsuitable for intensive chemotherapy [36]. A higher rate of disease non-progression, defined as CR/CRp rate or patients in stable disease at the end of induction course was reported in patients treated with the first drug schedule. Moreover, another phase III clinical trial revealed that the schedule 6 mg/mq on day 1 plus 3 mg/mq on day 8 of GO monotherapy significantly improved overall survival (OS) compared to best supportive care (1-year OS: 24.3% vs. 9.7%, hazard ratio (HR): 0.69, 95% confidence interval (CI): 0.53–0.90, p = 0.005) [37].
4.2. GO as Monotherapy for Relapsed/Refractory Pediatric AML
Regarding pediatric AML, the first phase I study of GO for compassionate use for children with relapse/refractory disease defined the effective dose of 4–9 mg/mq in up to 3 cycles in monotherapy [38]. Arceci and colleagues studied the effect of treatment with GO in monotherapy with doses of 6–9 mg/mq (2 doses, 2-week intervals) in children with relapse/refractory AML. Using this drug schedule, they found CR in 30% and 26% of AML patients with refractory and relapsed disease, respectively [39]. In 2010, a subsequent phase II study showed a significantly higher survival of children with advanced AML treated with two doses of 7.5 mg/mq of GO with 14-day intervals compared to children who did not receive the treatment (3-year probability of overall survival: 27.0% vs. 0.0%, respectively; p = 0.001) [40].
4.3. GO in Combination with Chemotherapy for Newly Diagnosed or Relapsed/Refractory Adult and Pediatric AML
During the last years, different clinical trials evaluated the efficacy of GO in combination with anti-leukemic drugs or with drug efflux pump inhibitors in both newly diagnosed or advanced AML patients. Unfortunately, due to the limited number of patients enrolled and/or absence of correct control arms, they failed to provide convincing proofs of the efficacy of GO in those settings.
Four randomized clinical trials evaluated the efficacy of GO in combination with the first cycle of intensive chemotherapy in AML patients:
SWOG S016: The Southwest Oncology Group conducted a phase III randomized trial to evaluate the clinical benefit of adding GO (6 mg/mq on day 4) to the standard 3 + 7 induction regimen in AML patients at first relapse. To balance toxicities patients allocated to the GO arm received lower dose of daunorubicin (45 mg/mq vs. 60 mg/mq) in comparison with patient treated with conventional induction regimen [41]. The interim analysis reported a higher number of fatal toxicities in the GO arm compared to the other, causing the premature end of the study and the withdrawal of GO from the market on June 2010. Moreover, the complete data analysis of the trial failed to demonstrate any clinical improvement by GO addition both in terms of relapse-free survival (RFS) and OS (GO vs. non-GO arm: 5-year RFS, 43.0% vs. 42.0%; p = 0.40; 5-year OS, 46.0% vs. 50.0%; p = 0.85).
Medical Research Council (MRC) AML15 and National Cancer Research Institute (NCRI) AML16: based on the results of a dose-finding trial that evaluated GO addition (3 mg/mq vs. 6 mg/mq) to intensive chemotherapy, with 3 mg/mq GO appearing effective and safe [42], two randomized phase III trials addressed the clinical consequences of adding GO 3 mg/mq to the induction regimen in young (predominantly ≤60 years, MRC AML15) [43] and older patients (NRCI AML16) [44]. In these trials, the GO arm showed an improved OS in elderly patients and in younger ones with favorable-risk AML.
Acute Leukemia French Association (ALFA)-0701: since the CD33 antigen is rapidly re-expressed on the surface of AML blasts after GO exposure, the acute leukemia French association tested the fractionated treatment (3 mg/mq on day 1, 4 and 7) in a phase I/II study in combination with chemotherapy for the treatment of relapsed AML [45][46]. Sixty-five to 75.0% of patients achieved CR/CRp. Based on these results, the randomized phase III ALFA-0701 trial compared fractionated GO (3 mg/mq on days 1,4 and 7 during induction and on day 1 of each consolidation course) plus standard chemotherapy vs. chemotherapy alone in newly diagnosed CD33+ AML patients aged 50 to 70 years [47]. The GO arm displayed an improvement of event free survival (EFS, median value 13.6 vs. −9.5 months, HR: 0.66; 95% CI: 0.49–0.89; p = 0.006), but no differences in terms of OS compared with the chemotherapy arm (HR: 0.81; 95% CI: 0.60–1.09; p = 0.16) [47][48].
