
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ger Rijkers | + 2056 word(s) | 2056 | 2021-09-14 09:37:35 | | | |
| 2 | Catherine Yang | Meta information modification | 2056 | 2021-10-11 03:20:27 | | |
Video Upload Options
Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causes Coronavirus Disease 2019 (COVID-19), which has reached pandemic proportions. A number of effective vaccines have been produced, including mRNA vaccines and viral vector vaccines, which are now being implemented on a large scale in order to control the pandemic. The mRNA vaccines are composed of viral Spike S1 protein encoding mRNA incorporated in a lipid nanoparticle and stabilized by polyethylene glycol (PEG). The mRNA vaccines are novel in many respects, including cellular uptake and the intracellular routing, processing, and secretion of the viral protein. Because of space restrictions, viral vector vaccines not discussed in detail. The antigen presentation routes in MHC class I and class II, in relation to the induction of virus-neutralizing antibodies and cytotoxic T-lymphocytes, will be reviewed. In rare cases, mRNA vaccines induce unwanted immune mediated side effects. In rare cases, the mRNA-based vaccines may lead to an anaphylactic reaction. This reaction may be triggered by PEG. The intracellular routing of PEG and potential presentation in the context of CD1 will be discussed.
1. Introduction
The high morbidity and mortality rate of coronavirus disease of 2019 (COVID-19) has triggered the rapid development of vaccines against its causative agent, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1][2]. Vaccines remain the most effective way to eliminate and control the COVID-19 virus. As of September 27, 2021, nearly 6 billion doses of highly effective vaccines have been administered (World Health Organization) [3]. The two major categories of SARS-COV-2 vaccines are messenger RNA-based (mRNA) vaccines and viral vector vaccines that both target the Spike protein of the virus [4]. Worldwide, the most used mRNA vaccines are those of Pfizer/BioNTech (BNT 162b2) and Moderna (mRNA-1273). The most frequently used viral vector vaccines are the ones of Oxford/AstraZeneca (ChAdOx 1 nCoV-19) and Jansen/Johnson and Johnson (Ad26.COV2:S), as well as the Sputnik-V and CanSino vaccines.
Both mRNA vaccines as well as viral vector based vaccines or SARS-COV2 have turned out to be highly effective for protection against mild and severe COVID-19 cases. After vaccination, cytotoxic T-cells are activated and high levels of (IgG and IgA) antibodies against the Spike protein are generated that show virus-neutralizing capacity in laboratory settings [5][6][7].
This entry describes the pathways, outside and inside of the cell, that lead to the presentation of Spike protein peptides to the immune system. Both the classical antigen presentation route via MHC class I to cytotoxic T cells (CD8+) and via MHC class II to T helper cells (CD4+), as well as the antigen-presenting routes for presentation to non-conventional T cells will be reviewed.
2. SARS-CoV-2 mRNA Vaccine Antigen Presentation
T cells only can recognize antigens when presented by molecules of the major histocompatibility complex (MHC). MHC molecules consist of an α chain, associated with β2 microglobulin (MHC class I) or of an α chain and β chain (class II). MHC class I present antigens which are produced intracellularly, and this includes viral peptides which are synthesized by virus infected cells. MHC class I is expressed by all cells of the body. Cytotoxic T cells (also known as CD8+ T cells), can recognize antigens presented in MHC class I, and upon differentiation can kill the virus-infected cells [8][9] . MHC class II molecules are expressed by the so-called professional antigen-presenting cells (dendritic cells, macrophages, and also B-lymphocytes) and present viral peptides to CD4+ T cells, the helper cells for CD8+ T cells, B cells, and other cells of the immune system (Figure 1) [9]. For activation of B cells, the B cell receptor (membrane immunoglobulin) can recognize and interact with either soluble or cell-bound antigens, but it does not require antigen presentation in MHC class I or II. However, the antibody response of B cells to protein antigens is dependent on T helper cells, in particular follicular helper T cells [10]. Next to MHC class I and II, non-conventional antigen presenting molecules exist which will be discussed below.
