
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krzysztof Stolarczyk | + 2321 word(s) | 2321 | 2021-08-26 07:48:59 | | | |
| 2 | Amina Yu | Meta information modification | 2321 | 2021-10-11 04:24:01 | | |
Video Upload Options
Pharmacological and nutraceutical effects of isoflavones are attributed to their antioxidant activity protecting cells against carcinogenesis. The knowledge of the oxidation mechanisms of an active substance is crucial to determine its pharmacological properties.
1. Introduction
Genistein (GE) belongs to the group of isoflavones found mainly in soybeans[1][2]. Some studies suggest that frequent consumption of soy-based products in Asian countries reduces the incidence of breast and prostate cancer compared to Western countries. Genistein also has antioxidant, anti-inflammatory, antiangiogenic, pro-apoptotic, and antiproliferative properties, which gives it great potential for use in anti-cancer therapy. It has been shown that genistein may influence the regulation of apoptosis, angiogenesis, metastasis, and various stages of the cell cycle. In addition to acting on transcription factors, genistein also induces stress on the endoplasmic reticulum, which in turn leads to the apoptosis of neoplastic cells. Additionally, it has been shown to induce apoptosis in cancer cells by targeting the PPAR (peroxisome proliferator-activated receptor) signaling cascade. Various molecular mechanisms of genistein in diverse cancer models were described by Tuli et al[3]. However, its low water-solubility and poor oral bioavailability severely hamper its use as an ingredient for food and the pharmaceutical industries [4]. Therefore, there is an urgent need for the study of a new analog of genistein, which additionally can be promising for an economical and feasible delivery system to enhance the solubility and dissolution rates, and bioavailability of genistein while maintaining its chemical stability [4].
Our previous studies showed that genistein interacts electrostatically with gold nanoparticles[5] which turned out to be interesting carriers for active substances. These studies were continued by Vodnik et al. [6], however the authors did not perform a purity study of conjugates in relation to the free GE and stability of new conjugates as well. It is known that electrostatic interactions are not sufficiently stable at extreme pH and in the presence of salt. For example, Dinkel and co-workers[7] described that citrates are only loosely bound to the gold nanoparticles (6.7 kJ/mol). However, sulfur-containing compounds have been used as excellent ligands for binding to flat gold surfaces as well as gold nanoparticles, because of a very strong interaction of sulfur nucleophiles with gold. Therefore, to increase the strength of the interaction, and improve the stability of AuNPs-GE conjugates, we focused on the investigation of an optimal thiol linker as a new derivative—thiolated genistein (TGE, 7-O-[2-(mercaptomethylcarboxy)ethyl-genistein) described by Sidoryk and co-workers [8] (compound No. 26). The structural formula of TGE is presented in Figure 1. TGE is composed of the genistein residue bound at the C7-OH site to the ethyl linker and thioglycolic acid residue. Such a construction assures that the TGE will be bound firmly to the Au surface via the Au-S chemical bond on the gold surface as well as gold nanoparticles.
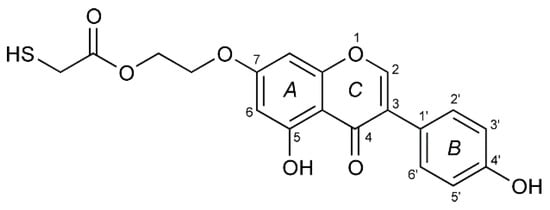
Figure 1. The chemical structure of TGE (the thiolated derivative of genistein). Three rings of the moiety are marked as A, B, and C.
Because pharmacological and nutraceutical effects of isoflavones are attributed to antioxidant mechanisms also, which protect cells against reducing carcinogenesis, the knowledge of the oxidation mechanisms is crucial to ascertain the influence of the redox behavior, on the pharmacological, nutritional, and chemical properties of our new thiolated analog of genistein. TGE was chemically attached to the gold surface as the self-assembled monolayers (SAMs). The study of SAMs of alkanethiol, alkanedithiol, sulphides, and disulfides on the Au surface allowed us to obtain data about processes occurring at the interface, as well as about interactions between molecules in the monolayer [9][10][11].