Thanks to these results, on September 2017, FDA re-approved GO for adult newly diagnosed CD33+ AML patients and for pediatric relapsed/refractory CD33+ AML patients (aged ≥2 years). GO received the marketing authorization of the European Medicine Agency (EMA) on April 2018 for the treatment of newly diagnosed de novo CD33+ AML patients aged ≥2 years, in combination with daunorubicin and cytarabine.
A meta-analysis of 3325 AML patients treated in the above reported clinical trials showed that the addition of GO had no impact on the overall remission rate. However, it reduced the 5-year cumulative incidence of relapse with an odds ratio (OR) of 0.81 (95% CI: 0.73–0.90, p < 0.001) and it improved survival (OR for death = 0.90, 95% CI: 0.82–0.98, p = 0.01) [49].
A subsequent trial (NCRI AML17) evaluated the impact of GO dosing 3 mg/mq vs. 6 mg/mq combined with intensive chemotherapy in a cohort of 788 newly diagnosed AML patients. The result of the study showed no correlation between the increase of GO dosage and clinical benefit. Indeed, the increased GO dosing did improve neither the response rate nor the patients’ outcome (OS: HR: 1.10; 95% CI: 0.90–1.34; p = 0.3; RFS: HR: 1.11; 95% CI: 0.91–1.35; p = 0.30 [50].
4.4. GO-related Toxicities
Most of GO-related toxicities have been reported after first infusion. Acute infusion-related toxicities, such as chills, fever, low or high blood pressure, nausea/vomiting were the most frequently observed events. However, all these events were usually transient and could be resolved using standard interventions. The most common adverse event reported in AML patients treated with GO in monotherapy was bone marrow myelosuppression, resulting in grade 3–4 neutropenia and thrombocytopenia. In the ALFA-0701 trial, similar toxicities were seen in patients treated with GO in combination with chemotherapy. In these patients, myeloid recovery was not significantly delayed following induction with GO and chemotherapy while platelet recovery was prolonged for days [47][48]. Severe intra-vascular hemolysis have been reported in some pediatric cases treated with GO [51]. The biological reason of this phenomenon may be ascribed to impaired hemoglobin scavenging coming from the elimination of CD33+ monocytes/macrophages expressing the CD163 hemoglobin scavenger receptor [14]. Other transient toxicities have been reported following GO therapy and, in particular, transient alterations of liver enzymes levels including hyper-bilirubinemia and increased level of aspartate and/or alanine aminotransferase (AST/ALT) [52][53].
Among GO-related toxicities, the development of veno-occlusive disease (VOD) has the highest clinical impact. Different clinical trials highlighted that the risk of VOD in patients treated with GO is dose-dependent. Indeed, a relatively low risk of VOD has been reported in AML patients receiving doses of GO lower than 3 mg/mq and in combination with conventional therapy [50]. Accordingly, a recent study on 137 GO-treated patients and 548 matched control subjects demonstrated that GO exposure before myeloablative allogenic transplantation does not associate with higher frequency of VOD or death [54]. On the other hand, the risk of VOD increases when GO is administered in heavily pre-treated AML patients or when the doses were higher than 3 mg/mq [55][56]. The biological reason for the development of VOD are still unknown, however similar observations come from patients treated with inotuzumab-ozogamicin, an anti-CD22 calicheamicin conjugate, used for the treatment of acute lymphoblastic leukemia (ALL) patients [57]. This phenomenon suggested that the mechanism of GO-associated VOD is CD33 independent but may be related to the structure of the antibody or related to calicheamicin toxicity. More than one aspect might be involved and their effective contribution might depend on the level of CD33-binding sites saturation achieved in the blood. Defibrotide, a drug commonly used to treat VOS, provided some benefits in the treatment of GO-induced VOD, with 17/27 patients (63.0%) surviving and/or showing a response, with a safety profile comparable to the one reported in other defibrotide studies [58].
4.5. GO Treatment before and after Transplantation in Adult AML Patients
Risk stratification in AML is currently used to tailor patients’ post-remission treatment, which may include transplant (autologous, autoSCT or allogeneic, alloSCT) or continued chemotherapy. Post-remission treatment for AML patients should be adjusted according to an assessment of transplant-related mortality (TRM) along with leukemia characteristics and minimal residual disease (MRD).
In this context, GO could be considered before harvesting, in order to achieve and/or consolidate MRD negativity, and during transplant, for intensifying conditioning regimens: of course, a modulation of GO dosage (and of conditioning regimens) should be evaluated. A list of clinical trials including GO treatment before or after transplantation is reported in Table 1 (clinicaltrials.gov updated to 1 September 2021).