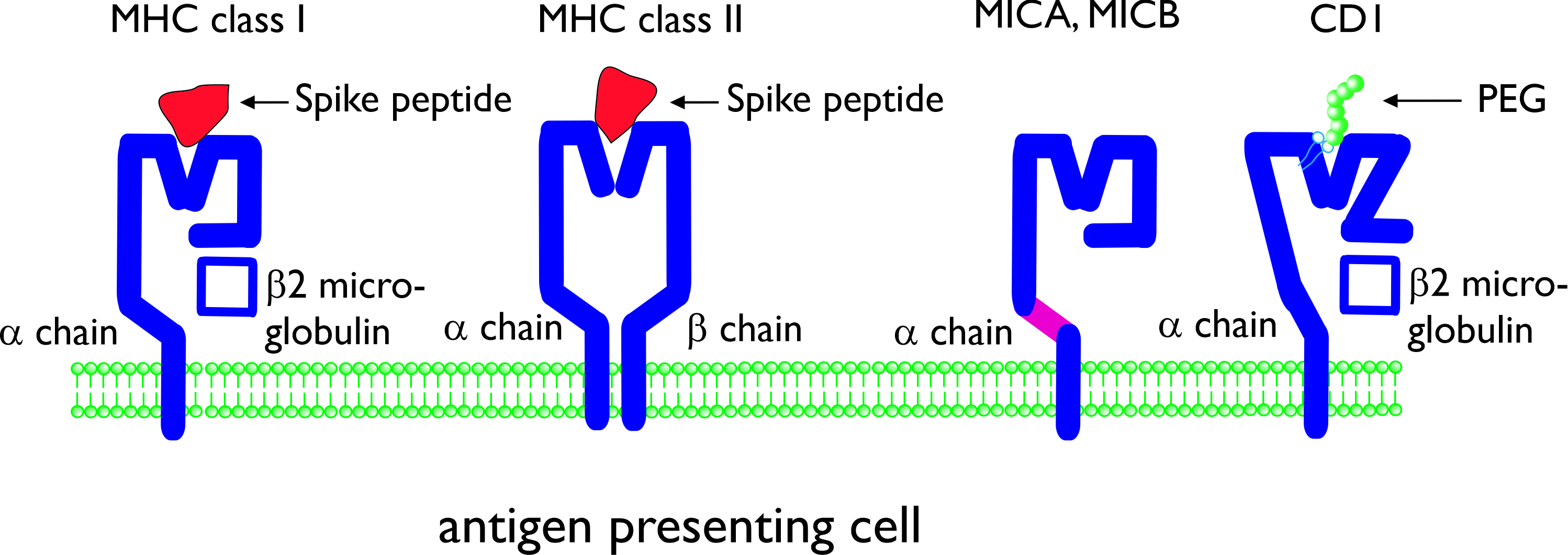
Figure 1. Schematic presentation of conventional and non-conventional antigen-presenting molecules major histocompatibility complex (MHC) class I and class II. The molecules are shown with (different) Spike peptides in the antigen-presenting groove. MHC class I-related molecules A and B (MICA, MICB) and CD1 are non-conventional antigen-presenting molecules consisting of a single α chain. CD1 is composed of an α chain, associated with β2 microglobulin. CD1 can present lipid antigens and (potentially) lipid-bound polyethylene glycol (PEG).
2.1 SARS-CoV-2 vaccines
DNA or RNA vaccines are delivered through the use of viral vectors (such as an adenovirus: another (harmless) virus with a nucleic acid of the virus that you are vaccinating for encoded in the genome) or non-viral delivery systems (e.g., using electroporation or lipid nanoparticles to enter cells) [11].