Our aim is to study the mechanism of the oxidation reaction of a potential new drug with promising biological activity. The selected anticancer drug is thiogenistein—a new analogue of genistein. For the first time, a self-assembled monolayer of such a complex compound attached to the gold electrode is studied. Three instrumental techniques are applied in order to determine products of the TGE oxidation on the Au electrode: electrochemistry, IR-ATR, and MALDI-MS. Selected experimental results are interpreted with the use of molecular modeling and quantum mechanical density functional calculations. We hope that understanding simulated electrochemical reactions will help to determine key molecular fragments involved in the subsequent biochemical processes in living organisms. Apart from these physico-chemical studies, a preliminary evaluation of the anticancer activity of TGE is undertaken based on one human prostate cancer (DU145 line) and safety for normal prostate epithelial cells (PNT2 line).
The topicality of our research is emphasized by the works of Kim and Rizzo [12][13], who confirmed the importance of genistein as an anticancer drug via oxidative processes.
2. In Vitro Study
Genistein is one of the isoflavones whose anti-cancer potential is extensively studied. It modulates various steps of the cell cycle, apoptosis, angiogenesis, and metastasis in different types of cancers. Therefore, the number of studies on the synthesis and biological evaluation of new genistein derivatives is increasing every year [3][3]. In our research, we focused on a new thioderivative of genistein and its biological properties. A preliminary study of the anti-tumor activity of thiogenistein (TGE) was carried out on DU145 prostate cancer cells to determine the properties of the new derivative and compared to genistein as the base compound. The concentration-dependent cell viability and cytotoxicity effect induced by TGE and GE (as a reference) were measured after 6, 24, and 72-h of incubation (Figure 2). After the addition of 50 µM TGE solution, the viability of prostate cancer cells is reduced to 81.49% (±0.73%) and 66.12% (±1.30%) after 6 and 24 hours of incubation, respectively. However, in the case of GE, after 6 h, cell viability drops only to 92.91% (±1.01%), and after 24 h to 74.32% (±0.75%). The obtained results suggest that TGE reduces the viability of prostate cancer cells faster compared to GE. No meaningful differences are observed at lower concentrations. However, the most significant differences between cells were observed after 6 h (Figure 2A1). The addition of 100 μM of the TGE solution reduces the viability of cancer cells to 60.8 % (±0.55%), relative to the untreated cells. In contrast, for GE, cell viability remains high at 91.3% (±0.26%). The results achieved by the ToxiLightTM assay are complementary to the analysis described above. The toxicity of TGE is shown in Figure 2B (compared with the effect of GE). The cytotoxicity for the three highest concentrations of TGE increased over time and reached 84% (±3.41%) after 72 h. In the case of the thiol derivative, a significant slow down in the proliferation of DU145 cells is observed (Figure 2B1–B3). The number of cells (estimated by ToxiLight 100 % Lysis Control) after 6, 24, and 72 h of incubation with the highest concentrations of TGE did not multiply. Based on these results, it could be concluded that TGE in a short time has far more negative effects on cellular respiration mechanisms and cytochromes of prostate cancer cells compared to GE. Additionally, the incubation of prostate cancer cells with TGE significantly affects their shape (Figure 2D1–D3). After only 6 h, cells become more rounded in contrast to GE-treated cells, which retain their characteristic, elongated shape. Due to early research, we are unable to determine the exact mechanism of TGE activity, which is responsible for reducing the rate of proliferation and reducing the viability of human prostate cancer cells.
