Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nongyue He | + 3518 word(s) | 3518 | 2021-09-24 11:46:54 | | | |
| 2 | Jessie Wu | Meta information modification | 3518 | 2021-10-08 08:09:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
He, N. Aptamer-Bound Nanomaterials in Cancer Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/14880 (accessed on 06 February 2026).
He N. Aptamer-Bound Nanomaterials in Cancer Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/14880. Accessed February 06, 2026.
He, Nongyue. "Aptamer-Bound Nanomaterials in Cancer Therapy" Encyclopedia, https://encyclopedia.pub/entry/14880 (accessed February 06, 2026).
He, N. (2021, October 08). Aptamer-Bound Nanomaterials in Cancer Therapy. In Encyclopedia. https://encyclopedia.pub/entry/14880
He, Nongyue. "Aptamer-Bound Nanomaterials in Cancer Therapy." Encyclopedia. Web. 08 October, 2021.
Copy Citation
Cancer is still a major disease that threatens human life. Although traditional cancer treatment methods are widely used, they still have many disadvantages. Aptamers, owing to their small size, low toxicity, good specificity, and excellent biocompatibility, have been widely applied in biomedical areas. Therefore, the combination of nanomaterials with aptamers offers a new method for cancer treatment.
aptamers
nanomaterials
cancer
treatment
targeting
1. Introduction
Cancer is one of the key threats to human health. Cancer is a disease caused by abnormal cell growth. Cancer cells may spread to different tissues and organs [1]. According to statistics from the World Health Organization, tens of millions of people worldwide are diagnosed with cancer each year. Most cancers are caused by the external environment, such as smoking, radiation, and environmental pollution. Until now, the main way to treat cancer in developed countries has been chemotherapy, radiotherapy, and surgical treatment [2][3]. However, these cancer treatments have a series of problems, such as easy recurrence, poor treatment effects, and large side effects. Hence, people are looking forward to developing new strategies to treat cancers.
In recent years, nanotechnology has developed rapidly, and more and more nanomaterials with excellent physical and chemical properties have been discovered [4] in the fields of electronics, magnetics, and optics. Many nanomaterials have been used by researchers in the field of biomedicine [5][6][7][8][9][10][11][12][13]. For example, nanomaterials with photothermal conversion properties are used for tumor treatment [14][15][16] and mesoporous materials are used for drug delivery [17][18][19], while magnetic nanomaterials have been found to have excellent superparamagnetic properties, and carbon nanomaterials have been found to feature broad absorbance regions [20][21][22][23]. The focus on nanomaterial applications has been moving from the cellular level toward the tissue level, including cellular imaging, drug delivery, and cancer diagnosis/therapy. Via the enhanced permeability and retention (EPR) effect, most nanomaterials accumulate in a targeted region. People have also proposed various strategies to improve the flow of nanomaterials in blood and improve their stability and biocompatibility [24][25]. However, the EPR effect is not always effective, and the targeting ability of nanomaterials is not good. These problems are accompanied by the issue of harmful toxicity to nontarget tissues or organs. Thus, this problem hinders the development of nanomaterials in bioapplications. Therefore, active targeting strategies have been considered as more powerful tools for cancer treatments, and much attention has been focused on selecting targeted moieties to endow antitumor agents with specific recognition of tumor cells via the affinity between ligands and receptors. There are many receptors overexpressed on the membrane of tumor cells. Based on this, various specific ligands have been discovered, including antibodies, transferrin, folic acid, and peptides. Apart from these ligands, aptamers have become one kind of the most attractive biomolecules.
It is well known that aptamers have a highly selective recognition ability that helps them recognize targets [26][27][28]. Aptamers are single-stranded DNAs or RNAs evolved through systematic evolution of ligands by exponential enrichment (SELEX) technology [29][30] and they are widely employed, as shown in Figure 1. Aptamers can fold into various secondary structures, further forming three-dimensional structures, which contribute to the interaction between aptamers and their targets through various forces, such as hydrophobic interaction and electrostatic attraction [31][32]. Moreover, compared with traditional targeting ligands, aptamers have their advantages, such as good reproducibility, convenient modification, small size, low toxicity, good stability, and low molecular weight [33][34][35]. In addition, aptamers have higher rates of tumor penetration, retention, and homogenous distribution, while the attachment process of aptamers to the surface of nanomaterials is more amenable and reproducible. In principle, an aptamer sequence can be deleted, added, or united flexibly to endow aptamers with tunable recognition ability to better identify targets. Stimulus–response strategies including light, pH, ligand binding, and other cues which have been developed into the design of aptamer-based nanomaterials [27][28][29]. In short, these merits make aptamers ideal candidates for disease diagnosis and therapeutics owing to their ability to deliver therapeutic cargoes into cancer cells, diseased tissue, and organs. Hence, the introduction of aptamers into the construction of nanomaterial systems will promote their bioapplications.
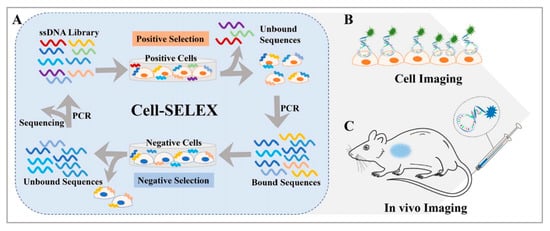
Figure 1. (A) Basic principle of the cell-SELEX method and applications for (B) cell imaging and (C) in vivo imaging. Reprinted with permission from [29]. Copyright © 2020 American Chemical Society.
In this review, we summarize several current applications of aptamer-bound nanomaterials in cancer therapy (Scheme 1). Today, there are various methods to treat cancer. Here, however, we mainly focus on photothermal therapy (PTT), photodynamic therapy (PDT), and improved drug delivery systems (DDSs). We chose several common cancers, including breast cancer, lung cancer, liver cancer, cervical cancer, gastric cancer, colorectal cancer, and prostate cancer. In the first section, we introduce the combination of aptamers with nanomaterials in different cancer therapies. Next, we list several commonly used nanomaterials in cancer therapies (Table 1). Finally, we discuss perspectives on challenges and future applications of aptamer-bound nanomaterials in cancer therapy.