The mRNA vaccines against SARS-CoV-2 Spike protein were developed by Moderna and Pfizer/BioNtech in record time, with the first vaccinations occurring less than a year after this novel coronavirus was sequenced [12][13][14][15]. Even though mRNA vaccines appear to be simple (consisting of a lipid envelope surrounding mRNA molecule encoding for the antigen protein of interest), hard work has gone into optimizing the safety and efficacy profiles by the pioneering work of multiple research, thereby enabling this success story.
2.2 Uptake, processing, and antigen presentation
The mechanism of action of an mRNA vaccine is very similar to the mechanism of viral infection. Through the use of the translational machinery of the host cells, the mRNA is translated into proteins. These proteins may undergo post-translational modification and either function within the cell or be secreted. Proteasomes degrade cytoplasmic proteins, thus generating antigenic peptide epitopes that are transported to the ER and loaded onto MHC class I molecules (Figure 2). MHC class I can present these peptides on the surface of the cell for specific CD8+ T cells. Alternatively, the secreted exogenous proteins can be taken up by professional antigen-presenting cells, either residing in the tissue or draining lymph nodes, and then be processed and presented in MHC class II [16]. In mRNA-vaccinated individuals (BNT162b1), T-cells can be detected to be secreting interferon-γ upon in vitro restimulation with SARS-CoV-2 peptides, which confirms the induction of CD4+ Th-cells through MHC class II [17]. Professional antigen-presenting cells also can present exogenous antigens, which are processed via alternative intracellular routing and presented via MHC class I (cross-presentation) [18][19].
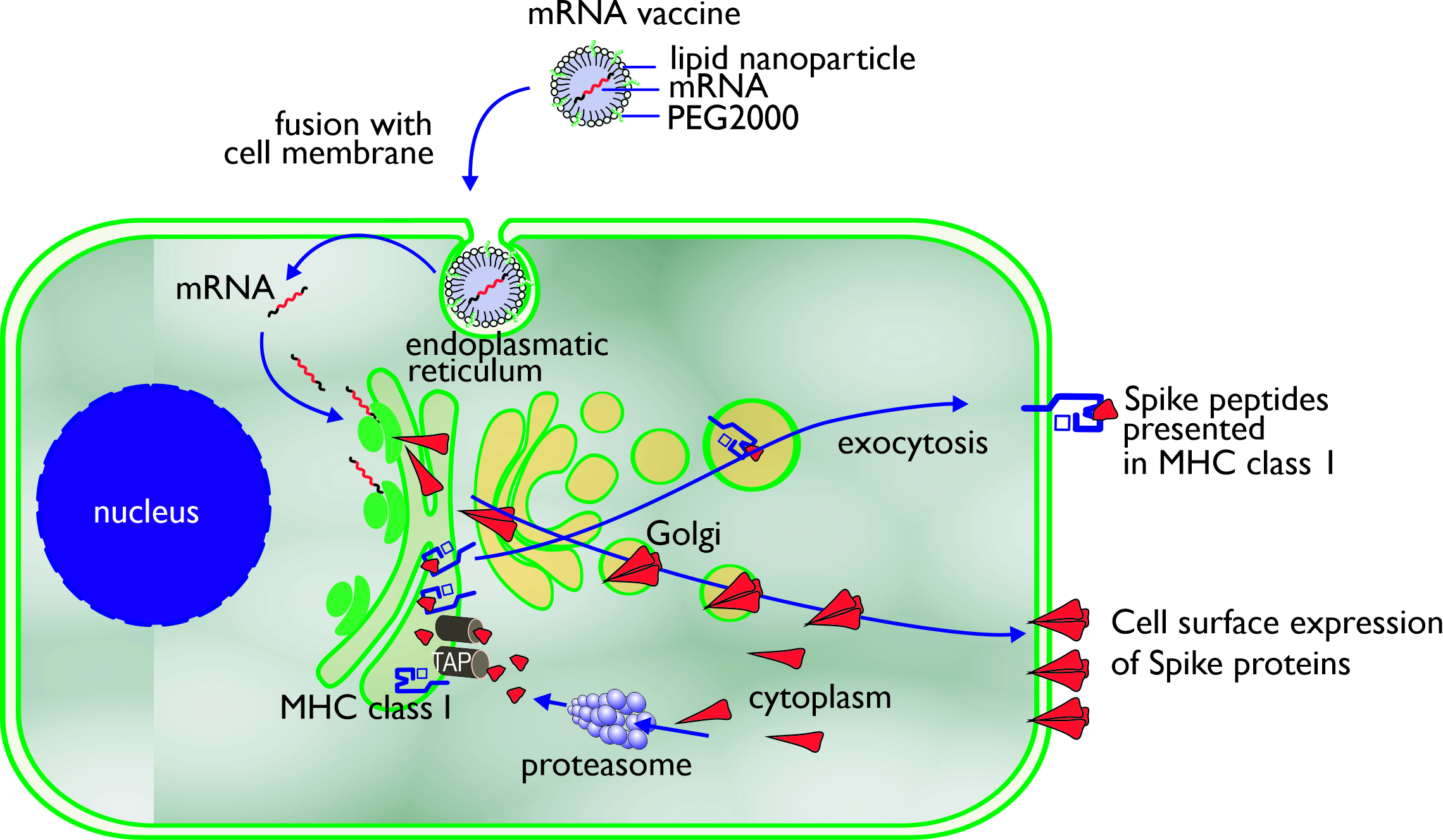
Figure 2. Uptake, processing, and MHC class I presentation of Spike proteins encoded by an mRNA vaccine.
There are multiple advantages to using mRNA-based vaccines over traditional vaccines. mRNA vaccines combine the simplicity, safety, and focused immunogenic properties of subunit vaccines with the favorable immunological properties of live viral vaccines. mRNA vaccines are molecularly defined to encode only the specific antigen of interest and no other excess information. This means that in the case of a SARS-CoV-2 vaccine, the mRNA does not encode