Table 1. Clinical trials of GO used before or after transplantation (clinicaltrials.gov updated to 1 September 2021).
| NCT Number | Intervention | Conditions | Age | Phase | Trial Status |
|---|---|---|---|---|---|
| NCT00044733 | GO at relapse after auto or alloSCT | AML | child, adult, older adult | II | Completed |
| NCT02221310 | GO+chemotherapy followed by alloSCT | high-risk AML/MDS | up to 25 years | II | Recruiting |
| NCT00669890 | GO+Busulfan and Cyclophosphamid before alloSCT | high-risk AML/MDS/JMML | up to 30 years | I | Terminated |
| NCT02117297 | GO consolidation after alloSCT | average-risk AML/MDS | up to 25 years | II | Recruiting |
| NCT01020539 | GO consolidation after alloSCT | average-risk AML/MDS/JMML | up to 30 years | I | Active, not recruiting |
| NCT00460447 | GO before alloSCT at relapse | AML | 18–70 years | I/II | Unknown |
| NCT00038831 | GO+Melphalan+Fludarabine before alloSCT in older or medically infirm patients | AML/MDS/CLL | 12–75 years | I/II | Completed |
| NCT00476541 | GO consolidation after SCT | AML | up to 18 years | III | Completed |
| NCT00008151 | GO+fludarabine+total-body irradiation before alloSCT | advanced AML/MDS | child, adult, older adult | II | Completed |
| NCT00038805 | GO+nonmyeloablative preparative regimen before mini-alloSCT in older or medically infirm patients | AML/ALL/CML/MDS | 55–75 years | II/III | Terminated |
| NCT01723657 | GO “in vivo purging” before autoSCT in patients with favorable/intermediate characteristics and without matched related donor | AML | 18–70 years | II | Completed |
| NCT00070174 | GO in remission induction, intensification therapy before alloSCT | AML | child, adult, older adult | II | Completed |
Allo: allogenic; AML: acute myeloid leukemia; auto: autologous; CLL: chronic lymphocytic leukemia; CML: chronic myeloid leukemia; GO: gemtuzumab ozogamicin, JMML: juvenile myelomonocytic leukemia; MDS: myelodysplastic syndrome.
In the favorable risk core binding factor (CBF) subtype, GO was not able to reduce the residual leukemia initiating clone that survived the consolidation therapy, thus showing no benefit in the setting of autoSCT [59].
The retrospective analysis of post-transplant outcomes in subjects who received HSCT as follow-up therapy in the ALFA-0701 trial showed that fractionated-dose GO in the induction and consolidation regimen did not induce higher rate of post-transplant VOD/sinusoidal obstruction syndrome or mortality [60]. Post-transplant outcomes were comparable between arms and the study failed to demonstrate the survival benefit observed in the GO vs. control arm in patients who did not receive HSCT. Taking together, these data indicate that HSCT can follow GO-based regimen, as consolidation treatment. Accordingly, in a “real-life” setting, the combination of fractionated GO with cytarabine and mitoxantrone (MYLODAM scheme) confirmed that a GO-based intensive regimen can be applied as bridge to alloSCT in relapsed/refractory AML [61]. Moreover, the efficacy of GO combinations as a potential bridge to transplant was confirmed in a retrospective study of 24 high-risk AML patients who received fractionated GO in combination with intermediate-dose cytarabine and daunorubicin as salvage therapy [62]. A recent study also reported that relapsed AML patients may also benefit of GO monotherapy as a conditioning regimen before second alloSCT from the same donor used in the first transplantation [63]. After transplantation, the disease relapse remains the major cause of therapy failure for AML patients. Moreover, the therapeutic options to treat relapsed patients after transplant are extremely limited, due to the rising of disease resistance and a higher risk of toxicities. Therefore, there is a clinical need for therapeutic strategies able to prevent or manage disease relapse. The optimal pharmacological compound should have a safe toxicity profile, an anti-tumor effect and an immune profile, which can be used to boost GVL and reduce GVHD. Several cases of treatment with GO or GO plus donor lymphocyte infusion (DLI) for AML relapsing after alloSCT have been previously reported. The available experiences [64][65][66][67] suggest that GO treatment followed by DLI is more effective when administered soon after relapse or, if possible, even in a pre-emptive setting. A recent study reported encouraging results by fractionated GO combined with intensive chemotherapy in adult CD33+ AML patients relapsing after alloHCT, as salvage regimen, with an overall response rate of 72.0% and OS of 42.0% at 2 years [68]. Moreover, the combination with additional targeted therapies, when available, has to be taken into account.
