
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anuja Bokare | + 1910 word(s) | 1910 | 2021-09-13 06:19:11 | | | |
| 2 | Nora Tang | + 158 word(s) | 2068 | 2021-10-13 10:44:09 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2068 | 2021-11-12 09:12:55 | | |
Video Upload Options
For anatase TiO2, the {001} crystal facets are the most reactive because they exhibit unique surface characteristics such as visible light responsiveness, dissociative adsorption, efficient charge separation capabilities and photocatalytic selectivity. In this review, a concise survey of the literature in the field of {001} dominated anatase TiO2 crystals and their composites is presented. Even though the design and morphologically controlled synthesis of TiO2-001 is considered to be a hot spot in scientific research, it still has some drawbacks like its wide band gap and high recombination rate. These drawbacks can easily be overcome by coupling them with other materials to form TiO2-001-based composites. This review focusses on the synthesis, properties and applications of TiO2-001-based composites.
1. Introduction
The rising demand for energy and the increase in environmental pollution have become extremely serious issues in recent years [1][2]. Harnessing the sun’s energy to produce electricity and to remediate environmental pollution through the use of advanced nanomaterials has proven to be a promising solution to the world’s energy crisis [3][4]. One of the first technologies that come to mind when discussing solar energy is photocatalysis. Photocatalysis relies on using the sunlight to promote the degradation of organic pollutants [5][6]. Among a wide spectrum of semiconductors, TiO2 is the most efficient photocatalyst due to its chemical stability, non-toxicity, strong oxidizing power, biocompatibility, large surface area, corrosion resistivity and cost effectiveness [7][8][9].
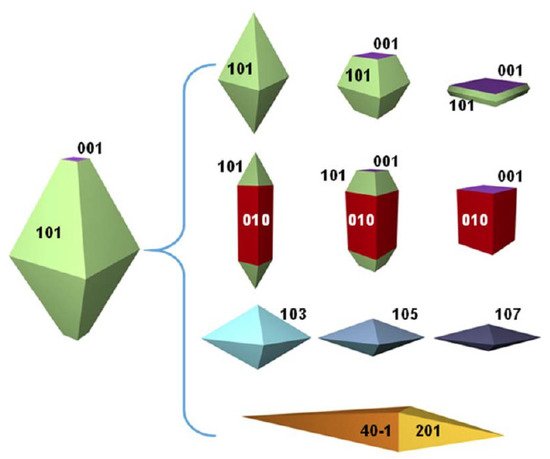
The intense interest in titanium dioxide (TiO 2) as a photocatalyst has spurred the successful synthesis and extensive investigations of a variety of its crystal facets. Exploring the application of the high energetic {001} facets of titania is a recent venture in TiO 2 photocatalysis [10][11][12][13]. A natural anatase crystal typically can exhibit many crystal facets, as shown in Figure 1 [14][15]. Among them, {001} surfaces are highly desired because they are theoretically predicted to possess more active sites (five coordinated Ti + ) and higher surface energy (0.90 J/m 2) relative to other energetically more favorable facets such as {100} 0.53 J/m 2 or {101} 0.44 J/m 2 [16]. Both theoretical and experimental studies demonstrate that the photocatalytic activity of the anatase {001} facets is higher than that of the thermodynamically stable {101} facets [17][18][19]. Apart from photocatalytic studies, many other promising applications such as photo- or electrocatalysis, photoelectrochemical or photovoltaic cells, lithium/sodium ion batteries, Li–S batteries and gas sensing can be significantly improved by morphological control and specifically exposed {001} facets on the surface [20][21][22][23]. Hence, more efforts are being made to synthesize TiO 2 nanomaterials with dominant high-energy {001} facets (TiO 2-001).