the entire virus, but only the S-protein. This greatly reduces the complications associated with biological vaccine production (such as genetic variability). An important benefit of RNA-based vaccines is the enormous flexibility of vaccine design and production. The antigen encoding sequence (the ORF) can be easily modified at specific locations or codon optimized to improve translation or engineered to guide the antigen to the desired intracellular compartments to improve antigen presentation. Modifications such as point mutations, deletions, or the removal of glycosylation sites could all potentially affect antigenicity, immunogenicity, and overall vaccine efficacy. Moreover, next to additions to the coding sequence, the half-life of mRNA, the pharmacokinetics of protein expression (such as magnitude and duration), and immunogenicity are all available for fine-tuning via modifications of, for example, the 5′ and 3′ UTRs and optimization of the length of the poly-A tail [20]. The mRNA could also be tailored in such a way to provide potent adjuvant stimuli to the innate immune system by direct activation of RNA-specific receptors, which may reduce the need for additional adjuvants [21].
3. Antigen Presentation of SARS-CoV-2 Vaccine Additives
3.1. Unconventional Antigen Presentation Molecules
3.2. Hypersensitivity and PEG Antigen Presentation
3.3. Antigen Presentation of PEG
3.4. Thrombosis and Vaccine-Induced Thrombocytopenia (VITP)
4. Conclusions
Both mRNA-based as well as viral vector vaccines with the genetic information for the SARS-CoV-2 Spike protein have turned out to induce an efficient humoral and cellular immune response. The design of these vaccines ensures that the antigens are presented to CD4+ T cells in MHC class II and to CD8+ T cells in MHC class I. The role of unconventional T cells, and the presentation of vaccine antigens to these unconventional T cells, is not completely understood at the moment. The benefits of vaccination in preventing COVID-19 must be emphasized, which far outweigh the risks of (severe) adverse events [29]. However, in rare cases, immune mediated side effects are observed, particularly hypersensitivity reactions including anaphylaxis. Delineation of the molecular mechanisms underlying these adverse effects will be required to reduce the incidence and to develop adequate testing and treatment modalities.