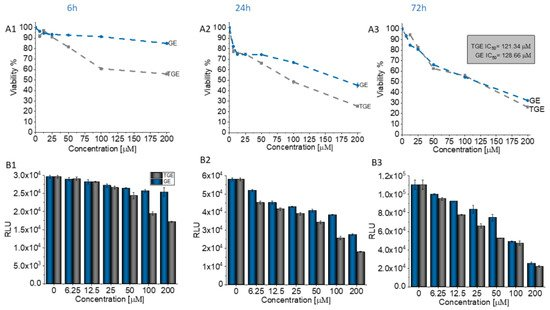
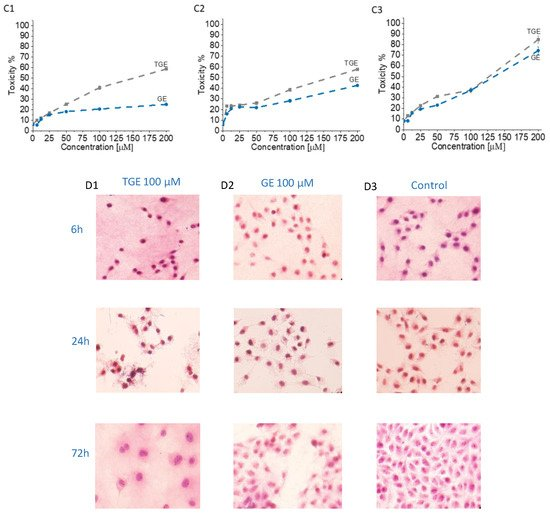
Figure 2. (A1–A3) Dependence of the viability (represented in % regarding control) on the concentration of TGE and GE incubated with DU145 cells for 6, 24, and 72 h. The viability was estimated based on the measurement of fluorescence intensity of the converted nonfluorescent resazurin to resorufin in metabolically active cells. (B1–B3) The luminescence intensity correlates with the adenylate kinase (AK) level obtained from all cells in the samples with ToxiLight 100% Lysate Control. The obtained results are proportional to the cell number of cells. (C1–C3) The cytotoxicity of the drugs was estimated based on the measurements of the AK level in the supernatant (released from the damaged cells) related to the AK level in the lysate (all cells in the samples). The data in (A–C) panels are representative of two independent experiments and are expressed as the mean ± SD. The error bars represent the ± SD. (D1–D3) Photomicrographs of the prostate cancer cells (DU145 line) morphology after 6, 24, and 72 h culture with the addition of 100 µM of TGE and GE as compared with the untreated group (control).
In addition to its anti-tumor properties, the new derivative should also show relative safety concerning healthy cells. Therefore, the initial characterization of TGE was supplemented with studies carried out on the PNT2 cell line—normal prostate epithelial cells. Figure 13 shows the concentration-dependent effect of TGE and GE on prostate epithelial cells. After 72 hours of incubation, the viability of PNT2 incubated with 50 µM GE decreased to 48.35% (±1.89%). With TGE, cell viability only drops to 79.88% (±0.86%). The results achieved by the ToxiLightTM assay are complementary to the analysis described above. The cytotoxicity of TGE against normal prostate epithelial cells is 19.07% (±0.50%), while GE is as high as 45.75% (±1.09%) (Figure 3C). This is clearly visible in the photomicrographs (Figure 3D), which demonstrate the morphology and the number of prostate epithelial cells. After 72 h, cells incubated with 50 µM GE lose the normal epithelial morphology and an arrest of proliferation is evident, relative to the control group. In the case of cells incubated with TGE, the changes in these two parameters are not observed in comparison to untreated cells. Based on the obtained results, we can conclude that TGE shows both a lower cytotoxic effect against normal prostate epithelial cells and an increased antitumor activity. In the case of cancer cells treated with GE and TGE, significant differences in their viability are visible at the dose of 100 µM, while in the case of normal cells, these differences are already visible at the dose of 50 µM.
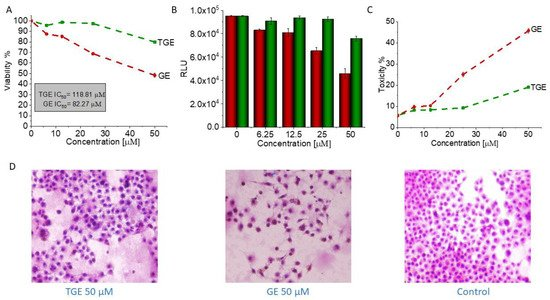
Figure 3. (A) The viability of PNT2 cells treated by different concentrations of GE and TGE for 72 h (determined by PrestoBlueTM test). (B,C) The cytotoxicity effect of TGE and GE was evaluated based on the luminescence intensity of the supernatant and lysate. (D) The morphology of prostate epithelial incubated cells with 50 μM of GE and TGE for 72 h. The data in (A–C) panels are representative of two independent experiments and are expressed as the mean ± SD. The error bars represent the ± SD.