Even though the design and morphologically controlled synthesis of TiO 2-001 is considered to be a hot spot in scientific research, it still has some drawbacks like its wide band gap and high recombination rate [24]. These drawbacks can easily be overcome by coupling them with other materials to form TiO 2-001-based composites. For example, doping TiO 2-001 with metal (transition or rare-earth metal) or non-metal ions (C, F, S, N) can make them responsive to visible light [25]. Higher rates of recombination can be suppressed by noble metal deposition on the TiO 2-001 surface [26][27]. When coupled with carbon-based materials, TiO 2-001-based composites show excellent properties, such as high surface area, high absorptivity of dyes and high charge separation [28][29]. These properties make them applicable for many areas of science and technology ranging from adsorption, catalysis and photocatalysis to biomedicine, environmental monitoring and cleanup, energy conversion and storage, etc [30][31]. Hence, it is crucial to highlight the recent progress in the photocatalytic performance of TiO 2-based composites with {001} facets as a future energy material.
This review begins by explaining the role of {001} facets in improving the photocatalytic performance of TiO 2-001 photocatalysts. Then, a brief discussion is given on the modification of the catalysts by doping and coupling mechanisms. Further, we focus on the synthesis routes to obtain TiO 2-001 and modified TiO 2-001-based composites. This is then followed by the various applications of these composites which include environmental remediation by dye degradation, H 2 generation, CO 2 reduction and energy generation through Li-ion batteries. Finally, we present a conclusion and future scope of this emerging field which highlights the major challenges and some invigorating perspectives for future research.
2. Properties of {001} Facets in TiO2
Many researchers have investigated the effect of dissociative adsorption on the photoactivity of TiO 2 crystals. They pointed out that the higher the percentage of {001} facets, the higher the photooxidation reactivity for similarly sized TiO 2 particles.
2.1. Dissociative Adsorption
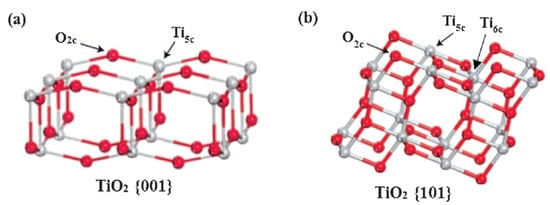
In a recent study, it was found that the {010} facets could only absorb water molecules on its surface while the {001} facets could dissociate the water molecules, producing hydroxyl and other reactive radicals [32]. It is proposed that the {001} facets could also facilitate transfer of these charge carriers showing higher photocatalytic efficiency [33]. The dissociative adsorption of reactant molecules on {001} facets appears to reduce their activation energy and affect the reaction mechanism at the molecular level in the photocatalytic reaction. Similar chemical activity has also been observed in a wide range of organic species adsorption and other applications such as bacterial inactivation, verifying the unique surface chemistry of the {001} facet [34][35].
2.2. Charge Separation
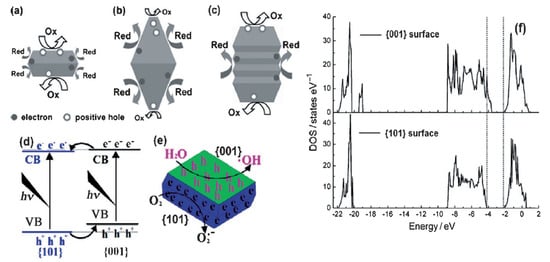
2.3. Optical Properties
Apart from preferential oxidation–reduction ability, the band gap of the TiO2 nanomaterials would also change according to the arrangements of crystal facets on the surface [39]. Based on X-ray photoelectron spectroscopy (XPS) VB spectra and DFT electronic structure calculations, Liu et al. revealed two significant points: (1) the band gap of {001} facets was smaller than that of {101} facets, and (2) the VB maximum of {001} facets was identical to that of {101} facets and thus the CB minimum of {001} facets was lower than that of {101} facets. (Figure 3d) [40]. Hence, the UV-Visible spectrum of anatase TiO2 with 72% {101} facets show blue shifts compared to that with 72% of {001} facets. This electronic band difference would certainly affect the photocatalytic activity of the catalysts in the visible light region [41]. However, up to now, no appreciable visible activity has been observed for pure anatase TiO2 exposed with {001} facets. To make visible light active, these nanomaterials are coupled with other materials.
2.4. Photocatalytic Selectivity
The selectivity of the photocatalysts towards pollutants/organics is a very important aspect for its conversion/transformation. The selectivity of the TiO2 can be enhanced by increasing the percentage of {001} facets on its surface. Li et al. demonstrated that the photocatalytic selectivity for the conversion of toluene to benzaldehyde can be enhanced by increasing the specific surface area of exposed {001} facets. [45]. Similarly, the rate of photoconversion of azo dyes is found to be higher for the TiO2-001 materials [46].