References
- Lindsey R. Baden; Hana M. El Sahly; Brandon Essink; Karen Kotloff; Sharon Frey; Rick Novak; David Diemert; Stephen A. Spector; Nadine Rouphael; C. Buddy Creech; et al.John McGettiganShishir KhetanNathan SegallJoel SolisAdam BroszCarlos FierroHoward SchwartzKathleen NeuzilLawrence CoreyPeter GilbertHolly JanesDean FollmannMary MarovichJohn MascolaLaura PolakowskiJulie LedgerwoodBarney S. GrahamHamilton BennettRolando PajonConor KnightlyBrett LeavWeiping DengHonghong ZhouShu HanMelanie IvarssonJacqueline MillerTal Zaks Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine 2021, 384, 403-416, 10.1056/nejmoa2035389.
- Eric J Haas; Frederick J Angulo; John M McLaughlin; Emilia Anis; Shepherd R Singer; Farid Khan; Nati Brooks; Meir Smaja; Gabriel Mircus; Kaijie Pan; et al.Jo SouthernDavid L SwerdlowLuis JodarYeheskel LevySharon Alroy-Preis Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet 2021, 397, 1819-1829, 10.1016/s0140-6736(21)00947-8.
- WHO Coronavirus (COVID-19) Dashboard . WHO Coronavirus (COVID-19) Dashboard. Retrieved 2021-10-10
- Birgit M. Prüβ; Current State of the First COVID-19 Vaccines. Vaccines 2021, 9, 30, 10.3390/vaccines9010030.
- Paola Rogliani; Alfredo Chetta; Mario Cazzola; Luigino Calzetta; SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines 2021, 9, 227, 10.3390/vaccines9030227.
- Leonidas Stamatatos; Julie Czartoski; Yu-Hsin Wan; Leah J. Homad; Vanessa Rubin; Hayley Glantz; Moni Neradilek; Emilie Seydoux; Madeleine F. Jennewein; Anna J. MacCamy; et al.Junli FengGregory MizeStephen C. De RosaAndrés FinziMaria P. LemosKristen W. CohenZoe MoodieM. Juliana McElrathAndrew T. McGuire mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, eabg9175, 10.1126/science.abg9175.
- Atil Bisgin; Ahter D. Sanlioglu; Yunus Emre Eksi; Thomas S. Griffith; Salih Sanlioglu; Current Update on Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Development with a Special Emphasis on Gene Therapy Viral Vector Design and Construction for Vaccination. Human Gene Therapy 2021, 32, 541-562, 10.1089/hum.2021.052.
- Vibhuti Kumar Shah; Priyanka Firmal; Aftab Alam; Dipyaman Ganguly; Samit Chattopadhyay; Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Frontiers in Immunology 2020, 11, 1949, 10.3389/fimmu.2020.01949.
- Ahmet Kursat Azkur; Mübeccel Akdis; Dilek Azkur; Milena Sokolowska; Willem Van De Veen; Marie-Charlotte Brüggen; Liam O’Mahony; Yadong Gao; Kari Nadeau; Cezmi A. Akdis; et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy 2020, 75, 1564-1581, 10.1111/all.14364.
- Wenzhi Song; Joe Craft; T follicular helper cell heterogeneity: Time, space, and function. Immunological Reviews 2019, 288, 85-96, 10.1111/imr.12740.
- Jeffrey B Ulmer; Andrew J Geall; Recent innovations in mRNA vaccines. Current Opinion in Immunology 2016, 41, 18-22, 10.1016/j.coi.2016.05.008.