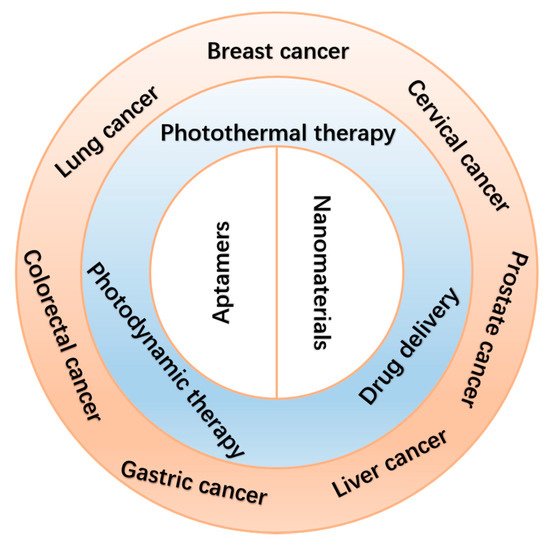
Scheme 1. Schematic illustration of the applications of aptamer-bound nanomaterials in cancer therapy.
2. Therapeutic Method
2.1. Photothermal Thearpy
PTT has gained much attention in the field of cancer therapy since Goldman used laser irradiation to remove tumors in 1966 [36][37][38][39][40][41][42][43]. Owing to its minimal toxicity, noninvasiveness, convenient operation, and low recurrence, PTT showed great promise for cancer therapy. After photosensitizing agents accumulate in the tumor site, a near-infrared (NIR) laser is used. Because of the high photothermal conversion efficiency of photosensitizing agents, the absorbed optical energy is converted into thermal energy, leading to either partial or complete ablation of the target tissues. For effective ablation of the tumors, PTT often requires the tumor center to reach high temperatures (≥50 ℃). Because of the deep-tissue penetration of NIR light, various organic and inorganic phototherapeutic agents have been developed. For example, Guo et al. prepared B quantum dots (BQDs) with good biocompatibility. BQDs can effectively produce photothermal effects under NIR light irradiation. Both in vitro and in vivo experimental studies showed that BQDs-PEG can significantly kill cancer cells and inhibit tumor growth through photothermal effects [36]. Leng et al. synthesized core-shell and dumbbell-like gold nanorods-Cu7S4 heterostructures. Both gold nanorods-Cu7S4 heterostructures exhibited significantly enhanced photothermal conversion efficiency (η = 56% and 62%) and good photothermal stability. The in vitro photothermal ablation of cancer cells featured low cytotoxicity and effective photothermal treatments [37]. Wang et al. developed a new dual-targeted small-molecule organic photothermal agent for enhanced photothermal therapy, which can target both biotin and mitochondria. The in vivo photothermal therapy experiments indicated that the dual-targeted photothermal agent performed much better in tumor inhibition [39].
2.2. Photodynamic Therapy
During the past few decades, PDT has been an attractive therapeutic method for tumor therapy [44][45][46][47][48] because it is noninvasive, spatially selective, and has negligible toxicity. After photosensitizers accumulate in the tumor site, reactive oxygen species (ROS) are produced by NIR laser irradiation. Toxic ROS damages tumor cells via the oxidation of protein, DNA, or RNA. Different photosensitizers have been prepared to overcome both accumulation issues and the hypoxic environment in the tumor. For example, Zhou et al. developed an activatable ROS generation system by modulating a biochemical reaction between linoleic acid hydroperoxide and catalytic iron (II) ions. The engineered nanoparticles were capable of inducing apoptotic cancer death both in vitro and in vivo through ROS generation [46]. Yang et al. prepared a Pt nanoenzyme with functionalized nanoplatform black phosphorus/Pt-Ce6/PEG nanosheets for synergistic photothermal and enhanced photodynamic therapy, in which the Pt nanoenzyme would decompose H2O2 into oxygen to enhance the photodynamic effect [48].
2.3. Drug Delivery Systems
A DDS can be defined as a method or process using the principles of chemistry, engineering, and biology for administering pharmaceutical compounds. Due to their special properties, DDSs have shown tremendous promise to improve the diagnostic and therapeutic effects of drugs, especially in enhancing the pharmaceutical effects of drugs and reducing the side effects of therapeutics in the treatment of various disease conditions. Various types of DDSs have been extensively investigated as potential drug carriers for the treatment of many diseases [49][50][51][52][53][54][55][56][57][58][59][60]. For example, Wang et al. successfully constructed a simple and novel gas generator with a high drug loading level that markedly facilitated doxorubicin release and O2 diffusion for amplifying PDT/PTT/chemotherapy combination therapy [49]. Lei et al. constructed a multifunctional mesoporous silica nanoparticle-based drug delivery platform to load indocyanine green and doxorubicin for NIR-triggered drug release and chemo/photothermal therapy [51].
Although the applications of nanomaterials in PTT, PDT, and DDS have been great successes, improving the targeting and specificity of these therapeutic methods is still a big challenge.
3. Aptamer-Bound Nanomaterials Used in Different Cancer Therapies
3.1. Breast Cancer Therapy
The incidence of breast cancer in female cancers worldwide is 24.2%, of which 52.9% occur in developing countries [61][62]. Developing an optimized treatment method is therefore of great importance. Conventional treatments include surgery, chemotherapy, and radiotherapy. Chemotherapy and radiotherapy treatments have serious side effects. For example, during chemotherapy, the patients’ white blood cells may be reduced, and there may be problems with their blood clotting function. It will also affect the normal function of the liver. Radiotherapy is a local treatment method that uses radiation to treat tumors. This process also risks damaging normal tissues and insufficiently killing off cancer cells. Moreover, radiation therapy may also cause wound complications [63][64][65]. Therefore, the novel treatment involving binding of nanomaterials with aptamers will contribute to the development of breast cancer therapy [66][67].
3.1.1. Photothermal Therapy
Molybdenum disulfide (MoS2) is a typical transition metal disulfide, which is a class of two-dimensional nanomaterials, that has many biomedical applications, owing to its simple preparation, good stability, large surface area, excellent water dispersibility, and biocompatibility [68][69][70]. MoS2 has especially high NIR absorbance. Because of this, it has high photothermal conversion efficiency. Based on this, many researchers have applied it in photothermal therapy [71][72]. However, its ability to recognize specific tumor cells needs to be improved. For example, Pang et al. developed a new method [73] which used MoS2 as the photothermal agent. Firstly, by mixing MoS2 and bovine serum albumin (BSA), researchers obtained MoS2-BSA nanosheets. Next, EDC and NHS were used to activate the free carboxyl group on the MoS2-BSA surface, and subsequently aptamers were modified to obtain composite MoS2-BSA-Apt nanosheets, which were stable and biocompatible. Owing to the good affinity between aptamers and receptors on the cell surface [74][75], the composition would distinguish MCF-7 human breast cancer cells from other cells and then enter the cells through endocytosis. Under the irradiation from an 808 nm laser, the heat generated kills cancer cells, which is on the basis of MoS2 nanosheets. After MoS2-BSA-Apt was cultured with MCF-7 human breast cancer cells and MCF-10A human breast cancer cells, the fluorescence-inverted microscope results showed that MCF-7 human breast cancer cells had uniformly distributed green fluorescence signals, while MCF-10A human breast cancer cells had almost no fluorescence signal. The results showed that under the same laser irradiation time, MoS2-BSA-Apt exhibited a better cell-killing effect than MoS2-BSA, indicating that MoS2-BSA-Apt could target MCF-7 human breast cancer cells. In addition, MoS2 nanosheets have magnetism, fluorescence, and other properties. These properties can be used to develop a combined accurate diagnosis and treatment platform that integrates photothermal therapy with imaging diagnosis.