4.6. GO Treatment before and after Transplantation in Pediatric AML
GO is currently being evaluated in this setting also in the pediatric population (Table 1). Data based on clinical experience showed that GO can be safely added (i) to a busulfan/cyclophosphamide conditioning regimen before alloSCT in children and adolescents affected by poor-risk AML [69]; (ii) to fludarabine and cytarabine (FLA) before HSCT for first-line refractory AML in children [70]; (iii) to fludarabine, cytarabine, granulocyte colony-stimulating factor and idarubicin (FLAG-IDA) as reinduction therapy before a KIR-ligand-mismatched cord blood transplant in pediatric relapsed/refractory AML [71]. Moreover, GO treatment, either as monotherapy or in combination with cytarabine or other agents, of relapsed/refractory pediatric AML patients enabled blast reduction [72] also to MRD negativity levels [73], thus allowing HSCT, without imposing major adverse events.
References
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73.
- Marin-Acevedo, J.A.; Soyano, A.E.; Dholaria, B.; Knutson, K.L.; Lou, Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 1–25.
- Kim, E.G.; Kim, K.M. Strategies and advancement in antibody-drug conjugate optimization for targeted cancer therapeutics. Biomol. Ther. 2015, 23, 493–509.
- Schumacher, D.; Hackenberger, C.P.R.; Leonhardt, H.; Helma, J. Current Status: Site-Specific Antibody Drug Conjugates. J. Clin. Immunol. 2016, 36, 100–107.
- Teicher, B.A.; Chari, R.V.J. Antibody conjugate therapeutics: Challenges and potential. Clin. Cancer Res. 2011, 17, 6389–6397.
- Lin, J.H.; Guo, Y.; Wang, W. Challenges of Antibody Drug Conjugates in Cancer Therapy: Current Understanding of Mechanisms and Future Strategies. Curr. Pharmacol. Rep. 2018, 4, 10–26.
- Von Gunten, S.; Bochner, B.S. Basic and clinical immunology of Siglecs. Ann. N. Y. Acad. Sci. 2008, 1143, 61–82.
- Lajaunias, F.; Dayer, J.M.; Chizzolini, C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur. J. Immunol. 2005, 35, 243–251.
- Orr, S.J.; Morgan, N.M.; Elliott, J.; Burrows, J.F.; Scott, C.J.; McVicar, D.W.; Johnston, J.A. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood 2007, 109, 1061–1068.
- Gbadamosi, M.O.; Shastri, V.M.; Hylkema, T.; Papageorgiou, I.; Pardo, L.; Cogle, C.R.; Doty, A.; Loken, M.R.; Meshinchi, S.; Lamba, J.K. Novel CD33 antibodies unravel localization, biology and therapeutic implications of CD33 isoforms. Future Oncol. 2021, 17, 263–277.
- Hernández-Caselles, T.; Martínez-Esparza, M.; Pérez-Oliva, A.B.; Quintanilla-Cecconi, A.M.; García-Alonso, A.; Alvarez-López, D.M.R.; García-Peñarrubia, P. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: Two isoforms of CD33 are generated by alternative splicing. J. Leukoc. Biol. 2006, 79, 46–58.
- Pérez-Oliva, A.B.; Martínez-Esparza, M.; Vicente-Fernández, J.J.; Corral-San Miguel, R.; García-Peñarrubia, P.; Hernández-Caselles, T. Epitope mapping, expression and post-translational modifications of two isoforms of CD33 (CD33M and CD33m) on lymphoid and myeloid human cells. Glycobiology 2011, 21, 757–770.
- Laszlo, G.S.; Harrington, K.H.; Gudgeon, C.J.; Beddoe, M.E.; Fitzgibbon, M.P.; Ries, R.E.; Lamba, J.K.; McIntosh, M.W.; Meshinchi, S.; Walter, R.B. Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia. Oncotarget 2016, 7, 43281–43294.
- Griffin, J.D.; Linch, D.; Sabbath, K.; Larcom, P.; Schlossman, S.F. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk. Res. 1984, 8, 521–534.
- Andrews, R.G.; Singer, J.W.; Bernstein, I.D. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J. Exp. Med. 1989, 169, 1721–1731.
- Hamann, P.R.; Hinman, L.M.; Hollander, I.; Beyer, C.F.; Lindh, D.; Holcomb, R.; Hallett, W.; Tsou, H.R.; Upeslacis, J.; Shochat, D.; et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-Calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 2002, 13, 47–58.
- Labrijn, A.F.; Buijsse, A.O.; Van Den Bremer, E.T.J.; Verwilligen, A.Y.W.; Bleeker, W.K.; Thorpe, S.J.; Killestein, J.; Polman, C.H.; Aalberse, R.C.; Schuurman, J.; et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat. Biotechnol. 2009, 27, 767–771.