- Lisa A. Jackson; Evan J. Anderson; Nadine G. Rouphael; Paul C. Roberts; Mamodikoe Makhene; Rhea N. Coler; Michele P. McCullough; James D. Chappell; Mark R. Denison; Laura J. Stevens; et al.Andrea J. PruijssersAdrian McDermottBritta FlachNicole A. Doria-RoseKizzmekia S. CorbettKaitlyn M. MorabitoSijy O’DellStephen D. SchmidtPhillip A. SwansonMarcelino PadillaJohn R. MascolaKathleen M. NeuzilHamilton BennettWellington SunEtza PetersMat MakowskiJim AlbertKaitlyn CrossWendy BuchananRhonda Pikaart-TautgesJulie E. LedgerwoodBarney S. GrahamJohn H. Beigel An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. New England Journal of Medicine 2020, 383, 1920, 10.1056/nejmoa2022483.
- Edward E. Walsh; Robert W. Frenck; Ann R. Falsey; Nicholas Kitchin; Judith Absalon; Alejandra Gurtman; Stephen Lockhart; Kathleen Neuzil; Mark J. Mulligan; Ruth Bailey; et al.Kena A. SwansonPing LiKenneth KouryWarren KalinaDavid CooperCamila Fontes-GarfiasPei-Yong ShiÖzlem TüreciKristin R. TompkinsKirsten E. LykeVanessa RaabePhilip R. DormitzerKathrin U. JansenUğur ŞahinWilliam C. Gruber Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine 2020, 383, 2439-2450, 10.1056/nejmoa2027906.
- Fernando P. Polack; Stephen J. Thomas; Nicholas Kitchin; Judith Absalon; Alejandra Gurtman; Stephen Lockhart; John L. Perez; Gonzalo Pérez Marc; Edson D. Moreira; Cristiano Zerbini; et al.Ruth BaileyKena A. SwansonSatrajit RoychoudhuryKenneth KouryPing C4591001 Clinical Trial GroupWarren V. KalinaDavid CooperJr. Robert W. FrenckLaura L. HammittÖzlem TüreciHaylene NellAxel SchaeferSerhat ÜnalDina B. TresnanSusan MatherPhilip R. DormitzerUğur ŞahinKathrin U. JansenWilliam C. Gruber Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 2020, 383, 2603-2615, 10.1056/nejmoa2034577.
- Mark J. Mulligan; Kirsten E. Lyke; Nicholas Kitchin; Judith Absalon; Alejandra Gurtman; Stephen Lockhart; Kathleen Neuzil; Vanessa Raabe; Ruth Bailey; Kena A. Swanson; et al.Ping LiKenneth KouryWarren KalinaDavid CooperCamila Fontes-GarfiasPei-Yong ShiÖzlem TüreciKristin R. TompkinsEdward E. WalshRobert FrenckAnn R. FalseyPhilip R. DormitzerWilliam C. GruberUğur ŞahinKathrin U. Jansen Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589-593, 10.1038/s41586-020-2639-4.
- Abishek Wadhwa; Anas Aljabbari; Abhijeet Lokras; Camilla Foged; Aneesh Thakur; Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics 2020, 12, 102, 10.3390/pharmaceutics12020102.
- Ugur Sahin; Alexander Muik; Isabel Vogler; Evelyna Derhovanessian; Lena M. Kranz; Mathias Vormehr; Jasmin Quandt; Nicole Bidmon; Alexander Ulges; Alina Baum; et al.Kristen E. PascalDaniel MaurusSebastian BrachtendorfVerena LörksJulian SikorskiPeter KochRolf HilkerDirk BeckerAnn-Kathrin EllerJan GrütznerManuel TonigoldCarsten BoeslerCorinna RosenbaumLudwig HeesenMarie-Cristine KühnleAsaf PoranJesse Z. DongUlrich LuxemburgerAlexandra Kemmer-BrückDavid LangerMartin BexonStefanie BolteTania PalancheArmin SchultzSybille BaumannAzita J. MahinyGábor BorosJonas ReinholzGábor T. SzabóKatalin KarikóPei-Yong ShiCamila Fontes-GarfiasJohn L. PerezMark CutlerDavid CooperChristos A. KyratsousPhilip R. DormitzerKathrin U. JansenÖzlem Türeci BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572-577, 10.1038/s41586-021-03653-6.