3. Methods to Modify the Properties of TiO2-001 Surfaces
3.1 Doping
One of the most common methods to modify the properties of TiO2-001 is through doping. Doping intentionally introduces impurities into pure TiO2-001 semiconductors and can be done for the purpose of modulating its physical, chemical and optical properties [47]. The choice of the dopant is dependent upon its properties, such as ionic radii, conductivity and chemical stability. Here, we will discuss metal and non-metal ion doping [48].
3.2 Codoping
Heterostructuring the TiO 2-001 by codoping with two or more dopants is reported to achieve significant synergistic effects compared to their single ion doped or undoped TiO 2 counterparts [49][50]. The strong interaction between these dopants within the TiO 2 matrix alters the charge carrier transfer-recombination dynamics and shifts the band gap absorption to the visible region [51]. In the case of Ni and N [52], N broadens the absorption profile, improving the photoutilization of TiO 2, and generates more electron–hole pairs, while Ni doping restrains the increase of grain growth and leads to crystal expansion, retarding the recombination of charge carriers and thus resulting in the faster degradation of MO compared with single ion doping or undoped TiO 2 under UV light.
3.3 Coupling TiO2-001 with semiconductor
Metal oxides, such as Cu 2O [53], Fe 3O 4 [54], Cds [55], MoO 3 [56], SnO 2 [57], and so on, have been considered for band gap engineering of TiO 2 which are discussed in detail in application section.
4. Synthesis of TiO2-001 and TiO2-001-Based Composites
F-terminated anatase TiO2 surfaces have the lowest γ for both {001} and {101} facets among the 12 non-metal-terminated surfaces, and (2) {001} facets are preferential and more stable compared to {101} facets for F-terminated anatase TiO2 crystals. There is strong preferential interaction between fluorine and the {001} facets of anatase TiO2 crystals which enhances the relative stability of the {001} facets during the crystal growth. Hence, researchers used HF as a capping agent and TiF 4 as raw material in a hydrothermal reaction to synthesize TiO 2-001.
However, F-containing compounds generate toxic and corrosive substances at elevated temperatures in hydrothermal synthesis [58]. Moreover, due to the strong interaction between TiO2-001 crystal surface and F− ions, removing them is very difficult. Thus, developing a fluorine-free synthesis methodology is very necessary. To date, there are some papers reporting fluorine-free reagents such as trategies for fabricating anatase TiO2-001, which are ethylene glycol, H2SO4 and H2O2.
5. Applications
Photocatalytic dye-degradation
Photocatalytic H2 generation
Li-ion batteries
Dye sensitized solar cells
Biomedical applications
Sensing
Drug delivery
6. Conclusions and Future Scope
Intensive research efforts on TiO 2-001-based materials, for their application in environmental remediation and energy conversion, have taken place over the last few years. The continuous advancement in the synthesis methods and modification techniques of TiO 2-001 materials have expanded the capabilities of TiO 2 to include visible light absorption, suppressed rate of recombination, higher electronic conductivity and higher surface area with more active sites. This expands the possibilities of TiO 2 applications into new and diverse areas. Despite the huge development in theoretical studies and experimental investigations involving TiO 2-001 and their composites, practical applications in this field are still in the preliminary stages and many challenges exist in several aspects. Some of the challenges are listed below.
The photocatalytic properties of TiO 2-001 materials can be amended by the interaction with new materials, which include light harvesters, charge transport materials, additives and interfacial modifiers. Moreover, many efforts must be made to develop large-scale preparation techniques for high-quality modified TiO 2-001 materials i.e., TiO 2-001-based composite materials.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM), as well as small-angle X-ray/neutron scattering (SANS/SAXS), are the widely used techniques to investigate the morphologies of {001} and {101} faceted anatase TiO 2 nanocrystals. However, these techniques can only provide selected local morphology information. Moreover, these techniques failed to probe the evolution of TiO 2 surface/interface structures in working conditions, which is crucial to study the complex phase transformation and device stability. Hence, more powerful techniques such as near edge X-ray absorption fine structure (NEXAFS) spectroscopy and neutron pair distribution function (PDF) should be used to obtain accurate average morphology and atomistic structures of {001} and {101} faceted anatase TiO 2 nanocrystals. Moreover, in situ characterization techniques must be developed to reveal the photocatalysis reaction process.