- Amico, D.; Barbui, A.M.; Erba, E.; Rambaldi, A.; Introna, M.; Golay, J. Differential response of human acute myeloid leukemia cells to gemtuzumab ozogamicin in vitro: Role of Chk1 and Chk2 phosphorylation and caspase 3. Blood 2003, 101, 4589–4597.
- Mårtensson, S.; Nygren, J.; Osheroff, N.; Hammarsten, O. Activation of the DNA-dependent protein kinase by drug-induced and radiation-induced DNA strand breaks. Radiat. Res. 2003, 160, 291–301.
- Elmroth, K.; Nygren, J.; Mårtensson, S.; Ismail, I.H.; Hammarsten, O. Cleavage of cellular DNA by calicheamicin γ1. DNA Repair 2003, 2, 363–374.
- Sullivan, N.; Lyne, L. Sensitivity of fibroblasts derived from ataxia-telangiectasia patients to calicheamicin γ1I. Mutat. Res. Lett. 1990, 245, 171–175.
- Prokop, A.; Wrasidlo, W.; Lode, H.; Herold, R.; Lang, F.; Henze, G.; Dörken, B.; Wieder, T.; Daniel, P.T. Induction of apoptosis by enediyne antibiotic calicheamicin θII proceeds through a caspase-mediated mitochondrial amplification loop in an entirely Bax-dependent manner. Oncogene 2003, 22, 9107–9120.
- Haag, P.; Viktorsson, K.; Lindberg, M.L.; Kanter, L.; Lewensohn, R.; Stenke, L. Deficient activation of Bak and Bax confers resistance to gemtuzumab ozogamicin-induced apoptotic cell death in AML. Exp. Hematol. 2009, 37, 755–766.
- Moore, J.; Seiter, K.; Kolitz, J.; Stock, W.; Giles, F.; Kalaycio, M.; Zenk, D.; Marcucci, G. A Phase II study of Bcl-2 antisense (oblimersen sodium) combined with gemtuzumab ozogamicin in older patients with acute myeloid leukemia in first relapse. Leuk. Res. 2006, 30, 777–783.
- McGavin, J.K.; Spencer, C.M. Gemtuzumab ozogamicin. Drugs 2001, 61, 1317–1322.
- Cowan, A.J.; Laszlo, G.S.; Estey, E.H.; Walter, R.B. Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin. Front. Biosci. 2013, 18, 1311–1334.
- Goemans, B.F.; Zwaan, C.M.; Vijverberg, S.J.H.; Loonen, A.H.; Creutzig, U.; Hählen, K.; Reinhardt, D.; Gibson, B.E.S.; Cloos, J.; Kaspers, G.J.L. Large interindividual differences in cellular sensitivity to calicheamicin may influence gemtuzumab ozogamicin response in acute myeloid leukemia. Leukemia 2008, 22, 2284–2285.
- Pagano, L.; Fianchi, L.; Caira, M.; Rutella, S.; Leone, G. The role of Gemtuzumab Ozogamicin in the treatment of acute myeloid leukemia patients. Oncogene 2007, 26, 3679–3690.
- Breccia, M.; Lo-Coco, F. Gemtuzumab ozogamicin for the treatment of acute promyelocytic leukemia: Mechanisms of action and resistance, safety and efficacy. Expert Opin. Biol. Ther. 2011, 11, 225–234.
- Htter, M.L.; Schlenk, R.F. Gemtuzumab ozogamicin in non-acute promyelocytic acute myeloid leukemia. Expert Opin. Biol. Ther. 2011, 11, 1369–1380.
- Takeshita, A. Efficacy and resistance of gemtuzumab ozogamicin for acute myeloid leukemia. Int. J. Hematol. 2013, 97, 703–716.
- Thol, F.; Schlenk, R.F. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin. Biol. Ther. 2014, 14, 1185–1195.
- Gottardi, M.; Sperotto, A.; Di Rorà, A.G.L.; Padella, A.; Cangini, D.; Giannini, M.B.; Simonetti, G.; Martinelli, G.; Cerchione, C. Gemtuzumab ozogamicin in acute myeloid leukemia: Past, present and future. Minerva Med. 2020, 111, 395–410.
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval Summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496.
- Laszlo, G.S.; Estey, E.H.; Walter, R.B. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014, 28, 143–153.