- Olivier Joffre; Elodie Segura; Ariel Savina; Sebastian Amigorena; Cross-presentation by dendritic cells. Nature Reviews Immunology 2012, 12, 557-569, 10.1038/nri3254.
- Pedro A. Reche; Potential Cross-Reactive Immunity to SARS-CoV-2 From Common Human Pathogens and Vaccines. Frontiers in Immunology 2020, 11, 586984, 10.3389/fimmu.2020.586984.
- Mohamad-Gabriel Alameh; Drew Weissman; Norbert Pardi; Messenger RNA-Based Vaccines Against Infectious Diseases.. Current Topics in Microbiology and Immunology 2020, 1, 1-35, 10.1007/82_2020_202.
- Thomas Kramps; Jochen Probst; Messenger RNA-based vaccines: progress, challenges, applications. WIREs RNA 2013, 4, 737-749, 10.1002/wrna.1189.
- John M. Kelso; Anaphylactic reactions to novel mRNA SARS-CoV-2/COVID-19 vaccines. Vaccine 2021, 39, 865-867, 10.1016/j.vaccine.2020.12.084.
- Tom Shimabukuro; Narayan Nair; Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA: The Journal of the American Medical Association 2021, 325, 780, 10.1001/jama.2021.0600.
- COVID-19: latest updates . European Medicines Agency. Retrieved 2021-10-10
- Cosby A. Stone; Yiwei Liu; Mary V. Relling; Matthew Krantz; Amanda L. Pratt; Andrew Abreo; Jonathan A. Hemler; Elizabeth J. Phillips; Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. The Journal of Allergy and Clinical Immunology: In Practice 2018, 7, 1533-1540.e8, 10.1016/j.jaip.2018.12.003.
- Gianfranco Calogiuri; Caterina Foti; Eustachio Nettis; Elisabetta Di Leo; Luigi Macchia; Angelo Vacca; Polyethylene glycols and polysorbates: Two still neglected ingredients causing true IgE-mediated reactions. The Journal of Allergy and Clinical Immunology: In Practice 2019, 7, 2509-2510, 10.1016/j.jaip.2019.05.058.
- Priya Sellaturay; Shuaib Nasser; Pamela Ewan; Polyethylene Glycol–Induced Systemic Allergic Reactions (Anaphylaxis). The Journal of Allergy and Clinical Immunology: In Practice 2020, 9, 670-675, 10.1016/j.jaip.2020.09.029.
- Eun Ji Park; Jiyoung Choi; Kang Choon Lee; Dong Hee Na; Emerging PEGylated non-biologic drugs. Expert Opinion on Emerging Drugs 2019, 24, 107-119, 10.1080/14728214.2019.1604684.
- Emily Wenande; Lene Heise Garvey; Immediate-type hypersensitivity to polyethylene glycols: a review. Clinical & Experimental Allergy 2016, 46, 907-922, 10.1111/cea.12760.
- Cines, D.B.; Bussel, J.B; SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. New England Journal of Medicine 2021, 384, e92, 10.1056/nejmx210006.
- Joshua K Salabei; Troy J Fishman; Zekarias T Asnake; Arroj Ali; Uma G Iyer; COVID-19 Coagulopathy: Current knowledge and guidelines on anticoagulation. Heart & Lung 2021, 50, 357-360, 10.1016/j.hrtlng.2021.01.011.
- Sukrita Bhattacharjee; Mainak Banerjee; Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Comprehensive Clinical Medicine 2020, 2, 2048-2058, 10.1007/s42399-020-00521-8.
- Vaccine Adverse Event Reporting System . Vaccine Adverse Event Reporting System. Retrieved 2021-10-10