Graphene oxides (GOs) and gold nanoparticles (AuNPs) have been widely employed in cancer therapies because of their excellent photothermal conversion efficiencies and biocompatibilities [76][77]. GOs are excellent nanomaterials as drug carriers due to their high loading capacity. Therefore, creating a nanomatrix by combining GOs with AuNPs in a single system may enhance the photothermal effects on tumors. Considering the high loading capacities of GOs, Yang et al. anchored AuNPs on GOs to enhance the photothermal effect [78]. As shown in Figure 2, to improve the targeting capability of this nanomatrix, thiolated MUC1 aptamers were immobilized on the surface of AuNPs via strong Au−S bond, which can specifically recognize breast cancer cells. Next, the Apt–AuNPs were absorbed onto GOs. Then, the generated heat kills cancer cells with irradiation of the laser. To evaluate the targeting of Apt-AuNPs-GOs, researchers used MCF-7 cells (MUC1-positive cell lines) and EA.hy926 cells (MUC1-negative cell lines). RB molecules were loaded onto Apt-AuNPs-GOs to form fluorescent RB-Apt-AuNPs-GOs. The results indicated that EA.hy926 cells showed very weak fluorescence while MCF-7 cells showed strong red fluorescence. Owing to the excellent loading capacities of GOs, this strategy could be extended to the construction of heat shock protein inhibitor-loaded Apt-AuNPs-GOs to strengthen the effect of photothermal therapy.
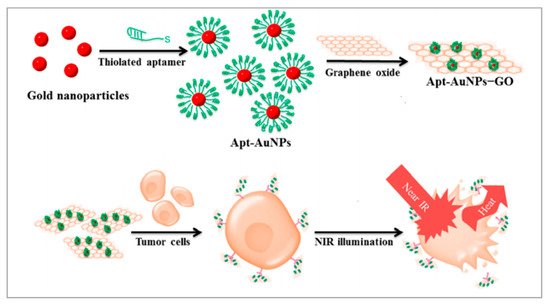
Figure 2. Schematic representation of the preparation of Apt-AuNP-GO and its application with NIR laser irradiation for photothermal therapy of cancer cells. Reprinted with permission from [78]. Copyright © 2015 American Chemical Society.
As shown in Figure 3, Wu et al. also proposed a method with metal nanomaterials [79]. Ag-Au nanostructures have high photothermal conversion efficiencies and are applied in PTT. By modifying the S2.2 aptamer on the surface of Ag-Au nanostructures, the Apt-Ag-Au nanostructures could interact with breast cancer cells on whose membrane MUC1 proteins are overexpressed and realized photothermal therapy. Besides, Ag-Au nanostructures are attractive surface-enhanced Raman scattering (SERS) substrates because of the synergism of these metals, the tunability of the plasmon resonance, and the superior SERS activity. The synthesized Apt-Ag-Au nanostructures will contribute to developing a protocol to specifically recognize and sensitively detect the cancer cells and facilitate the synergistic treatment of diagnosis and photothermal therapy.
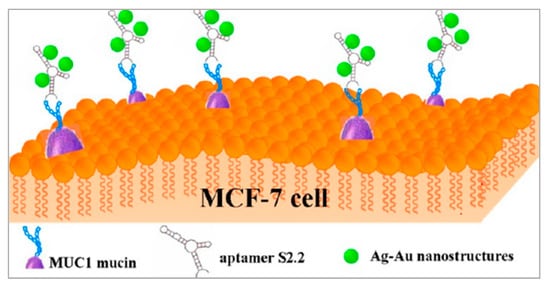
Figure 3. Schematic representation of the preparation of Apt-Ag-Au nanostructure and its application. Reprinted with permission from [79]. Copyright © 2012 American Chemical Society.
3.1.2. Photodynamic Therapy
Manganese dioxide (MnO2) has been applied more and more in biomedical areas due to its excellent loading capacities and convenient surface functionalization [80][81]. Owing to its quenching property, MnO2 has been used as a carrier of photosensitizer to construct novel activatable PDT systems. MnO2 is a unique type of tumor microenvironment-responsive nanomaterial that can react with GSH, and thus overcome the problems of PDT treatment [82][83][84]. Liu et al. proposed a new strategy [85] that used photosensitizer HMME with mesoporous MnO2 (mMnO2) functioning as the carrier of HMME. The photosensitizers were in the quenching state when loaded on the surface of mMnO2 nanoparticles and sealed by the aptamers on the particle surface. The aptamers were able to selectively recognize the specific membrane protein MUC1 on the tumor cell, and when this happened the photosensitizers were released. When it interacted with normal cells lacking MUC1, the HMME were not released and the PDT did not work. On the contrary, in the presence of MUC1-overexpressed breast cancer cells, the aptamer bound with MUC1 protein, and HMME was released [86][87]. Then ROS was produced under laser irradiation, which killed cancer cells. To examine the tumor-targeting release of HMME, researchers used MCF-7 cells and Hs578bst cells. The confocal laser scanning microscopy (CLSM) results showed that the fluorescence of HMME in MCF-7 cells was very bright, while it was very low in Hs578bst cells. Furthermore, after irradiation of the laser, the ROS level in MCF-7 (56.4%) was much higher than that in Hs578Bst (1.24%), which confirmed the HMME imaging results. Compared with the conventional PDT method, this constructed system provides a simple but effective approach for the selective killing of tumor cells, with infinitesimal toxicity to normal cells, and paves a new way for utilizing PDT in precise cancer treatment.