Overall, we believe that future research efforts and developments can benefit TiO 2-001 composites as a next generation material for energy conversion and environmental remediation.
References
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage 2021, 3, e135.
- Rogdakis, K.; Karakostas, N.; Kymakis, E. Up-scalable emerging energy conversion technologies enabled by 2D materials: From miniature power harvesters towards grid-connected energy systems. Energy Environ. Sci. 2021, 14, 3352–3392.
- Detz, R.J.; Reek, J.N.H.; van der Zwaan, B.C.C. The future of solar fuels: When could they become competitive? Energy Environ. Sci. 2018, 11, 1653–1669.
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in solar energy conversion. Chem. Soc. Rev. 2019, 48, 1862–1864.
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694.
- Xu, Y.-J. Promises and Challenges in Photocatalysis. Front. Catal. 2021, 1, 6.
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790.
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.-H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.-V.N.; Kim, S.Y.; Van Le, Q. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671.
- Bokare, A.; Pai, M.; Athawale, A.A. Surface modified Nd doped TiO2 nanoparticles as photocatalysts in UV and solar light irradiation. Sol. Energy 2013, 91, 111–119.
- Weon, S.; Choi, E.; Kim, H.; Kim, J.Y.; Park, H.-J.; Kim, S.-M.; Kim, W.; Choi, W. Active Facet Exposed TiO2 Nanotubes Photocatalyst Filter for Volatile Organic Compounds Removal: From Material Development to Commercial Indoor Air Cleaner Application. Environ. Sci. Technol. 2018, 52, 9330–9340.
- Chu, L.; Qin, Z.; Yang, J.; Li, X. Anatase TiO2 Nanoparticles with Exposed Facets for Efficient Dye-Sensitized Solar Cells. Sci. Rep. 2015, 5, 12143.
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204.
- Du, Y.-E.; Niu, X.; Li, W.; An, J.; Liu, Y.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance. Materials 2019, 12, 3614.
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.M.; Cheng, H.-M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612.
- Andrés, J.; Gracia, L.; Gouveia, A.F.; Ferrer, M.M.; Longo, E. Effects of surface stability on the morphological transformation of metals and metal oxides as investigated by first-principles calculations. Nanotechnology 2015, 26, 405703.
- Martsinovich, N.; Troisi, A. How TiO2 crystallographic surfaces influence charge injection rates from a chemisorbed dye sensitiser. Phys. Chem. Chem. Phys. 2012, 14, 13392–13401.
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H.; et al. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 8156–8166.
- Liu, X.; Du, G.; Li, M. True Photoreactivity Origin of Ti3+-Doped Anatase TiO2 Crystals with Respectively Dominated Exposed , , and Facets. ACS Omega 2019, 4, 14902–14912.
- Zheng, J.-Y.; Bao, S.-H.; Guo, Y.; Jin, P. Anatase TiO2 films with dominant facets fabricated by direct-current reactive magnetron sputtering at room temperature: Oxygen defects and enhanced visible-light photocatalytic behaviors. ACS Appl. Mater. Interfaces 2014, 6, 5940–5946.
- Yu, Y.; Wang, X.; Sun, H.; Ahmad, M. 3D anatase TiO2 hollow microspheres assembled with high-energy facets for lithium-ion batteries. RSC Adv. 2012, 2, 7901–7905.
- Niu, L.; Zhang, Q.; Liu, J.; Qian, J.; Zhou, X. TiO2 nanoparticles embedded in hollow cube with highly exposed 001 facets: Facile synthesis and photovoltaic applications. J. Alloys Compd. 2016, 656, 863–870.
- Pei, D.-N.; Gong, L.; Zhang, A.-Y.; Zhang, X.; Chen, J.-J.; Mu, Y.; Yu, H.Q. Defective titanium dioxide single crystals exposed by high-energy facets for efficient oxygen reduction. Nat. Commun. 2015, 6, 8696.