According to the literature, genistein is metabolized mainly through oxidation, sulfation, glucuronidation, hydroxylation, or methylation[14]. However, the influence of genistein metabolites on its anti-cancer properties is still not fully understood. Kiriakidis et al. in their work identified two genistein metabolites in T47D cells of the breast epithelium, such as 5,7,39,49-tetrahydroxyisoflavone (THIF)—orobol and 2 glutathinyl conjugates of THI[15]. THIF has also been shown to inhibit angiogenesis and the proliferation of endothelial cells. This suggests that THIF formation during genistein treatment may play a major role in cell cycle arrest, inhibition of cell proliferation, and activation of signaling pathways such as p38 MAPK that have been observed in T47D cells. According to the literature, over time, THIF oxidizes to o-quinone with the formation of hydrogen peroxides and reactive oxygen, which induces DNA strand breakage [16][17]. The synthetic derivatives, such as genistein glycosides, are also reported to possess anticancer activity when assessed in vitro. The anticancer potency of genistein glycosides varies depending on the sugar groups attached. For example, the addition of acetylated sugar hydroxyls to genistein resulted in more selectivity toward tumor cells. It is worthwhile to note that the anticancer potency of genistein and its derivatives differs in various types of cancer, depending on their selectivity toward the target molecule[18] .
In vitro studies would suggest that changing the hydroxyl for an ether containing an -SH group on the C7 carbon in ring A may increase the cytotoxic properties of TGE. This effect can be attributed to the presence of a highly reactive -SH group [19]. Additionally, the presence of the -SH group in the new substituent may be responsible for the correct thiol-disulfide balance and the associated oxidation-reduction potential of cells. A similar mechanism is observed for glutathione which is a natural antioxidant [20].
3. Conclusions
This work contributes to the design of a simple and feasible strategy for thio-compounds in nano-drugs for use in oncology. In our previous publication, we described the synthesis of the thiolated genistein analog (TGE). In this work, while continuing the research on this new, promising compound, we examined its properties after oxidation as a process related to the reduction of cancer proliferation. The properties of TGE and its oxidation products were studied on a model system, i.e., the TGE monolayer on the Au electrode. In the course of the voltammetric experiments, it was proven that TGE is electrochemically active. The TGE monolayer undergoes irreversible oxidation reaction of the hydroxyl group in the A ring and the attachment of the -OH group to the B ring in the first stage, and then the quasi-reversible process of the quinone/hydroquinone system in the next stage. The formation of electroactive products that undergo redox reactions were observed. Electroactive centers of TGE were identified and its oxidation mechanisms were analyzed. The structure of TGE with a greater number of hydroxyl groups and additionally in a polymer configuration were discussed through MALDI-TOF MS and IR. Molecular modeling and the quantum mechanical density functional calculations supported this discussion. Our observations from the voltammetric experiments, supported with spectrometric data, are consistent with the literature regarding natural phenolic antioxidants described by the group of Oliveira-Brett [21]. In a preliminary in-vitro study, it was recognized that TGE has a higher cytotoxic activity towards DU145 prostate cancer cells and is safer for normal prostate epithelial cells (PNT2) than genistein (GE) itself. Moreover, the formation of TGE trimers upon oxidation deduced from the present study may enhance the biological response than the parent drug due to the synergizing of this response[22][23][24]. Most often, dimeric/trimeric drugs are not released (or cleaved) within the cell, so they can act as a completely new molecular unit inside the targeted cells[24]. The TGE monolayer firmly bound to the Au surface described in the present work may contribute to the design and development of the carriers of medicines in nanotechnology of biological applications.