Upconversion nanoparticles (UCNPs) are often used as photosensitizer energy donors and delivery vectors in PDT therapy. Moreover, UCNPs functionalized with recognition moieties can also be conferred with the cell-targeting ability for the specific delivery of photosensitizer to enhance the efficiency of PDT. Jin et al. developed a novel method [88]. As shown in Figure 4, a long, single-stranded DNA (ssDNA) with an AS1411 aptamer and a DNAzyme was prepared using rolling circle amplification (RCA). UCNPs functioned as the carrier on which to load the ssDNA. The multivalence of the ssDNA endowed the upconversion nanoplatform with high recognition and drug loading capacity and DNAzyme inhibited the expression of survivin by gene interfering tools. In this nanosystem, AS1411 aptamer was not only used to load the photosensitizer TMPyP4, but it also functioned as the targeting agent to recognize the nucleolin that was overexpressed on breast cancer cells. PDT was triggered by NIR irradiation and generated ROS to kill the cancer cells. To evaluate the targeting of this nanosystem, the uptake of UCNP-ApDz-TMPyP4 in MCF-7 cells (the target cancer cell) and BRL 3A cells (the control cell) was detected by flow cytometry and CLSM. The CLSM results showed that the fluorescence intensity in the MCF-7 cells was significantly stronger than that of BRL 3A cells, which were consistent with the flow cytometry studies. To evaluate the cytotoxicity of MCF-7 cells, MTT assays, LIVE/DEAD viability/cytotoxicity assay were performed. The results showed that UCNP@PVP did not show any cytotoxicity, while in the UCNP-ApDz-TMPyP4 groups the cell survival rate was 36.3%. Emerging evidence has indicated that PDT is always adversely attenuated by the development of cancer cells resistance. However, this multifunctional upconversion nanoplatform, collaborating with PDT and DNAzyme-based gene therapy when used on tumor tissues, exhibits excellent antitumor response in vivo and in vitro and might act as an admirable alternative strategy for treating cancer.
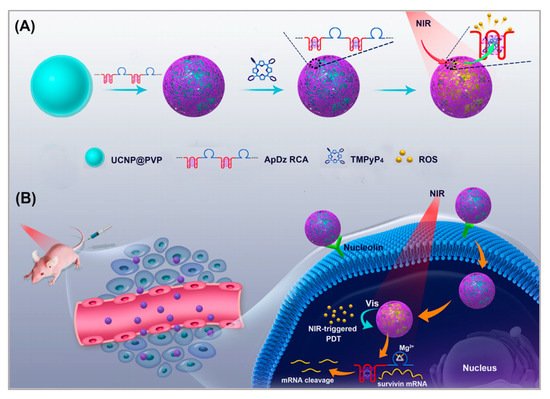
Figure 4. Illustration of: (A) the synthesis of the multifunctional DNA polymer-assisted upconversion therapeutic nanoplatform; and (B) the targeted photodynamic nanoplatform for highly efficient photodynamic therapy. Reprinted with permission from [88]. Copyright © 2020 American Chemical Society.
3.1.3. Drug Delivery System
Similar to the application of mMnO2 by Liu [85], Si et al. proposed another strategy [89]. With the help of mesoporous silica nanoparticles (MSNs), they loaded DNA sensor-capped doxorubicin (DOX). DNA sensors on the targeted nanoparticles could trigger DOX release through a conformational switch induced by MUC-1 protein. They modified the aptamer on the surface of MSNs, which can recognize MUC-1 protein [90]. When the composition was endocytosed into MCF-7 cells, in which MCU-1 was overexpressed, DOX would be released and would kill cancer cells. This caused a significant difference in cell viability between breast cancer MCF-7 and normal breast Hs578bst cells (24.8% and 86.0%). The selectivity and efficiency of treatment were improved greatly. Although the MUC-1 adaptor has been used for drug delivery, the adaptor only served as a targeted ligand and could not accomplish the controlled release of drugs. In this nanosystem, DOX release could be specifically “turned on” in tumor cells according to the MUC1-induced conformational change. This nanosystem provides a new idea for the drug delivery system.
Gene therapy is a promising therapeutic strategy to combat many serious gene-related diseases. Liu et al. developed a novel drug delivery system to combine gene therapy with chemotherapy [91]. During long-term chemotherapy, tumors show drug resistance. Researchers have found that the p53 gene can enhance the sensitivities of drug-resistant tumors to chemotherapeutics. It is well known that DNA nanostructure can be designed to assemble a variety of functional components. As shown in Figure 5, a biocompatible triangle DNA origami was chosen to efficiently load DOX (TOD) and p53 genes (TODP), and then the MUC1 aptamer was modified on the surface of the DNA nanostructure to improve targeted delivery and controlled release. To evaluate the targeting of TODP, delivery vectors with aptamers and without aptamers were used. The biodistribution of these delivery vectors was studied in an animal imaging system utilizing the fluorescent signal of Cy5.5-labeled DNA origami. The results showed that those with aptamers located mainly in the tumor tissue had substantially higher intensity than those without aptamers. Furthermore, with the application of DNA nanostructure, additional functional groups such as RNA-based drugs, gene editing systems, and imaging diagnosis components may also be introduced into this codelivery system for synergistic theranostics. We think this is a promising platform for the development of a new generation of therapeutics for the treatment of cancers.
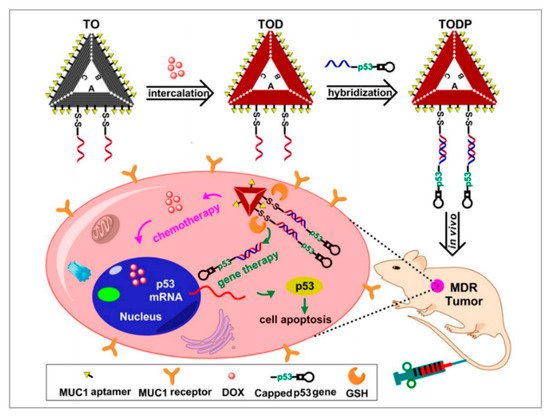
Figure 5. Schematic illustration showing the DNA nanostructure-based combination of gene therapy and chemotherapy. Reprinted with permission from [91]. Copyright © 2018 American Chemical Society.