- Hu, C.; Zhang, X.; Li, W.; Yan, Y.; Xi, G.; Yang, H.; Yu, H.-Q. Large-scale, ultrathin and (001) facet exposed TiO2 nanosheet superstructures and their applications in photocatalysis. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 2040–2043.
- Umar, A.A.; Md Saad, S.K.; Ali Umar, M.I.; Rahman, M.Y.A.; Oyama, M. Advances in porous and high-energy (001)-faceted anatase TiO2 nanostructures. Opt. Mater. 2018, 75, 390–430.
- Di Liberto, G.; Tosoni, S.; Pacchioni, G. Nitrogen doping in coexposed (001)-(101) anatase TiO2 surfaces: A DFT study. Phys. Chem. Chem. Phys. 2019, 21, 21497–21505.
- Banerjee, B.; Amoli, V.; Maurya, A.; Sinha, A.K.; Bhaumik, A. Green synthesis of Pt-doped TiO2 nanocrystals with exposed (001) facets and mesoscopic void space for photo-splitting of water under solar irradiation. Nanoscale 2015, 7, 10504–10512.
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.; Balčytis, A.; Nitta, A.; Mendez-Medrano, M.-G.; Colbeau-Justin, C.; Juodkazis, S.; Othani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B 2018, 237, 574–587.
- Lu, T.; Zhang, R.; Hu, C.; Chen, F.; Duo, S.; Hu, Q. TiO2-graphene composites with exposed facets produced by a one-pot solvothermal approach for high performance photocatalyst. Phys. Chem. Chem. Phys. 2013, 15, 12963–12970.
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nanomaterials 2018, 8, 105.
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Facet-dependent photocatalytic properties of TiO2-based composites for energy conversion and environmental remediation. ChemSusChem 2014, 7, 690–719.
- Chen, J.S.; Lou, X.W. SnO2 and TiO2 nanosheets for lithium-ion batteries. Mater. Today 2012, 15, 246–254.
- Vittadini, A.; Selloni, A.; Rotzinger, F.P.; Grätzel, M. Structure and Energetics of Water Adsorbed at TiO2 Anatase (101) and (001) Surfaces. Phys. Rev. Lett. 1998, 81, 2954–2957.
- Selloni, A. Crystal growth: Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615.
- Liu, N.; Li, K.; Li, X.; Chang, Y.; Feng, Y.; Sun, X.; Cheng, Y.; Wu, Z.; Zhang, H. Crystallographic Facet-Induced Toxicological Responses by Faceted Titanium Dioxide Nanocrystals. ACS Nano 2016, 10, 6062–6073.
- Liu, N.; Chang, Y.; Feng, Y.; Cheng, Y.; Sun, X.; Jian, H.; Feng, Y.; Li, X.; Zhang, H. - Surface Heterojunction-Enhanced Antibacterial Activity of Titanium Dioxide Nanocrystals Under Sunlight Irradiation. ACS Appl. Mater. Interfaces 2017, 9, 5907–5915.
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with dominant high-energy 001 facets: Synthesis, properties, and applications. Chem. Mater. 2011, 23, 4085–4093.
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641.
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Lu, H.; Wang, L.; Lu, G.Q.; Cheng, H.M. Enhanced Photoactivity of Oxygen-Deficient Anatase TiO2 Sheets with Dominant Facets. J. Phys. Chem. C 2009, 113, 21784–21788.
- Wang, J.; Lin, W.; Zhou, S.; Li, Z.; Hu, H.; Tao, Y.; Zhou, S.; Zhao, X.; Kong, Y. Probing the formation and optical properties of Ti3+–TiO2 with (001) exposed crystal facet by ethanol-assisted fluorination. New J. Chem. 2021, 45, 12453–12463.
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.M.; Cheng, H.M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757.
- Angeline Dorothy, A.; Panigrahi, P. Tuning optical properties of TiO2 by dimension reduction: From 3D bulk to 2D sheets along and plane. Mater. Res. Express 2020, 6, 1250f1.