References
- Xiong, P.; Wang, R.; Zhang, X.; DeLa Torre, E.; Leon, F.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.-H. Design, Synthesis, and Evaluation of Genistein Analogues as Anti-Cancer Agents. Anti-Cancer Agents Med. Chem. 2015, 15, 1197–1203. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Kleczkowska, P.; Olędzka, E.; Figat, R.; Sobczak, M. Poly(Chitosan-ester-ether-urethane) hydrogels as highly controlled genistein release systems. Int. J. Mol. Sci. 2021, 22, 3339. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Xiao, Y.; Ho, C.-T.; Chen, Y.; Wang, Y.; Wei, Z.; Dong, M.; Huang, Q. Synthesis, Characterization, and Evaluation of Genistein-Loaded Zein/Carboxymethyl Chitosan Nanoparticles with Improved Water Dispersibility, Enhanced Antioxidant Activity, and Controlled Release Property. Foods 2020, 9, 1604. [Google Scholar] [CrossRef]
- Stolarczyk, E.U.; Stolarczyk, K.; Łaszcz, M.; Kubiszewski, M.; Maruszak, W.; Olejarz, W.; Bryk, D. Synthesis and characterization of genistein conjugated with gold nanoparticles and the study of their cytotoxic properties. Eur. J. Pharm. Sci. 2017, 96, 176–185. [Google Scholar] [CrossRef]
- Vodnik, V.V.; Mojić, M.; Stamenović, U.; Otoničar, M.; Ajdžanović, V.; Maksimović-Ivanić, D.; Mijatović, S.; Marković, M.M.; Barudžija, T.; Filipović, B.; et al. Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater. Sci. Eng. C 2021, 124, 112078. [Google Scholar] [CrossRef] [PubMed]
- Dinkel, R.; Braunschweig, B.; Peukert, W. Fast and slow ligand exchange at the surface of colloidal gold nanoparticles. J. Phys. Chem. C 2016, 120, 1673–1682. [Google Scholar] [CrossRef]
- Sidoryk, K.; Michalak, O.; Kubiszewski, M.; Leś, A.; Cybulski, M.; Stolarczyk, E.U.; Doubsky, J. Synthesis of thiol derivatives of biological active compounds for nanotechnology application. Molecules 2020, 25, 3470. [Google Scholar] [CrossRef]
- Jiang, C.; Elliott, J.M.; Cardin, D.J.; Tsang, S.C. An electrochemical study of 4-aminothiophenol/Pt nanoparticle multilayers on gold electrodes. Langmuir 2008, 25, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, K.; Bilewicz, R. Catalytic oxidation of ascorbic acid on 2D and 3D monolayers of 4-hydroxythiophenol. Electroanalysis 2004, 16, 1609–1615. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Pałys, B.; Bilewicz, R. Catalytic properties of 4-hydroxythiophenol protected gold nanoclusters supported on gold electrodes. J. Electroanal. Chem. 2004, 564, 93–98. [Google Scholar] [CrossRef]
- Kim, I. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Rizzo, G. The antioxidant role of soy and soy foods in human health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hernandez-Montes, E.; Vauzour, D.; Schönthal, A.H.; Rice-Evans, C.; Cadenas, E.; Spencer, J.P.E. The intracellular genistein metabolite 5,7,3′,4′-tetrahydroxyisoflavone mediates G2-M cell cycle arrest in cancer cells via modulation of the p38 signaling pathway. Free Radic. Biol. Med. 2006, 41, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Kiriakidis, S.; Högemeier, O.; Starcke, S.; Dombrowski, F.; Hahne, J.C.; Pepper, M.; Jha, H.C.; Wernert, N. Novel tempeh (fermented soyabean) isoflavones inhibit in vivo angiogenesis in the chicken chorioallantoic membrane assay. Br. J. Nutr. 2005, 93, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Markovits, J.; Linassier, C.; Fossé, P.; Couprie, J.; Pierre, J.; Jacquemin-Sablon, A.; Saucier, J.M.; Le Pecq, J.B.; Larsen, A.K. Inhibitory Effects of the Tyrosine Kinase Inhibitor Genistein on Mammalian DNA Topoisomerase II. Cancer Res. 1989, 49, 5111–5117. [Google Scholar]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs—Genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, L.; Zhang, X.; He, J.; Wei, G. Electropolymerization Treatment of Phenol Wastewater and the Reclamation of Phenol. Water Environ. Res. 2012, 84, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Grosso, R.; De-Paz, M.-V. Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid. Pharmaceutics 2021, 13, 854. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Berube, G. Natural and Synthetic Biologically Active Dimeric Molecules: Anticancer Agents, Anti-HIV Agents, Steroid Derivatives and Opioid Antagonists. Curr. Med. Chem. 2005, 13, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Murakami, Y.; Kadoma, Y. Dimerization, ROS formation, and biological activity of o-methoxyphenols. Arch. Immunol. Ther. Exp. 2005, 53, 28–38. [Google Scholar]
- Paquin, A.; Reyes-Moreno, C.; Bérubé, G. Recent advances in the use of the dimerization strategy as a means to increase the biological potential of natural or synthetic molecules. Molecules 2021, 26, 2340. [Google Scholar] [CrossRef] [PubMed]