- Amadori, S.; Suciu, S.; Selleslag, D.; Stasi, R.; Alimena, G.; Baila, L.; Rizzoli, V.; Borlenghi, E.; Gaidano, G.; Magro, D.; et al. Randomized trial of two schedules of low-dose gemtuzumab ozogamicin as induction monotherapy for newly diagnosed acute myeloid leukaemia in older patients not considered candidates for intensive chemotherapy. A phase II study of the EORTC and GIMEMA leuka. Br. J. Haematol. 2010, 149, 376–382.
- Amadori, S.; Venditti, A.; Voso, M.T.; Annino, L.; De Fabritiis, P.; Alimena, G.; Mancini, M.; Paoloni, F.; Vignetti, M.; Fazi, P.; et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC-GIMEMA AML-19 Trial. J. Clin. Oncol. 2016, 34, 972–979.
- Zwaan, C.M.; Reinhardt, D.; Corbacioglu, S.; Van Wering, E.R.; Bökkerink, J.P.M.; Tissing, W.J.E.; Samuelsson, U.; Feingold, J.; Creutzig, U.; Kaspers, G.J.L. Gemtuzumab ozogamicin: First clinical experiences in children with relapsed/refractory acute myeloid leukemia treated on compassionate-use basis. Blood 2003, 101, 3868–3871.
- Arceci, R.J.; Sande, J.; Lange, B.; Shannon, K.; Franklin, J.; Hutchinson, R.; Vik, T.A.; Flowers, D.; Aplenc, R.; Berger, M.S.; et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood 2005, 106, 1183–1188.
- Zwaan, C.M.; Reinhardt, D.; Zimmerman, M.; Hasle, H.; Stary, J.; Stark, B.; Dworzak, M.; Creutzig, U.; Kaspers, G.J.L. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: Results of a phase II study. Br. J. Haematol. 2010, 148, 768–776.
- Malfuson, J.V.; Konopacki, J.; Thepenier, C.; Eddou, H.; Foissaud, V.; De Revel, T. Fractionated doses of gemtuzumab ozogamicin combined with 3+7 induction chemotherapy as salvage treatment for young patients with acute myeloid leukemia in first relapse. Ann. Hematol. 2012, 91, 1871–1877.
- Kell, W.J.; Burnett, A.K.; Chopra, R.; Yin, J.A.L.; Clark, R.E.; Rohatiner, A.; Culligan, D.; Hunter, A.; Prentice, A.G.; Milligan, D.W. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood 2003, 102, 4277–4283.
- Burnett, A.K.; Hills, R.K.; Milligan, D.; Kjeldsen, L.; Kell, J.; Russell, N.H.; Yin, J.A.L.; Hunter, A.; Goldstone, A.H.; Wheatley, K. Identification of Patients with Acute Myeloblastic Leukemia Who Benefit from the Addition of Gemtuzumab Ozogamicin: Results of the MRC AML15 Trial. J. Clin. Oncol. 2011, 29, 369–377.
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Kell, J.; Freeman, S.; Kjeldsen, L.; Hunter, A.E.; Yin, J.; Craddock, C.F.; Dufva, I.H.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 3924–3931.
- Farhat, H.; Reman, O.; Raffoux, E.; Berthon, C.; Pautas, C.; Kammoun, L.; Chantepie, S.; Gardin, C.; Rousselot, P.; Chevret, S.; et al. Fractionated doses of gemtuzumab ozogamicin with escalated doses of daunorubicin and cytarabine as first acute myeloid leukemia salvage in patients aged 50–70-year old: A phase 1/2 study of the acute leukemia French association. Am. J. Hematol. 2012, 87, 62–65.
- Pilorge, S.; Rigaudeau, S.; Rabian, F.; Sarkozy, C.; Taksin, A.L.; Farhat, H.; Merabet, F.; Ghez, S.; Raggueneau, V.; Terré, C.; et al. Fractionated gemtuzumab ozogamicin and standard dose cytarabine produced prolonged second remissions in patients over the age of 55 years with acute myeloid leukemia in late first relapse. Am. J. Hematol. 2014, 89, 399–403.
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516.
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119.
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. The addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996.
- Burnett, A.; Cavenagh, J.; Russell, N.; Hills, R.; Kell, J.; Jones, G.; Nielsen, O.J.; Khwaja, A.; Thomas, I.; Clark, R. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: A comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 trial. Haematologica 2016, 101, 724–731.
- Maniecki, M.B.; Hasle, H.; Friis-Hansen, L.; Lausen, B.; Nielsen, O.J.; Bendix, K.; Moestrup, S.K.; Møller, H.J. Impaired CD163-mediated hemoglobin-scavenging and severe toxic symptoms in patients treated with gemtuzumab ozogamicin. Blood 2008, 112, 1510–1514.