3.1.4. Photothermal Therapy/Photodynamic Therapy/Chemotherapy
Among various combined treatments, combined chemotherapy/phototherapy is a practicable and promising strategy for cancer treatments due to its favorable synergistic effects and clinical realizability. Xu et al. developed a strategy to combine PTT, PDT, and chemotherapy [92]. As shown in Figure 6, they assembled DOX, indocyanine green (ICG), and bovine serum albumin (BSA) molecules to form nanosized DOX/ICG/BSA nanoparticles. To improve the targeting of the nanoparticles, AS1411 aptamers and a cell-penetrating peptide (KALA) were modified on the surface of the DOX/ICG/BSA nanoparticles through electrostatic interaction. Finally, under the irradiation of the laser, phototherapy was applied and DOX was released to realize chemotherapy. Researchers chose nucleolin overexpressed MCF-7 cells to study the targeting of DOX/ICG/BSA/KALA/Apt. After 4 h of incubation, compared with DOX/ICG/BSA, DOX/ICG/BSA/KALA/Apt showed higher intracellular concentrations of both DOX and ICG. However, in no nucleolin overexpressed 293T cells, the cellular uptakes of DOX/ICG/BSA and DOX/ICG/BSA/KALA/Apt were nearly the same. Besides, the cytotoxicity of DOX/ICG/BSA/KALA/Apt was stronger than DOX/ICG/BSA in MCF-7 cells. Studies showed improved antitumor efficiency of DOX/ICG/BSA/KALA/Apt nanoparticles and demonstrated that the functional theranostic system had great promise in tumor treatment. Although integrated therapeutic systems have developed a lot, the toxicity, biodegradability, and cumbersome assembly processes are still big problems. In future study, researchers should focus on the facile and biocompatible assembly of multifunctional nanosystems.
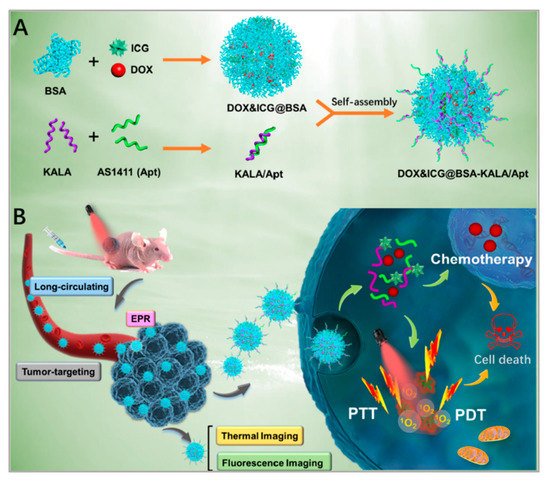
Figure 6. Schematic illustration of: (A) the preparation of DOX/ICG/BSA/KALA/Apt nanoparticles; and (B) the theranostic process based on DOX/ICG/BSA/KALA/Apt nanoparticles. Reprinted with permission from [92]. Copyright © 2019 American Chemical Society.
References
- He, J.; Fu, L.-H.; Qi, C.; Lin, J.; Huang, P. Metal peroxides for cancer treatment. Bioact. Mater. 2021, 6, 2698–2710.
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313.
- Li, T.; Yang, J.; Ali, Z.; Wang, Z.; Mou, X.; He, N.; Wang, Z. Synthesis of aptamer-functionalized Ag nanoclusters for MCF-7 breast cancer cells imaging. Sci. China Chem. 2016, 60, 370–376.
- Barani, M.; Mukhtar, M.; Rahdar, A.; Sargazi, S.; Pandey, S.; Kang, M. Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma. Biosensors 2021, 11, 55.
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906.
- Berlina, A.N.; Zherdev, A.V.; Pridvorova, S.M.; Gaur, M.; Dzantiev, B.B. Rapid Visual Detection of Lead and Mercury via Enhanced Crosslinking Aggregation of Aptamer-Labeled Gold Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5489–5495.
- Cheon, H.J.; Lee, S.M.; Kim, S.-R.; Shin, H.Y.; Seo, Y.H.; Cho, Y.K.; Lee, S.P.; Kim, M.I. Colorimetric Detection of MPT64 Antibody Based on an Aptamer Adsorbed Magnetic Nanoparticles for Diagnosis of Tuberculosis. J. Nanosci. Nanotechnol. 2019, 19, 622–626.
- Khan, R.A.; Barani, M.; Rahdar, A.; Sargazi, S.; Cucchiarini, M.; Pandey, S.; Kang, M. Multi-Functionalized Nanomaterials and Nanoparticles for Diagnosis and Treatment of Retinoblastoma. Biosensors 2021, 11, 97.
- Jiang, Q.; Shi, Y.; Zhang, Q.; Li, N.; Zhan, P.; Song, L.; Dai, L.; Tian, J.; Du, Y.; Cheng, Z.; et al. A Self-Assembled DNA Origami-Gold Nanorod Complex for Cancer Theranostics. Small 2015, 11, 5134–5141.
- Chen, W.; Kang, Y.; Qin, L.; Jiang, J.; Zhao, Y.; Zhao, Y.; Yang, Z. Aptasensor for the Detection of Ochratoxin A Using Graphene Oxide and Deoxyribonuclease I-Aided Signal Amplification. J. Nanosci. Nanotechnol. 2021, 21, 4573–4578.
- Gu, M.; Liu, J.; Li, D.; Wang, M.; Chi, K.; Zhang, X.; Deng, Y.; Ma, Y.; Hu, R.; Yang, Y. Development of Ochratoxin Aptasensor Based on DNA Metal Nanoclusters. Nanosci. Nanotechnol. Lett. 2019, 11, 1139–1144.
- Liu, D.-L.; Li, Y.; Sun, R.; Xu, J.-Y.; Chen, Y.; Sun, C.-Y. Colorimetric Detection of Organophosphorus Pesticides Based on the Broad-Spectrum Aptamer. J. Nanosci. Nanotechnol. 2020, 20, 2114–2121.
- Xia, Y.; Wu, X.; Zhao, J.; Zhao, J.; Li, Z.; Ren, W.; Tian, Y.; Li, A.; Shen, Z.; Wu, A. Three dimensional plasmonic assemblies of AuNPs with an overall size of sub-200 nm for chemo-photothermal synergistic therapy of breast cancer. Nanoscale 2016, 8, 18682–18692.
- Chen, C.H.; Wu, Y.-J.; Chen, J.-J. Gold Nanotheranostics: Photothermal Therapy and Imaging of Mucin 7 Conjugated Antibody Nanoparticles for Urothelial Cancer. BioMed Res. Int. 2015, 2015, 813632.
- Jia, Q.; Zhao, Z.; Liang, K.; Nan, F.; Li, Y.; Wang, J.; Ge, J.; Wang, P. Recent advances and prospects of carbon dots in cancer nanotheranostics. Mater. Chem. Front. 2019, 4, 449–471.
- Lu, Q.; Lu, T.; Xu, M.; Yang, L.; Song, Y.; Li, N. SO2 prodrug doped nanorattles with extra-high drug payload for “collusion inside and outside” photothermal/pH triggered-gas therapy. Biomaterials 2020, 257, 120236.