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-Controlled Anatase Titanium(IV) Oxide Particles Prepared by Hydrothermal Treatment of Peroxo Titanic Acid in the Presence of Polyvinyl Alcohol. J. Phys. Chem. C 2009, 113, 3062–3069.
- Ye, L.; Liu, J.; Tian, L.; Peng, T.; Zan, L. The replacement of by facets inhibits the photocatalytic activity of anatase TiO2. Appl. Catal. B 2013, 134–135, 60–65.
- Zheng, Z.; Huang, B.; Lu, J.; Qin, X.; Zhang, X.; Dai, Y. Hierarchical TiO2 microspheres: Synergetic effect of and facets for enhanced photocatalytic activity. Chemistry 2011, 17, 15032–15038.
- Zhu, J.; Wang, S.; Bian, Z.; Xie, S.; Cai, C.; Wang, J.; Yang, H.; Li, H. Solvothermally controllable synthesis of anatase TiO2 nanocrystals with dominant facets and enhanced photocatalytic activity. CrystEngComm 2010, 12, 2219–2224.
- Liu, S.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed facets. J. Am. Chem. Soc. 2010, 132, 11914–11916.
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201.
- Sulaiman, S.N.A.; Noh, M.Z.; Adnan, N.N.; Bidin, N.; Razak, S.N.A. Effects of photocatalytic activity of metal and non-metal doped TiO2 for Hydrogen production enhancement—A Review. J. Phys. Conf. Ser. 2018, 1027, 012006.
- Zhuang, H.; Zhang, Y.; Chu, Z.; Long, J.; An, X.; Zhang, H.; Lin, H.; Zhang, Z.; Wang, X. Synergy of metal and nonmetal dopants for visible-light photocatalysis: A case-study of Sn and N co-doped TiO2. Phys. Chem. Chem. Phys. 2016, 18, 9636–9644.
- Suriyachai, N.; Chuangchote, S.; Laosiripojana, N.; Champreda, V.; Sagawa, T. Synergistic Effects of Co-Doping on Photocatalytic Activity of Titanium Dioxide on Glucose Conversion to Value-Added Chemicals. ACS Omega 2020, 5, 20373–20381.
- Xiang, Q.; Yu, J.; Jaroniec, M. Nitrogen and sulfur co-doped TiO2 nanosheets with exposed facets: Synthesis, characterization and visible-light photocatalytic activity. Phys. Chem. Chem. Phys. 2011, 13, 4853–4861.
- Zhang, Y.; Li, C.; Pan, C. N + Ni codoped anatase TiO2 nanocrystals with exposed 001 facets through two-step hydrothermal route. J. Am. Ceram. Soc. 2012, 95, 2951–2956.
- Liu, L.; Gu, X.; Sun, C.; Li, H.; Deng, Y.; Gao, F.; Lin, D. In situ loading of ultra-small Cu2O particles on TiO2 nanosheets to enhance the visible-light photoactivity. Nanoscale 2012, 4, 6351–6359.
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Magnetic composite microspheres with exposed faceted TiO2 shells: A highly active and selective visible-light photocatalyst. J. Mater. Chem. 2012, 22, 13341–13347.
- Qi, L.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of CdS-sensitized Pt/TiO2 nanosheets with exposed (001) facets. Phys. Chem. Chem. Phys. 2011, 13, 8915–8923.
- Moon, S.H.; Kim, M.J.; Im, S.H. Synthesis of lustering two-dimensional α-MoO3 van der Waals crystals by TiO2 assisted selective facet passivation. J. Ind. Eng. Chem. 2020, 84, 358–365.
- Chen, G.; Ji, S.; Li, H.; Kang, X.; Chang, S.; Wang, Y.; Yu, G.; Lu, J.; Claverie, J.; Sang, Y.; et al. High.-Energy Faceted SnO2-Coated TiO2 Nanobelt Heterostructure for Near-Ambient Temperature-Responsive Ethanol Sensor. ACS Appl. Mater. Interfaces 2015, 7, 24950–24956.
- Han, X.; Wang, X.; Xie, S.; Kuang, Q.; Ouyang, J.; Xie, Z.; Zheng, L. Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets. RSC Adv. 2012, 2, 3251–3253.