- Tsimberidou, A.; Estey, E.; Cortes, J.; Thomas, D.; Faderl, S.; Verstovsek, S.; Garcia-Manero, G.; Keating, M.; Albitar, M.; O’Brien, S.; et al. Gemtuzumab, fludarabine, cytarabine, and cyclosporine in patients with newly diagnosed acute myelogenous leukemia or high-risk myelodysplastic syndromes. Cancer 2003, 97, 1481–1487.
- Larson, R.A.; Sievers, E.L.; Stadtmauer, E.A.; Löwenberg, B.; Estey, E.H.; Dombret, H.; Theobald, M.; Voliotis, D.; Bennett, J.M.; Richte, M.; et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005, 104, 1442–1452.
- Ho, V.T.; Martin, A.S.; Pérez, W.S.; Steinert, P.; Zhang, M.J.; Chirnomas, D.; Hoang, C.J.; Loberiza, F.R.; Saber, W. Prior Gemtuzumab Ozogamicin Exposure in Adults with Acute Myeloid Leukemia Does Not Increase Hepatic Veno-Occlusive Disease Risk after Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Analysis. Biol. Blood Marrow Transplant. 2020, 26, 884–892.
- Selby, C.; Yacko, L.R.; Glode, A.E. Gemtuzumab Ozogamicin: Back Again. J. Adv. Pract. Oncol. 2019, 10, 68–82.
- De Witte, T.; Suciu, S.; Meert, L.; Halkes, C.; Selleslag, D.; Bron, D.; Amadori, S.; Willemze, R.; Muus, P.; Baron, F. Idarubicin and cytarabine in combination with gemtuzumab ozogamicin (IAGO) for untreated patients with high-risk MDS or AML evolved from MDS: A phase II study from the EORTC and GIMEMA Leukemia Groups (protocol 06013). Ann. Hematol. 2015, 94, 1981–1989.
- Wynne, J.; Wright, D.; Stock, W. Inotuzumab: From preclinical development to success in B-cell acute lymphoblastic leukemia. Blood Adv. 2019, 3, 96–104.
- Richardson, P.G.; Corbacioglu, S. Veno-occlusive disease/sinusoidal obstruction syndrome in patients with prior gemtuzumab ozogamicin: Literature analysis of survival after defibrotide treatment. Blood Cancer J. 2020, 10.
- Van Der Reijden, B.A.; Simons, A.; Luiten, E.; Van Der Poel, S.C.; Hogenbirk, P.E.; Tönnissen, E.; Valk, P.J.M.; Löwenberg, B.; De Greef, G.E.; Breuning, M.H.; et al. Minimal residual disease quantification in patients with acute myeloid leukaemia and inv(16)/CBFB-MYH11 gene fusion. Br. J. Haematol. 2002, 118, 411–418.
- Pautas, C.; Raffoux, E.; Lambert, J.; Legrand, O.; Chantepie, S.; Gastaud, L.; Marolleau, J.P.; Thomas, X.; Turlure, P.; Benner, R.J.; et al. Outcomes following hematopoietic stem cell transplantation in patients treated with standard chemotherapy with or without gemtuzumab ozogamicin for acute myeloid leukemia. Bone Marrow Transplant. 2021, 56, 1474–1477.
- Debureaux, P.E.; Labopin, M.; Mamez, A.C.; Lapusan, S.; Isnard, F.; Adaeva, R.; Bonnin, A.; Hirsch, P.; Delhommeau, F.; Battipaglia, G.; et al. Fractionated gemtuzumab ozogamicin in association with high dose chemotherapy: A bridge to allogeneic stem cell transplantation in refractory and relapsed acute myeloid leukemia. Bone Marrow Transplant. 2020, 55, 452–460.
- Paubelle, E.; Ducastelle-Leprêtre, S.; Labussière-Wallet, H.; Nicolini, F.E.; Barraco, F.; Plesa, A.; Salles, G.; Wattel, E.; Thomas, X. Fractionated gemtuzumab ozogamicin combined with intermediate-dose cytarabine and daunorubicin as salvage therapy in very high-risk AML patients: A bridge to reduced intensity conditioning transplant? Ann. Hematol. 2017, 96, 363–371.
- Sumiyoshi, R.; Tashiro, H.; Saito, S.; Matsuo, T.; Yamamoto, T.; Matsumoto, K.; Ooi, J.; Shirafuji, N. Gemtuzumab ozogamicin monotherapy prior to stem cell infusion induces sustained remission in a relapsed acute myeloid leukemia patient after allogeneic stem cell transplantation: A case report. Medicine 2020, 99, e22064.