- Lantero, E.; Belavilas-Trovas, A.; Biosca, A.; Recolons, P.; Moles, E.; Sulleiro, E.; Zarzuela, F.; Ávalos-Padilla, Y.; Ramírez, M.; Fernàndez-Busquets, X. Development of DNA Aptamers Against Plasmodium falciparum Blood Stages Using Cell-Systematic Evolution of Ligands by EXponential Enrichment. J. Biomed. Nanotechnol. 2020, 16, 315–334.
- Ma, X.; Zhao, Y.; Ng, K.W.; Zhao, Y. Integrated Hollow Mesoporous Silica Nanoparticles for Target Drug/siRNA Co-Delivery. Chem. Eur. J. 2013, 19, 15593–15603.
- Yu, S.H.; Kim, T.H. T-T Mismatch-Based Electrochemical Aptasensor for Ultratrace Level Detection of Hg2+ Using Electrochemically Reduced Graphene Oxide-Modified Electrode. J. Biomed. Nanotechnol. 2019, 15, 1824–1831.
- Duan, Q.; Yang, M.; Zhang, B.; Li, Y.; Zhang, Y.; Li, X.; Wang, J.; Zhang, W.; Sang, S. Gold nanoclusters modified mesoporous silica coated gold nanorods: Enhanced photothermal properties and fluorescence imaging. J. Photochem. Photobiol. B Biol. 2021, 215, 112111.
- Zhao, J.; Wang, A.; Si, T.; Hong, J.-D.; Li, J. Gold nanorods based multicompartment mesoporous silica composites as bioagents for highly efficient photothermal therapy. J. Colloid Interface Sci. 2019, 549, 9–15.
- Liu, J.; Detrembleur, C.; De Pauw-Gillet, M.-C.; Mornet, S.; Jérôme, C.; Duguet, E. Gold Nanorods Coated with Mesoporous Silica Shell as Drug Delivery System for Remote Near Infrared Light-Activated Release and Potential Phototherapy. Small 2015, 11, 2323–2332.
- Sivaram, A.J.; Wardiana, A.; Howard, C.; Mahler, S.M.; Thurecht, K.J. Recent Advances in the Generation of Antibody–Nanomaterial Conjugates. Adv. Healthc. Mater. 2017, 7, 1700607.
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.; Xu, J.; Cui, C.; Liu, Y.; Hou, W.; Wang, Y.; Zhang, L.; et al. Self-assembly of DNA Nanohydrogels with Controllable Size and Stimuli-Responsive Property for Targeted Gene Regulation Therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415.
- Liu, M.; Xi, L.; Tan, T.; Jin, L.; Wang, Z.; He, N. A novel aptamer-based histochemistry assay for specific diagnosis of clinical breast cancer tissues. Chin. Chem. Lett. 2020, 32, 1726–1730.
- Liu, Y.; Yang, G.; Li, T.; Deng, Y.; Chen, Z.; He, N. Selection of a DNA aptamer for the development of fluorescent aptasensor for carbaryl detection. Chin. Chem. Lett. 2021, 32, 1957–1962.
- Huang, R.; He, L.; Li, S.; Liu, H.; Jin, L.; Chen, Z.; Zhao, Y.; Li, Z.; Deng, Y.; He, N. A simple fluorescence aptasensor for gastric cancer exosome detection based on branched rolling circle amplification. Nanoscale 2019, 12, 2445–2451.
- Lin, N.; Wu, L.; Xu, X.; Wu, Q.; Wang, Y.; Shen, H.; Song, Y.; Wang, H.; Zhu, Z.; Kang, D.; et al. Aptamer Generated by Cell-SELEX for Specific Targeting of Human Glioma Cells. ACS Appl. Mater. Interfaces 2020, 13, 9306–9315.
- He, L.; Huang, R.; Xiao, P.; Liu, Y.; Jin, L.; Liu, H.; Li, S.; Deng, Y.; Chen, Z.; Li, Z.; et al. Current signal amplification strategies in aptamer-based electrochemical biosensor: A review. Chin. Chem. Lett. 2021, 32, 1593–1602.
- Kim, Y.; Yang, J.; Hur, H.; Oh, S.; Lee, H. Highly Sensitive Colorimetric Assay of Cortisol Using Cortisol Antibody and Aptamer Sandwich Assay. Biosensors 2021, 11, 163.
- Malam, Y.; Loizidou, M.; Seifalian, A. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599.
- Hianik, T. Advances in Electrochemical and Acoustic Aptamer-Based Biosensors and Immunosensors in Diagnostics of Leukemia. Biosensors 2021, 11, 177.
- Liu, M.; Zhang, B.; Li, Z.; Wang, Z.; Li, S.; Liu, H.; Deng, Y.; He, N. Precise discrimination of Luminal A breast cancer subtype using an aptamer in vitro and in vivo. Nanoscale 2020, 12, 19689–19701.
- Liu, M.; Khan, A.; Wang, Z.; Liu, Y.; Yang, G.; Deng, Y.; He, N. Aptasensors for pesticide detection. Biosens. Bioelectron. 2019, 130, 174–184.
- Guo, T.; Tang, Q.; Guo, Y.; Qiu, H.; Dai, J.; Xing, C.; Zhuang, S.; Huang, G. Boron Quantum Dots for Photoacoustic Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 13, 306–311.
- Leng, C.; Zhang, X.; Xu, F.; Yuan, Y.; Pei, H.; Sun, Z.; Li, L.; Bao, Z. Engineering Gold Nanorod-Copper Sulfide Heterostructures with Enhanced Photothermal Conversion Efficiency and Photostability. Small 2018, 14, e1703077.
- Ha, M.; Nam, S.H.; Sim, K.; Chong, S.-E.; Kim, J.; Kim, Y.; Lee, Y.; Nam, J.-M. Highly Efficient Photothermal Therapy with Cell-Penetrating Peptide-Modified Bumpy Au Triangular Nanoprisms using Low Laser Power and Low Probe Dose. Nano Lett. 2020, 21, 731–739.
- Wang, H.; Chang, J.; Shi, M.; Pan, W.; Li, N.; Tang, B. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 58, 1057–1061.
- Hu, K.; Xie, L.; Zhang, Y.; Hanyu, M.; Yang, Z.; Nagatsu, K.; Suzuki, H.; Ouyang, J.; Ji, X.; Wei, J.; et al. Marriage of black phosphorus and Cu2+ as effective photothermal agents for PET-guided combination cancer therapy. Nat. Commun. 2020, 11, 1–15.
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A New Era for Cancer Treatment: Gold-Nanoparticle-Mediated Thermal Therapies. Small 2011, 7, 169–183.