- Mosna, F.; Papayannidis, C.; Martinelli, G.; Di Bona, E.; Bonalumi, A.; Tecchio, C.; Candoni, A.; Capelli, D.; Piccin, A.; Forghieri, F.; et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up. Am. J. Hematol. 2015, 90, 515–523.
- Terwijn, M.; Kelder, A.; Huijgens, P.C.; Dräger, A.M.; Oussoren, Y.J.M.; Scholten, W.J.; Snel, A.N.; Ossenkoppele, G.J.; Schuurhuis, G.J.; Biemond, B.J.; et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: Data from the HOVON/SAKK AML 42A study. J. Clin. Oncol. 2013, 31, 3889–3897.
- Walter, R.B.; Gooley, T.A.; Wood, B.L.; Milano, F.; Fang, M.; Sorror, M.L.; Estey, E.H.; Salter, A.I.; Lansverk, E.; Chien, J.W.; et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 1190–1197.
- Pollard, J.A.; Alonzo, T.A.; Loken, M.; Gerbing, R.B.; Ho, P.A.; Bernstein, I.D.; Raimondi, S.C.; Hirsch, B.; Franklin, J.; Walter, R.B.; et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood 2012, 119, 3705–3711.
- Genthon, A.; Brissot, E.; Malard, F.; Van de Wyngaert, Z.; Bonnin, A.; Banet, A.; Marjanovic, Z.; Ikhlef, S.; Lapusan, S.; Sestili, S.; et al. Gemtuzumab Ozogamicin Combined With Intensive Chemotherapy in Patients With Acute Myeloid Leukemia Relapsing After Allogenic Stem Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2020, 20, 791–796.
- Satwani, P.; Bhatia, M.; Garvin, J.H.; George, D.; Dela Cruz, F.; Le Gall, J.; Jin, Z.; Schwartz, J.; Duffy, D.; Van de Ven, C.; et al. A Phase I Study of Gemtuzumab Ozogamicin (GO) in Combination with Busulfan and Cyclophosphamide (Bu/Cy) and Allogeneic Stem Cell Transplantation in Children with Poor-Risk CD33+ AML: A New Targeted Immunochemotherapy Myeloablative Conditioning (MAC) Regim. Biol. Blood Marrow Transplant. 2012, 18, 324–329.
- Penel-Page, M.; Plesa, A.; Girard, S.; Marceau-Renaut, A.; Renard, C.; Bertrand, Y. Association of fludarabin, cytarabine, and fractioned gemtuzumab followed by hematopoietic stem cell transplantation for first-line refractory acute myeloid leukemia in children: A single-center experience. Pediatr. Blood Cancer 2020, 67, e28305.
- Toyama, D.; Matsuno, R.; Sugishita, Y.; Kaneko, R.; Okamoto, N.; Koganesawa, M.; Fujita, S.; Akiyama, K.; Isoyama, K.; Yamamoto, S. Successful Treatment of Pediatric Refractory/Relapsed AML with KIR-Ligand-Mismatched Cord Blood Transplant after FLAG-IDA Reinduction Therapy with or without the GO Regimen. Case Rep. Hematol. 2020.
- Niktoreh, N.; Lerius, B.; Zimmermann, M.; Gruhn, B.; Escherich, G.; Bourquin, J.P.; Dworzak, M.; Sramkova, L.; Rossig, C.; Creutzig, U.; et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: A report by Berlin-Frankfurt-Münster study group. Haematologica 2019, 104, 120–127.
- O’Hear, C.; Inaba, H.; Pounds, S.; Shi, L.; Dahl, G.; Paul Bowman, W.; Taub, J.W.; Pui, C.H.; Ribeiro, R.C.; Coustan-Smith, E.; et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer 2013, 119, 4036–4043.
- Roman, E.; Cooney, E.; Harrison, L.; Militano, O.; Wolownik, K.; Hawks, R.; Foley, S.; Satwani, P.; Unal, E.; Bhatia, M.; et al. Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia. Clin. Cancer Res. 2005, 11, 7164s–7170s.
- Zahler, S.; Bhatia, M.; Ricci, A.; Roy, S.; Morris, E.; Harrison, L.; van de Ven, C.; Fabricatore, S.; Wolownik, K.; Cooney-Qualter, E.; et al. A Phase I Study of Reduced-Intensity Conditioning and Allogeneic Stem Cell Transplantation Followed by Dose Escalation of Targeted Consolidation Immunotherapy with Gemtuzumab Ozogamicin in Children and Adolescents with CD33+ Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 698–704.
More
Information
Subjects:
Hematology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
819
Revisions:
2 times
(View History)
Update Date:
15 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