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.-Y.; Zhang, H.; Xia, Y.; Li, X. Immuno Gold Nanocages with Tailored Optical Properties for Targeted Photothermal Destruction of Cancer Cells. Nano Lett. 2007, 7, 1318–1322.
- Piao, J.-G.; Wang, L.; Gao, F.; You, Y.-Z.; Xiong, Y.; Yang, L. Erythrocyte Membrane Is an Alternative Coating to Polyethylene Glycol for Prolonging the Circulation Lifetime of Gold Nanocages for Photothermal Therapy. ACS Nano 2014, 8, 10414–10425.
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer. CA Cancer J. Clin. 2011, 61, 250–281.
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2016, 27, 1604053.
- Zhou, Z.; Song, J.; Tian, R.; Yang, Z.; Yu, G.; Lin, L.; Zhang, G.; Fan, W.; Zhang, F.; Niu, G.; et al. Activatable Singlet Oxygen Generation from Lipid Hydroperoxide Nanoparticles for Cancer Therapy. Angew. Chem. 2017, 129, 6592–6596.
- He, T.; Jiang, C.; He, J.; Zhang, Y.; He, G.; Wu, J.; Lin, J.; Zhou, X.; Huang, P. Manganese-Dioxide-Coating-Instructed Plasmonic Modulation of Gold Nanorods for Activatable Duplex-Imaging-Guided NIR-II Photothermal-Chemodynamic Therapy. Adv. Mater. 2021, 33, 2008540.
- Yang, X.; Liu, R.; Zhong, Z.; Huang, H.; Shao, J.; Xie, X.; Zhang, Y.; Wang, W.; Dong, X. Platinum nanoenzyme functionalized black phosphorus nanosheets for photothermal and enhanced-photodynamic therapy. Chem. Eng. J. 2020, 409, 127381.
- Wang, X.; Mao, Y.; Sun, C.; Zhao, Q.; Gao, Y.; Wang, S. A versatile gas-generator promoting drug release and oxygen replenishment for amplifying photodynamic-chemotherapy synergetic anti-tumor effects. Biomaterials 2021, 276, 120985.
- Kwon, O.S.; Song, H.S.; Conde, J.; Kim, H.-I.; Artzi, N.; Kim, J.-H. Dual-Color Emissive Upconversion Nanocapsules for Differential Cancer Bioimaging In Vivo. ACS Nano 2016, 10, 1512–1521.
- Lei, Q.; Qiu, W.-X.; Hu, J.-J.; Cao, P.-X.; Zhu, C.-H.; Cheng, H.; Zhang, X.-Z. Multifunctional Mesoporous Silica Nanoparticles with Thermal-Responsive Gatekeeper for NIR Light-Triggered Chemo/Photothermal-Therapy. Small 2016, 12, 4286–4298.
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60.
- Ali, Z.; Wang, J.; Tang, Y.; Liu, B.; He, N.; Li, Z. Simultaneous detection of multiple viruses based on chemiluminescence and magnetic separation. Biomater. Sci. 2016, 5, 57–66.
- Kaur, H. Aptamer Conjugated Quantum Dots for Imaging Cellular Uptake in Cancer Cells. J. Nanosci. Nanotechnol. 2019, 19, 3798–3803.
- Wan, L.; Zhao, Q.; Zhao, P.; He, B.; Jiang, T.; Zhang, Q.; Wang, S. Versatile hybrid polyethyleneimine–mesoporous carbon nanoparticles for targeted delivery. Carbon 2014, 79, 123–134.
- Yu, S.; Bi, X.; Yang, L.; Wu, S.; Yu, Y.; Jiang, B.; Zhang, A.; Lan, K.; Duan, S. Co-Delivery of Paclitaxel and PLK1-Targeted siRNA Using Aptamer-Functionalized Cationic Liposome for Synergistic Anti-Breast Cancer Effects In Vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148.
- Zhen, D.; Zhong, F.; Yang, D.; Cai, Q.; Liu, Y. Photoelectrochemical aptasensor based on a ternary CdS/Au/TiO2 nanotube array for ultrasensitive detection of cytochrome c. Mater. Express 2019, 9, 319–327.
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364.
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827.
- Xi, Z.; Huang, R.; Li, Z.; He, N.; Wang, T.; Su, E.; Deng, Y. Selection of HBsAg-Specific DNA Aptamers Based on Carboxylated Magnetic Nanoparticles and Their Application in the Rapid and Simple Detection of Hepatitis B Virus Infection. ACS Appl. Mater. Interfaces 2015, 7, 11215–11223.
- Fang, X.; Cao, J.; Shen, A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2020, 57, 101662.
- Hu, J.J.; Liu, M.D.; Gao, F.; Chen, Y.; Peng, S.Y.; Li, Z.H.; Cheng, H.; Zhang, X.Z. Photo-controlled liquid metal nanoparticle-enzyme for starvation/photothermal therapy of tumor by win-win cooperation. Biomaterials 2019, 217, 119303.
- Cheng, Y.-J.; Zhang, A.-Q.; Hu, J.-J.; He, F.; Zeng, X.; Zhang, X.-Z. Multifunctional Peptide-Amphiphile End-Capped Mesoporous Silica Nanoparticles for Tumor Targeting Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 2093–2103.
- Wan, X.; Min, Y.; Bludau, H.; Keith, A.; Sheiko, S.S.; Jordan, R.; Wang, A.Z.; Sokolsky-Papkov, M.; Kabanov, A.V. Drug Combination Synergy in Worm-like Polymeric Micelles Improves Treatment Outcome for Small Cell and Non-Small Cell Lung Cancer. ACS Nano 2018, 12, 2426–2439.
- Tan, Y.; Li, Y.; Qu, Y.-X.; Su, Y.; Peng, Y.; Zhao, Z.; Fu, T.; Wang, X.-Q.; Tan, W. Aptamer-Peptide Conjugates as Targeted Chemosensitizers for Breast Cancer Treatment. ACS Appl. Mater. Interfaces 2020, 13, 9436–9444.
- Hu, X.; Zhang, M.; Xue, Q.; Cai, T. ATP Aptamer-Modified Quantum Dots with Reduced Glutathione/Adenosine Triphosphate Dual Response Features as a Potential Probe for Intracellular Drug Delivery Monitoring of Vesicular Nanocarriers. J. Biomed. Nanotechnol. 2019, 15, 319–328.
- Liu, M.; Yu, X.; Chen, Z.; Yang, T.; Yang, D.; Liu, Q.; Du, K.; Li, B.; Wang, Z.; Li, S.; et al. Aptamer selection and applications for breast cancer diagnostics and therapy. J. Nanobiotechnology 2017, 15, 1–16.
- Choi, H.; Shin, C. Negative Capacitance Transistor with Two-Dimensional Channel Material (Molybdenum disulfide, MoS2). Phys. Status Solidi 2019, 216, 1900177.
- Zhao, X.; Li, Z.; Zhang, J.; Gong, F.; Huang, B.; Zhang, Q.; Yan, Q.-L.; Yang, Z. Regulating safety and energy release of energetic materials by manipulation of molybdenum disulfide phase. Chem. Eng. J. 2021, 411, 128603.
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.-J.; Chen, H.-Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741.
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug Delivery with PEGylated MoS2Nano-sheets for Combined Photothermal and Chemotherapy of Cancer. Adv. Mater. 2014, 26, 3433–3440.
- Kim, J.; Kim, H.; Kim, W.J. Single-Layered MoS2 -PEI-PEG Nanocomposite-Mediated Gene Delivery Controlled by Photo and Redox Stimuli. Small 2015, 12, 1184–1192.
- Pang, B.; Yang, H.; Wang, L.; Chen, J.; Jin, L.; Shen, B. Aptamer modified MoS2 nanosheets application in targeted photothermal therapy for breast cancer. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 608, 125506.
- Yang, D.; Liu, M.; Xu, J.; Yang, C.; Wang, X.; Lou, Y.; He, N.; Wang, Z. Carbon nanosphere-based fluorescence aptasensor for targeted detection of breast cancer cell MCF-7. Talanta 2018, 185, 113–117.
- Liu, M.; Yang, T.; Chen, Z.; Wang, Z.; He, N. Differentiating breast cancer molecular subtypes using a DNA aptamer selected against MCF-7 cells. Biomater. Sci. 2018, 6, 3152–3159.
- Cole, J.; Mirin, N.A.; Knight, M.; Goodrich, G.P.; Halas, N. Photothermal Efficiencies of Nanoshells and Nanorods for Clinical Therapeutic Applications. J. Phys. Chem. C 2009, 113, 12090–12094.
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831.
- Yang, L.; Tseng, Y.-T.; Suo, G.; Chen, L.; Yu-Ting, T.; Chiu, W.-J.; Huang, C.-C.; Lin, C.-H. Photothermal Therapeutic Response of Cancer Cells to Aptamer–Gold Nanoparticle-Hybridized Graphene Oxide under NIR Illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106.
- Wu, P.; Gao, Y.; Zhang, H.; Cai, C. Aptamer-Guided Silver–Gold Bimetallic Nanostructures with Highly Active Surface-Enhanced Raman Scattering for Specific Detection and Near-Infrared Photothermal Therapy of Human Breast Cancer Cells. Anal. Chem. 2012, 84, 7692–7699.
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177.
- Siminzar, P.; Omidi, Y.; Golchin, A.; Aghanejad, A.; Barar, J. Targeted delivery of doxorubicin by magnetic mesoporous silica nanoparticles armed with mucin-1 aptamer. J. Drug Target. 2019, 28, 92–101.
- Zhong, L.; Gan, L.; Deng, Z.; Liu, X.; Peng, H.; Tang, H.; Liu, X.; Fang, F.; Yao, F.; Li, W.; et al. Antitumor Activity of Lipid-DNA Aptamer Modified T Lymphocytes in Carcinoma. J. Biomed. Nanotechnol. 2020, 16, 1110–1118.
- Zhao, Z.; Fan, H.; Zhou, G.; Bai, H.; Liang, H.; Wang, R.; Zhang, X.; Tan, W. Activatable Fluorescence/MRI Bimodal Platform for Tumor Cell Imaging via MnO2 Nanosheet–Aptamer Nanoprobe. J. Am. Chem. Soc. 2014, 136, 11220–11223.
- Wang, Y.; Chang, K.; Yang, C.; Li, S.; Wang, L.; Xu, H.; Zhou, L.; Zhang, W.; Tang, X.; Wang, Y.; et al. Highly Sensitive Electrochemical Biosensor for Circulating Tumor Cells Detection via Dual-Aptamer Capture and Rolling Circle Amplification Strategy. J. Biomed. Nanotechnol. 2019, 15, 1568–1577.
- Liu, W.; Zhang, K.; Zhuang, L.; Liu, J.; Zeng, W.; Shi, J.; Zhang, Z. Aptamer/photosensitizer hybridized mesoporous MnO2 based tumor cell activated ROS regulator for precise photodynamic therapy of breast cancer. Colloids Surfaces B Biointerfaces 2019, 184, 110536.
- Liu, M.; Wang, Z.; Tan, T.; Chen, Z.; Mou, X.; Yu, X.; Deng, Y.; Lu, G.; He, N. An Aptamer-Based Probe for Molecular Subtyping of Breast Cancer. Theranostics 2018, 8, 5772–5783.
- Xi, Z.; Huang, R.; Deng, Y.; He, N. Progress in Selection and Biomedical Applications of Aptamers. J. Biomed. Nanotechnol. 2014, 10, 3043–3062.
- Jin, Y.; Wang, H.; Li, X.; Zhu, H.; Sun, D.; Sun, X.; Liu, H.; Zhang, Z.; Cao, L.; Gao, C.; et al. Multifunctional DNA Polymer-Assisted Upconversion Therapeutic Nanoplatform for Enhanced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 26832–26841.
- Si, P.; Shi, J.; Zhang, P.; Wang, C.; Chen, H.; Mi, X.; Chu, W.; Zhai, B.; Li, W. MUC-1 recognition-based activated drug nanoplatform improves doxorubicin chemotherapy in breast cancer. Cancer Lett. 2019, 472, 165–174.
- Tang, Y.; Liu, H.; Chen, H.; Chen, Z.; Liu, Y.; Jin, L.; Deng, Y.; Li, S.; He, N. Advances in Aptamer Screening and Drug Delivery. J. Biomed. Nanotechnol. 2020, 16, 763–788.
- Liu, J.; Song, L.; Liu, S.; Jiang, Q.; Liu, Q.; Li, N.; Wang, Z.-G.; Ding, B. A DNA-Based Nanocarrier for Efficient Gene Delivery and Combined Cancer Therapy. Nano Lett. 2018, 18, 3328–3334.
- Xu, L.; Wang, S.-B.; Xu, C.; Han, D.; Ren, X.-H.; Zhang, X.-Z.; Cheng, S.-X. Multifunctional Albumin-Based Delivery System Generated by Programmed Assembly for Tumor-Targeted Multimodal Therapy and Imaging. ACS Appl. Mater. Interfaces 2019, 11, 38385–38394.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
08 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




